1.3.6. More About Protein
Defining Protein
Protein makes up approximately 20 percent of the human body and is present in every single cell. The word protein is a Greek word, meaning “of utmost importance.” Proteins are called the workhorses of life as they provide the body with structure and perform a vast array of functions. You can stand, walk, run, skate, swim, and more because of your protein-rich muscles. Protein is necessary for proper immune system function, digestion, and hair and nail growth, and is involved in numerous other body functions. In fact, it is estimated that more than one hundred thousand different proteins exist within the human body. In this chapter you will learn about the components of protein, the important roles that protein serves within the body, how the body uses protein, the risks and consequences associated with too much or too little protein, and where to find healthy sources of it in your diet.
What Is Protein?
Proteins, simply put, are macromolecules composed of amino acids. Amino acids are commonly called protein’s building blocks. Proteins are crucial for the nourishment, renewal, and continuance of life. Proteins contain the elements carbon, hydrogen, and oxygen just as carbohydrates and lipids do, but proteins are the only macronutrient that contains nitrogen.
Essential and Nonessential Amino Acids
Amino acids are further classified based on nutritional aspects. Recall that there are twenty different amino acids, and we require all of them to make the many different proteins found throughout the body. Eleven of these are called nonessential amino acids because the body can synthesize them. However, nine of the amino acids are called essential amino acids because we cannot synthesize them either at all or in sufficient amounts. These must be obtained from the diet. The nutritional value of a protein is dependent on what amino acids it contains and in what quantities.
The Role of Proteins in Foods
In addition to having many vital functions within the body, proteins perform different roles in our foods by adding certain functional qualities to them. Protein provides food with structure and texture and enables water retention. For example, proteins foam when agitated. (Picture whisking egg whites to make angel food cake. The foam bubbles are what give the angel food cake its airy texture.) Yogurt is another good example of proteins providing texture. Milk proteins called caseins coagulate, increasing yogurt’s thickness. Cooked proteins add some color and flavor to foods as the amino group binds with carbohydrates and produces a brown pigment and aroma. Eggs are between 10 and 15 percent protein by weight. Most cake recipes use eggs because the egg proteins help bind all the other ingredients together into a uniform cake batter. The proteins aggregate into a network during mixing and baking that gives cake structure.

Protein Digestion and Absorption
How do the proteins from foods, denatured or not, get processed into amino acids that cells can use to make new proteins? When you eat food the body’s digestive system breaks down the protein into the individual amino acids, which are absorbed and used by cells to build other proteins and a few other macromolecules, such as DNA. We previously discussed the general process of food digestion, let’s follow the specific path that proteins take down the gastrointestinal tract and into the circulatory system (Figure 1.3.6.1 “Digestion and Absorption of Protein”). Eggs are a good dietary source of protein and will be used as our example to describe the path of proteins in the processes of digestion and absorption. One egg, whether raw, hard-boiled, scrambled, or fried, supplies about six grams of protein.
Figure 1.3.6.1 Digestion and Absorption of Protein
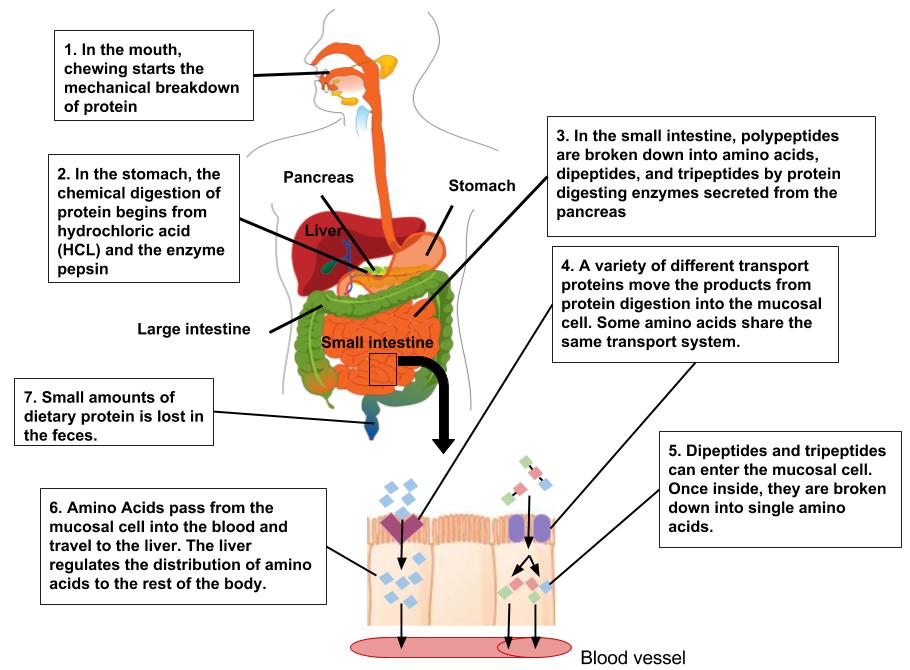
From the Mouth to the Stomach
Unless you are eating it raw, the first step in egg digestion (or any other protein food) involves chewing. The teeth begin the mechanical breakdown of the large egg pieces into smaller pieces that can be swallowed. The salivary glands provide some saliva to aid swallowing and the passage of the partially mashed egg through the esophagus. The mashed egg pieces enter the stomach through the esophageal sphincter. The stomach releases gastric juices containing hydrochloric acid and the enzyme, pepsin, which initiate the breakdown of the protein. The acidity of the stomach facilitates the unfolding of the proteins that still retain part of their three-dimensional structure after cooking and helps break down the protein aggregates formed during cooking. Pepsin, which is secreted by the cells that line the stomach, dismantles the protein chains into smaller and smaller fragments. Egg proteins are large globular molecules and their chemical breakdown requires time and mixing. The powerful mechanical stomach contractions churn the partially digested protein into a more uniform mixture called chyme. Protein digestion in the stomach takes a longer time than carbohydrate digestion, but a shorter time than fat digestion. Eating a high-protein meal increases the amount of time required to sufficiently break down the meal in the stomach. Food remains in the stomach longer, making you feel full longer.
From the Stomach to the Small Intestine
The stomach empties the chyme containing the broken down egg pieces into the small intestine, where the majority of protein digestion occurs. The pancreas secretes digestive juice that contains more enzymes that further break down the protein fragments. The cells that line the small intestine release additional enzymes that finally break apart the smaller protein fragments into the individual amino acids. The muscle contractions of the small intestine mix and propel the digested proteins to the absorption sites. In the lower parts of the small intestine, the amino acids are transported from the intestinal lumen through the intestinal cells to the blood. This movement of individual amino acids requires special transport proteins and the cellular energy molecule, adenosine triphosphate (ATP). Once the amino acids are in the blood, they are transported to the liver. As with other macronutrients, the liver is the checkpoint for amino acid distribution and any further breakdown of amino acids, which is very minimal. Recall that amino acids contain nitrogen, so further catabolism of amino acids releases nitrogen-containing ammonia. Because ammonia is toxic, the liver transforms it into urea, which is then transported to the kidney and excreted in the urine. Urea is a molecule that contains two nitrogens and is highly soluble in water. This makes it a good choice for transporting excess nitrogen out of the body. Because amino acids are building blocks that the body reserves in order to synthesize other proteins, more than 90 percent of the protein ingested does not get broken down further than the amino acid monomers.
Amino Acids Are Recycled
Just as some plastics can be recycled to make new products, amino acids are recycled to make new proteins. All cells in the body continually break down proteins and build new ones, a process referred to as protein turnover. Every day over 250 grams of protein in your body are dismantled and 250 grams of new protein are built. To form these new proteins, amino acids from food and those from protein destruction are placed into a “pool.” Though it is not a literal pool, when an amino acid is required to build another protein it can be acquired from the additional amino acids that exist within the body. Amino acids are used not only to build proteins, but also to build other biological molecules containing nitrogen, such as DNA, RNA, and to some extent to produce energy. It is critical to maintain amino acid levels within this cellular pool by consuming high-quality proteins in the diet, or the amino acids needed for building new proteins will be obtained by increasing protein destruction from other tissues within the body, especially muscle.
This amino acid pool is less than one percent of total body-protein content. Thus, the body does not store protein as it does with carbohydrates (as glycogen in the muscles and liver) and lipids (as triglycerides in adipose tissue).
Figure 1.3.6.2 Options for Amino Acid Use in the Human Body
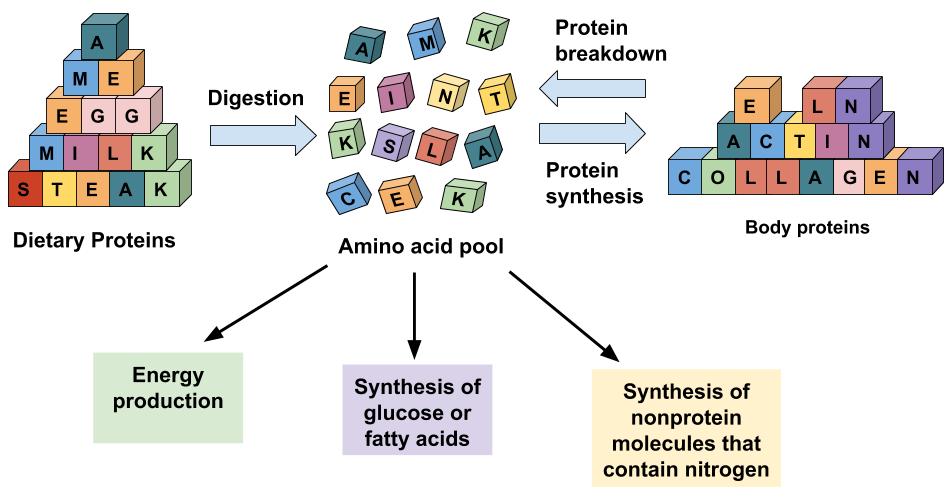
Amino acids in the cellular pool come from dietary protein and from the destruction of cellular proteins. The amino acids in this pool need to be replenished because amino acids are outsourced to make new proteins, energy, and other biological molecules.
The Functions of Proteins in the Body
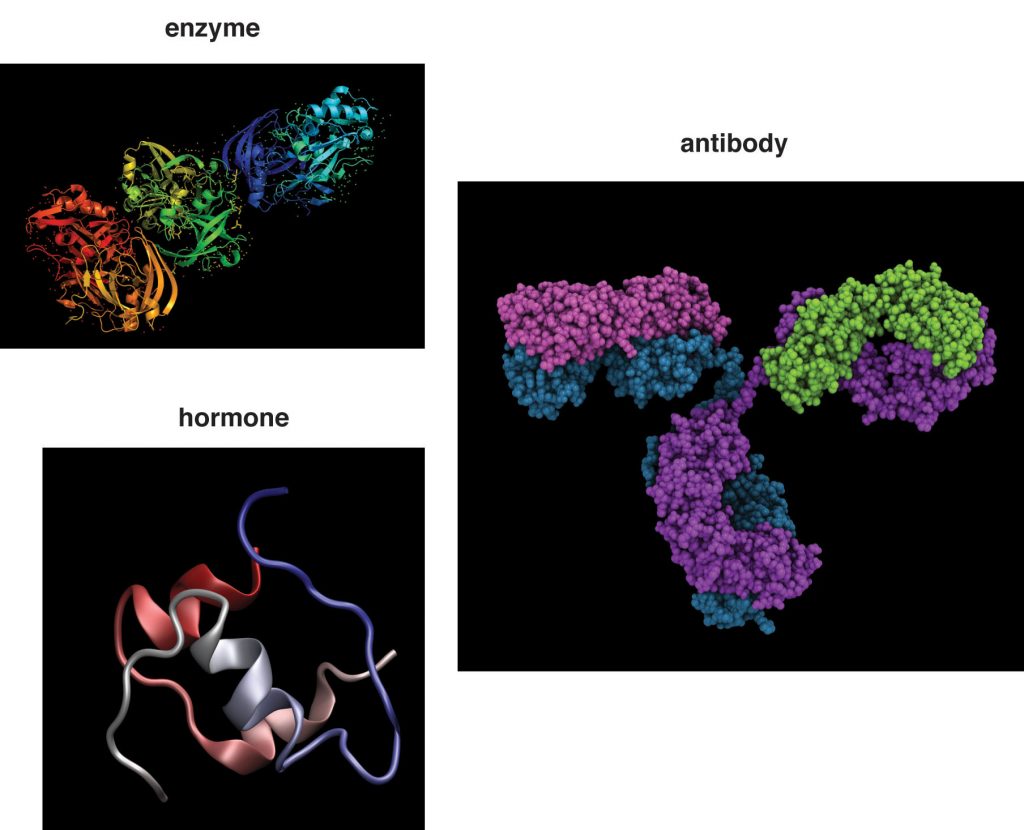
Figure 1.3.6.3 Proteins
Structure and Motion
Figure 1.3.6.4 Collagen Structure
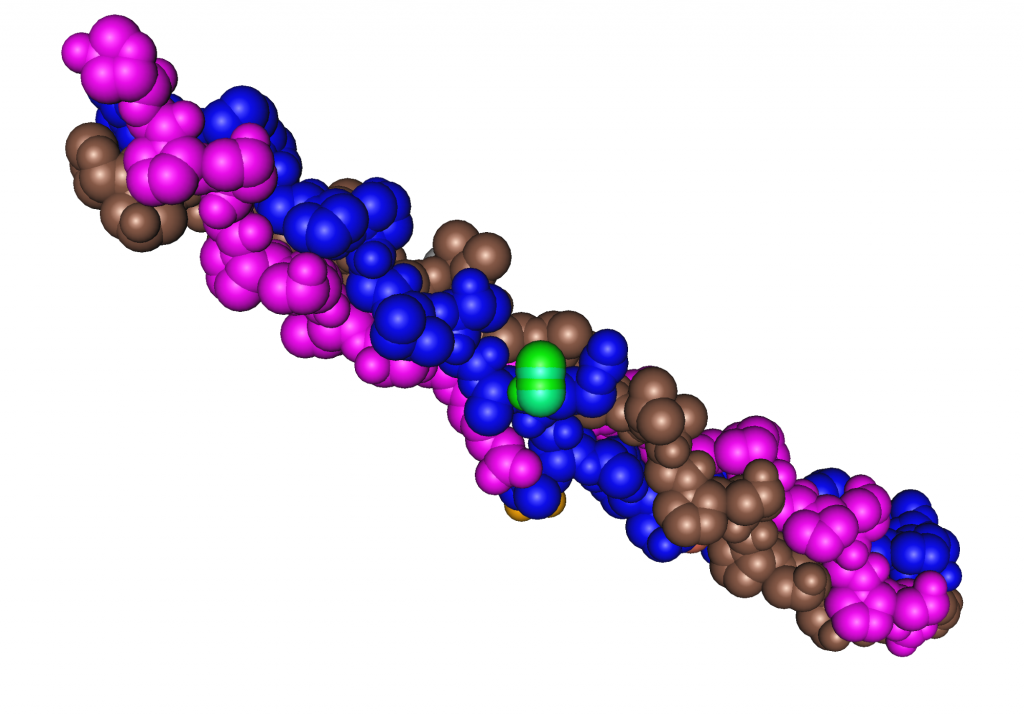
More than one hundred different structural proteins have been discovered in the human body, but the most abundant by far is collagen, which makes up about 6 percent of total body weight. Collagen makes up 30 percent of bone tissue and comprises large amounts of tendons, ligaments, cartilage, skin, and muscle. Collagen is a strong, fibrous protein in a highly ordered structure even stronger than steel fibers of the same size. Collagen makes bones strong, but flexible. Collagen fibers in the skin’s dermis provide it with structure, and the accompanying elastin protein fibrils make it flexible. Pinch the skin on your hand and then let go; the collagen and elastin proteins in skin allow it to go back to its original shape. Smooth-muscle cells that secrete collagen and elastin proteins surround blood vessels, providing the vessels with structure and the ability to stretch back after blood is pumped through them. Another strong, fibrous protein is keratin, which is what skin, hair, and nails are made of. The closely packed collagen fibrils in tendons and ligaments allow for synchronous mechanical movements of bones and muscle and the ability of these tissues to spring back after a movement is complete.
Enzymes
Although proteins are found in the greatest amounts in connective tissues such as bone, their most extraordinary function is as enzymes. Enzymes are proteins that conduct specific chemical reactions. An enzyme’s job is to provide a site for a chemical reaction and to lower the amount of energy and time it takes for that chemical reaction to happen (this is known as “catalysis”). On average, more than one hundred chemical reactions occur in cells every single second and most of them require enzymes. The liver alone contains over one thousand enzyme systems. Enzymes are specific and will use only particular substrates that fit into their active site, similar to the way a lock can be opened only with a specific key. Nearly every chemical reaction requires a specific enzyme. Fortunately, an enzyme can fulfill its role as a catalyst over and over again, although eventually it is destroyed and rebuilt. All bodily functions, including the breakdown of nutrients in the stomach and small intestine, the transformation of nutrients into molecules a cell can use, and building all macromolecules, including protein itself, involve enzymes.
Hormones
Proteins are responsible for hormone synthesis. Hormones are the chemical messages produced by the endocrine glands. When an endocrine gland is stimulated, it releases a hormone. The hormone is then transported in the blood to its target cell, where it communicates a message to initiate a specific reaction or cellular process. For instance, after you eat a meal, your blood glucose levels rise. In response to the increased blood glucose, the pancreas releases the hormone insulin. Insulin tells the cells of the body that glucose is available and to take it up from the blood and store it or use it for making energy or building macromolecules. A major function of hormones is to turn enzymes on and off, so some proteins can even regulate the actions of other proteins. While not all hormones are made from proteins, many of them are.
Fluid and Acid-Base Balance
Proper protein intake enables the basic biological processes of the body to maintain the status quo in a changing environment. Fluid balance refers to maintaining the distribution of water in the body. If too much water in the blood suddenly moves into a tissue, the results are swelling and, potentially, cell death. Water always flows from an area of high concentration to one of a low concentration. As a result, water moves toward areas that have higher concentrations of other solutes, such as proteins and glucose. To keep the water evenly distributed between blood and cells, proteins continuously circulate at high concentrations in the blood. The most abundant protein in blood is the butterfly-shaped protein known as albumin. Albumin’s presence in the blood makes the protein concentration in the blood similar to that in cells. Therefore, fluid exchange between the blood and cells is not in the extreme, but rather is minimized to preserve the status quo.
Protein is also essential in maintaining proper pH balance (the measure of how acidic or basic a substance is) in the blood. Blood pH is maintained between 7.35 and 7.45, which is slightly basic. Even a slight change in blood pH can affect body functions.
Albumin acts as a buffer against abrupt changes in the concentrations of other molecules, thereby balancing blood pH and maintaining the status quo. The protein hemoglobin also participates in acid-base balance by binding and releasing protons.
Transport
Albumin and hemoglobin also play a role in molecular transport. Albumin chemically binds to hormones, fatty acids, some vitamins, essential minerals, and drugs, and transports them throughout the circulatory system. Each red blood cell contains millions of hemoglobin molecules that bind oxygen in the lungs and transport it to all the tissues in the body.
A cell’s plasma membrane is usually not permeable to large polar molecules, so to get the required nutrients and molecules into the cell many transport proteins exist in the cell membrane. Some of these proteins are channels that allow particular molecules to move in and out of cells. Others act as one-way taxis and require energy to function.
Protection
Earlier we discussed that the strong collagen fibers in skin provide it with structure and support. The skin’s dense network of collagen fibers also serves as a barricade against harmful substances. The immune system’s attack and destroy functions are dependent on enzymes and antibodies, which are also proteins. An enzyme called lysozyme is secreted in the saliva and attacks the walls of bacteria, causing them to rupture. Certain proteins circulating in the blood can be directed to build a molecular knife that stabs the cellular membranes of foreign invaders. The antibodies secreted by the white blood cells survey the entire circulatory system looking for harmful bacteria and viruses to surround and destroy. Antibodies also trigger other factors in the immune system to seek and destroy unwanted intruders.
Wound Healing and Tissue Regeneration
Proteins are involved in all aspects of wound healing, a process that takes place in three phases: inflammatory, proliferative, and remodeling. For example, if you were sewing and pricked your finger with a needle, your flesh would turn red and become inflamed. Within a few seconds bleeding would stop. The healing process begins with proteins, which dilate blood vessels at the site of injury. An additional protein helps to secure platelets that form a clot to stop the bleeding. Next, in the proliferative phase, cells move in and mend the injured tissue by installing newly made collagen fibers. The collagen fibers help pull the wound edges together. In the remodeling phase, more collagen is deposited, forming a scar. Scar tissue is only about 80 percent as functional as normal uninjured tissue. If a diet is insufficient in protein, the process of wound healing is markedly slowed.
While wound healing takes place only after an injury is sustained, a different process called tissue regeneration is ongoing in the body. The main difference between wound healing and tissue regeneration is in the process of regenerating an exact structural and functional copy of the lost tissue. Thus, old, dying tissue is not replaced with scar tissue but with brand new, fully functional tissue. Some cells (such as skin, hair, nails, and intestinal cells) have a very high rate of regeneration, while others, (such as heart-muscle cells and nerve cells) do not regenerate at any appreciable levels. Tissue regeneration is the creation of new cells (cell division), which requires many different proteins including enzymes that synthesize RNA and proteins, transport proteins, hormones, and collagen. In a hair follicle, cells divide and a hair grows in length. Hair growth averages 1 centimeter per month and fingernails about 1 centimeter every one hundred days. The cells lining the intestine regenerate every three to five days. Protein-inadequate diets impair tissue regeneration, causing many health problems including impairment of nutrient digestion and absorption and, most visibly, hair and nail growth.
Energy Production
Some of the amino acids in proteins can be disassembled and used to make energy (Figure 1.3.6.2 “Options for Amino Acid Use in the Human Body”). Only about 10 percent of dietary proteins are catabolized each day to make cellular energy.
If a person’s diet does not contain enough carbohydrates and fats their body will use more amino acids to make energy, which compromises the synthesis of new proteins and destroys muscle proteins. Alternatively, if a person’s diet contains more protein than the body needs, the extra amino acids will be broken down and transformed into fat.
Diseases Involving Proteins
As you may recall, moderation refers to having the proper amount of a nutrient—having neither too little nor too much. A healthy diet incorporates all nutrients in moderation. Low protein intake has several health consequences, and a severe lack of protein in the diet eventually causes death. Although severe protein deficiency is a rare occurrence in children and adults in North America, it is estimated that more than half of the elderly in nursing homes are protein-deficient. The Acceptable Macronutrient Distribution Range (AMDR) for protein for adults is between 10 and 35 percent of kilocalories, which is a fairly wide range. The percent of protein in the diet that is associated with malnutrition and its health consequences is less than 10 percent, but this is often accompanied by deficiencies in calories and other micronutrients.
Health Consequences of Protein Deficiency
Recall that one of protein’s functional roles in the body is fluid balance. Diets extremely low in protein do not provide enough amino acids for the synthesis of albumin. One of the functions of albumin is to hold water in the blood vessels, so having lower concentrations of blood albumin results in water moving out of the blood vessels and into tissues, causing swelling. Primary symptoms include not only swelling, but also diarrhea, fatigue, peeling skin, and irritability. Severe protein deficiency in addition to other micronutrient deficiencies, such as folate (vitamin B9), iodine, iron, and vitamin C all contribute to the many health manifestations of this syndrome.
Although organ system function is compromised by undernutrition, the ultimate cause of death from protein deficiency is usually infection. Undernutrition is intricately linked with suppression of the immune system at multiple levels, so undernourished children commonly die from severe diarrhea and/or pneumonia resulting from bacterial or viral infection. The United Nations Children’s Fund (UNICEF), the most prominent agency with the mission of changing the world to improve children’s lives, reports that undernutrition causes at least one-third of deaths of young children. As of 2008, the prevalence of children under age five who were underweight was 26 percent. The percentage of underweight children has declined less than 5 percent in the last eighteen years despite the Millennium Development Goal of halving the proportion of people who suffer from hunger by the year 2015.
Figure 1.3.6.6 Causes of Death for Children Under the Age of Five, Worldwide
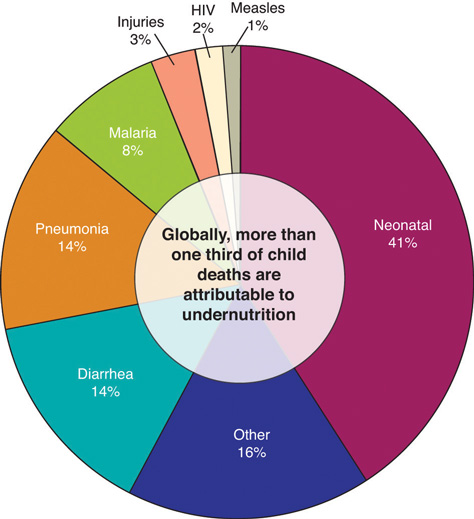
Source: Diseases Involving Proteins
Health Consequences of Too Much Protein in the Diet
An explicit definition of a high-protein diet has not yet been developed by the Food and Nutrition Board of the Institute of Medicine (IOM), but typically diets high in protein are considered as those that derive more than 30 percent of calories from protein. Many people follow high-protein diets because marketers tout protein’s ability to stimulate weight loss. It is true that following high-protein diets increases weight loss in some people. However the number of individuals that remain on this type of diet is low and many people who try the diet and stop will regain the weight they had lost. Additionally, there is a scientific hypothesis that there may be health consequences of remaining on high-protein diets for the long-term, but clinical trials are ongoing or scheduled to examine this hypothesis further. As the high-protein diet trend arose so did the intensely debated issue of whether there are any health consequences of eating too much protein. Observational studies conducted in the general population suggest diets high in animal protein, specifically those in which the primary protein source is red meat, are linked to a higher risk for kidney stones, kidney disease, liver malfunction, colorectal cancer, and osteoporosis. However, diets that include lots of red meat are also high in saturated fat and cholesterol and sometimes linked to unhealthy lifestyles, so it is difficult to conclude that the high protein content is the culprit.
High protein diets appear to only increase the progression of kidney disease and liver malfunction in people who already have kidney or liver malfunction, and not to cause these problems. However, the prevalence of kidney disorders is relatively high and under diagnosed. In regard to colon cancer, an assessment of more than ten studies performed around the world published in the June 2011 issue of PLoS purports that a high intake of red meat and processed meat is associated with a significant increase in colon cancer risk.[1] Although there are a few ideas, the exact mechanism of how proteins, specifically those in red and processed meats, causes colon cancer is not known and requires further study.
Some scientists hypothesize that high-protein diets may accelerate bone-tissue loss because under some conditions the acids in protein block absorption of calcium in the gut, and, once in the blood, amino acids promote calcium loss from bone; however even these effects have not been consistently observed in scientific studies. Results from the Nurses’ Health Study suggest that women who eat more than 95 grams of protein each day have a 20 percent higher risk for wrist fracture.[2]
Other studies have not produced consistent results. The scientific data on high protein diets and increased risk for osteoporosis remains highly controversial and more research is needed to come to any conclusions about the association between the two.[3]
High-protein diets can restrict other essential nutrients. The American Heart Association (AHA) states that “High-protein diets are not recommended because they restrict healthful foods that provide essential nutrients and do not provide the variety of foods needed to adequately meet nutritional needs. Individuals who follow these diets are therefore at risk for compromised vitamin and mineral intake, as well as potential cardiac, renal, bone, and liver abnormalities overall.”[4]
As with any nutrient, protein must be eaten in proper amounts. Moderation and variety are key strategies to achieving a healthy diet and need to be considered when optimizing protein intake. While the scientific community continues its debate about the particulars regarding the health consequences of too much protein in the diet, you may be wondering just how much protein you should consume to be healthy.
- Chan DS, Lau R, Aune D et al. Red and Processed Meat and Colorectal Cancer Incidence: Meta-Analysis of Prospective Studies. PLoS One. 2011; 6(6), e20456. http://dx.plos.org/10.1371/journal.pone.0020456. Accessed September 30, 2017. ↵
- Uriel S. Barzel, Linda K. Massey. Excess Dietary Protein Can Adversely Affect Bone. J Nutr. 1998; 128(6), 1051–53. http://jn.nutrition.org/content/128/6/1051.long. Accessed September 28, 2017. ↵
- St. Jeor ST, Howard BV, Prewitt TE et al. Dietary Protein and Weight Reduction: A Statement for Healthcare Professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001; 104, 1869–74. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11591629. Accessed September 28, 2017. ↵
- St. Jeor ST, Howard BV, Prewitt TE et al. Dietary Protein and Weight Reduction: A Statement for Healthcare Professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001; 104, 1869–74. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=11591629. Accessed September 28, 2017. ↵

