7 Primate Ecology and Behavior
Karin Enstam Jaffe, Ph.D., Sonoma State University
This chapter is a revision from “Chapter 6: Primate Ecology and Behavior” by Karin Enstam Jaffe. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Describe the variables that affect primate diets.
- Explain how primates interact with other organisms in their environment.
- Discuss why primates live in groups, types of primate groups, and components of their social systems.
- Describe the reproductive strategies of males and females.
- Explain the ways in which primates communicate.
- Discuss the evidence for primate cultural traditions.
Nonhuman primates (hereafter, “primates”) are a fascinating group of animals, whose similarity to humans can be striking. Because of this similarity, studying primates helps anthropologists to gain insight into how our human ancestors may have behaved. It also allows us to better understand our own behavior through comparison (examining similarities and differences) with other primates as well as by comparing different species of primates to one another. In this way, studying primates helps anthropologists comprehend humanity from a biological perspective, which contributes to anthropology’s commitment to holism, the idea that the parts of a system interconnect and interact to make up the whole.

Ethology is the study of animal behavior, while primatology is the study of primate behavior. People who study primates are called primatologists. Research on primates can be conducted in the field (i.e., on wild primates) or in captivity (i.e., zoos) and may or may not involve experiments, such as playing recorded alarm calls to see how individuals react. Unlike some other Science, Technology, Engineering, and Math (STEM) fields, primatology has a long history of research conducted by women (see “Special Topic: Women in Primatology”). Primatologists come from many different disciplines, have diverse backgrounds, and study primates for different reasons. Biologists study primates as examples of evolutionary theories like natural selection, and to understand behaviors as adaptations, or traits with a function that increases fitness, i.e. an individual’s survival and/or reproduction. Primate intelligence is of interest to psychologists who want to learn more about deception or cooperation and to linguists interested in the principles of communication and language. Ecologists consider how primates interact with the habitats they occupy, and conservationists examine how primates are affected by deforestation, poaching, or illegal animal trade (see Appendix B: Primate Conservation for more information on these topics). Biological anthropologists, like myself (Figure 7.1), who study primates are interested in learning about their social complexity, and ecological and behavioral variation, to better understand the biological basis of human behavior. And, similar to biologists, we also explore how primate behavior is adaptive and contributes to individual fitness. Like other sciences, primatology is only as strong as its researchers, methods, and theories, and the field has benefitted recently from efforts to increase diversity and reckon with its colonialist past, as discussed below in “Special Topic: Women in Primatology.”
Humans share many traits in common with primates. As you learned in Chapter 5, some of these traits are similar due to homology, traits both species inherited from a common primate ancestor. For example, like most other primates, humans are social animals who live in groups. Group living did not evolve independently in humans and other primates. Rather, group living is a trait that evolved in a primate ancestor, and because it benefited survival, it was retained in the species’ descendants (or the species that come after the ancestor species). In contrast, humans and other primates can have similar traits that evolved independently, which is called analogy. For example, both humans and Japanese macaques (Macaca fuscata) use natural hot springs (Figures 7.2a-b). Research on these monkeys indicates that sitting in hot springs reduces stress and helps keep them warm, much as it does for humans (Takeshita et al. 2018). But this behavior is not the result of humans and Japanese macaques having a shared ancestor who used hot springs. Rather, the behavior arose independently in two species that both occupy northerly environments and adapted to cold climates using a similar behavior. Studying the homologous traits we share with other primates, like living in groups, helps us develop hypotheses about human behaviors as adaptations, which in turn helps us develop models for the behavior of our human ancestors. Studying analogous traits, like hot springs use, allows us to better understand the effects of ecological variables on morphology and behavior of both primates and humans, living and extinct.

Special Topic: Women in Primatology
While many STEM fields have traditionally been, and continue to be, dominated by men, primatology has a long history of significant research conducted by women. This is due, in part, to the fact that three of the most well-known primatologists are women. In the early 1960s, British paleoanthropologist Louis Leakey (discussed in Chapters 9 and 10) was looking for students to study the great apes in hopes of shedding light on the behaviors of our early ancestors. He chose Jane Goodall (Figure 7.3a) to study chimpanzees (Pan troglodytes), Birute Galdikas (Figure 7.3b) to study Bornean orangutans (Pongo pygmaeus), and Dian Fossey (Figure 7.3c) to study mountain gorillas (Gorilla beringei beringei). The work of these three women, sometimes referred to as Leakey’s “Trimates,” has transformed our understanding of ape (and primate) behavior.

Arriving at the Gombe Stream Reserve in Tanzania in 1960, Jane Goodall was one of the first scientists to conduct a long-term study of wild nonhuman primates. Before then, most studies lasted less than a year and were often zoo-based. By 1961, she had made two astounding observations that forced us to reconsider what differentiates humans from the rest of the primate order. She observed chimpanzees eating a colobus monkey, the first reported evidence of meat eating in our closest relatives (she later observed them hunting and sharing meat). And she discovered that chimpanzees make and use tools by stripping leaves off twigs to “fish” for termites. Her work, spanning several decades, has produced long-term data on chimpanzee mating strategies, mother-infant bonds, and aggression. In the mid-1980s, Goodall transitioned from field researcher to conservationist and activist, advocating for the humane use of nonhuman animals (Stanford 2017).
Birute Galdikas began her study of orangutans in Kalimantan, Borneo, in 1971. Hers was the first long-term study conducted on the Bornean orangutan. Galdikas and her colleagues have collected over 150,000 hours of observational data, focusing on the life histories of individual orangutans. While conducting behavioral research, Galdikas discovered that the pet trade and habitat loss were adversely affecting the orangutan population. Eventually, Galdikas’s conservation efforts began to extend beyond advocacy and into rehabilitation and forest preservation (Bell 2017). If you would like to learn more about primate conservation efforts, please see Appendix B: Primate Conservation.
In 1967, Dian Fossey began her long-term study of mountain gorillas and founded the Karisoke Research Center in Rwanda. Her and her colleagues’ research, over several decades, revealed much about gorilla social behavior, ecology, and life history. Her efforts also led to the development of mountain gorilla conservation programs. However, she was a controversial figure, as discussed below. Fossey was murdered in December 1985; the case remains unsolved (Stewart 2017).
Decolonizing Primatology
Recently, the movement to decolonize primatology, by understanding and highlighting the theories and research of non-Western individuals and perspectives, has gathered steam. This movement draws attention to the maltreatment of local people by Western primatologists. For example, Michelle Rodrigues (2019) argues that it’s time we stop focusing on the scientific and conservation contributions of Dian Fossey and acknowledge that her “active conservation” techniques included kidnapping and torturing local Rwandans who were known as, or suspected to be, gorilla poachers. Rodrigues (2019) argues:
The image of Fossey, a white American woman, whipping and torturing black African poachers is evocative of the behavior of white slaveholders in the American South. It is appalling enough to think of that behavior occurring in the 1850s; there is no way we can explain Fossey’s behavior in the 1970s as the product of “a different time.” Yet, almost three decades later, the romantic notion of a noble martyr who died for her devotion to gorillas prevails, and these terrifying actions are often described as simply unorthodox methods. Perhaps these truths are softened due to fears that the reality of this legacy would harm gorilla conservation efforts. But memorializing her as a martyr and patron saint of gorilla conservation demands that we forget the cruel acts she advocated for and performed.
Further, Louis Leakey’s installment of Goodall, Galdikas, and Fossey to study chimpanzees, orangutans, and mountain gorillas, respectively, is itself viewed as recapitulating the colonial legacy in Africa and Asia. Given that Leakey was the offspring of British missionaries, Rodrigues (2019) argues, it is no accident that he was willing to mentor British and American women, while overlooking women from Africa and Asia as potential researchers. This leads us to another level of the decolonizing movement, which aims to highlight the research of non-Western primatologists, particularly those living in what primatologists refer to as “habitat countries” that are home to living primates. As you will see in this chapter, scientists from diverse backgrounds are active contributors to exciting research on primates around the world.
Ecology
The more than 600 species and subspecies of living primates are highly diverse in their dietary preferences and the habitats they occupy. In this section we’ll briefly discuss aspects of ecology, or the relationship between organisms and their physical surroundings, that impact a primate’s life, the foods they eat, and the other species with whom they interact.
Primate Diets
Diet may be the most important variable influencing variation in primate morphology, behavior, and ecology. Most primates are omnivores who ingest a variety of foods in order to obtain appropriate levels of protein, carbohydrates, fats, and fluids, but one type of food often makes up the majority of each species’ diet. You learned about the dental and digestive adaptations of frugivores (who feed primarily on fruit), folivores (whose diet consists mostly of leaves), and insectivores (who eat mainly insects) in Chapter 5, so we will not discuss them again here.
Body Size and Diet

Insects are a high-quality food, full of easily digestible protein and high in calories that meet most of a primate’s dietary needs. Although all primates will eat insects if they come upon them, those species that rely most heavily on insects tend to be the smallest. Why? Because larger primates simply cannot capture and consume enough insects every day to survive. Because of their small size (less than 150 g), spectral tarsiers (Tarsius spectrum) have a fast metabolism, which means they turn food to energy quickly, but they do not need to consume large amounts of food each day. It does not matter to a spectral tarsier that a grasshopper only weighs 300 mg, because the tarsier (Tarsius) itself is so small that one grasshopper is a good-size meal (Figure 7.4a). That same grasshopper is not even a snack for an adult male mountain gorilla (Gorilla beringei beringei), who may weigh up to 200 kg. Fortunately for gorillas (Gorilla), their large body size means they have a slow metabolism, converting food into energy much more slowly, so they can eat lower quality food that takes longer to digest, provided there is a lot of it. For gorillas, leaves, which are hard to digest but plentiful, fit the bill (Figure 7.4b). Most medium-sized primates are highly frugivorous, and supplement their fruit based diet in ways that correspond with their size: Smaller frugivores tend to supplement with insects, while larger frugivores tend to supplement with leaves.
Food Abundance and Distribution
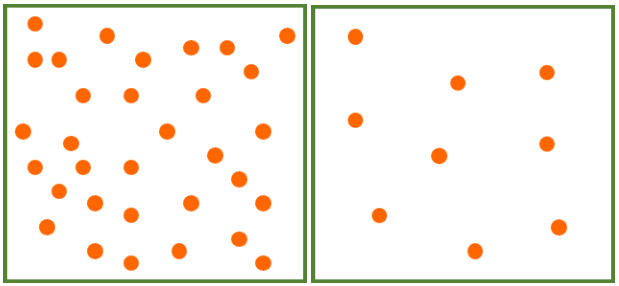
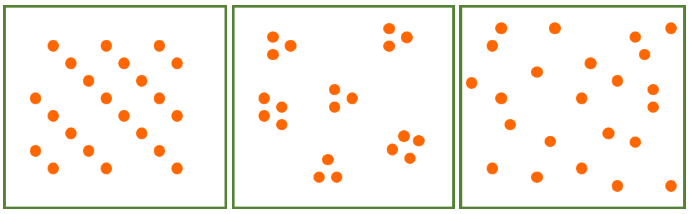
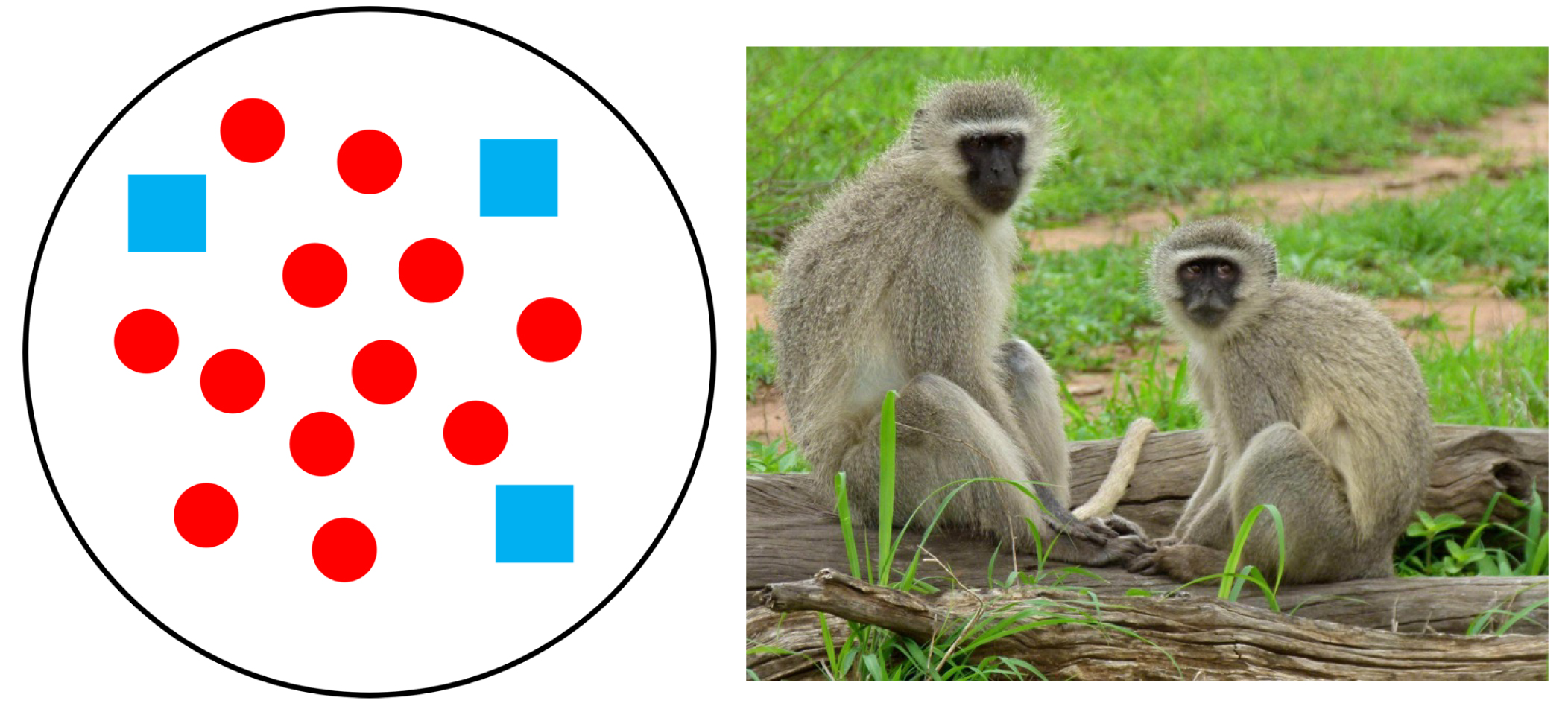
Nutrients are not the only dietary considerations primates must make. They must also ensure that they consume more calories than they use. The abundance and distribution of food affect energy expenditure and calorie intake because they determine how far animals must travel in search of food and how much they must compete to obtain it. Abundance refers to how much food is available in a given area while distribution refers to how food is spread out. In terms of abundance, food is either plentiful or scarce (Figure 7.5a–b). Food is distributed in one of three ways: uniformly (Figure 7.6a), in clumps (Figure 7.6b), or randomly (Figure 7.6c). In general, higher-quality foods, like fruit and insects, are less abundant and have patchier distributions than lower-quality foods, like leaves. Primates who eat fruit or insects usually have to travel farther to find food and burn more calories in the process. Abundance and distribution of food is another reason why larger primates tend to rely more heavily on leaves than either fruit or insects.
Community Ecology
Primates are members of broader ecological communities composed of other species, including other primates, predators, parasites, and even humans. Community ecology deals with the relationships and interactions between different organisms that occupy the same habitat. Interactions with conspecifics (members of the same species) and heterospecifics (members of different species) are critical aspects of ecological communities. Some habitats support highly diverse primate communities consisting of 10 or more primate species. How can so many primate species occupy the same area and avoid competition? In most cases, the primate species that live together occupy different niches, which means they do not meet their needs for food and shelter in the exact same way. Two species can avoid competition by eating different kinds of food, living at different levels of a forest, or even searching for food at different times of day. Because tropical rainforests, like Manu National Park in Peru, are highly variable, with many habitats and many sources of food and shelter, there are many different niches for multiple species to exploit, and large primate communities can result (Figure 7.7).

Competitive Interactions
Although species living in the same location often occupy different niches to avoid competition, when a resource that is important for survival or reproduction is scarce, individuals will compete to obtain that resource. This is a central tenet of Charles Darwin’s theory of evolution by natural selection (see Chapter 2). Competition between primates takes two forms: Individuals engage in direct competition, which involves physical interaction between individuals (such as fighting), over resources that are large and worth defending (fruit is a good example of a food resource over which primates will fight). Individuals engage in indirect competition, in which there is no physical interaction between individuals, when a resource is small. Primates often engage in indirect competition for insects, like grasshoppers, that are eaten quickly, often before another individual arrives on the scene. Primates may engage in direct and/or indirect competition with members of their own group, with members of other groups of conspecifics, or with heterospecifics.
Predator-Prey Interactions
The plants and animals that primates eat are an important part of their ecological community. In addition to insects, many primates incorporate some vertebrate (animals with an internal spinal column or backbone) prey into their diet. Often, predation by primates is opportunistic, occurring because the prey happens to be in the right place at the right time. I’ve observed vervets (Chlorocebus pygerythrus) opportunistically killing lizards by smashing them against a rock or tree trunk and eating them. More rarely, hunting is deliberate and cooperative. In some chimpanzee (Pan troglodytes) populations, hunts involve multiple individuals, each of whom plays a specific role and is rewarded afterward with a share of the prey that has been captured (Samuni et al. 2018).
All primates are susceptible to predation by mammalian carnivores (animals whose diet consists primarily of animal tissue (e.g., Figure 7.8a), reptiles (e.g., Figure 7.8b), or birds of prey (e.g., Figure 7.8c). Although the specific predators found in an ecological community differ based on geography, smaller primates always fall prey to a wider range of predators. Because predators are diverse in their hunting tactics, primates have evolved a wide range of tactics to avoid or escape them. We will discuss some of these behavioral adaptations later in this chapter in the section titled “Why Do Primates Live in Groups?.”

Mutualistic Interactions
So far, we’ve discussed competitive and predator-prey interactions in primate communities. But there are some interactions (between different primate species and between primates and other species) that are mutualistic, which is when organisms of different species work together, each benefiting from the interaction or relationship. One example is seed dispersal, which is the process by which seeds move away from the plant that produced them in preparation for germination and becoming a new plant. When seeds are dispersed by animals, like primates, it is an example of mutualism. The primate eats the fruit of a plant, which provides nutrients for its body, and in the process ingests the plant’s seeds. Later, it deposits the seeds at another location as a pile of fertilizer.
Another example of mutualism is polyspecific associations, which are associations between two or more different species that are maintained by behavioral changes by at least one of the species. While some associations are short in duration, others are semi-permanent. The mutualistic benefits of polyspecific associations include one species gaining access to food that would otherwise have been inaccessible or being alerted to the presence of predators that they would not have not have known were present otherwise. In some cases, individuals seem to recognize and seek out specific members of another species. Twenty years of observations on chimpanzees and Western lowland gorillas (Gorilla gorilla gorilla) in the Republic of Congo has revealed social ties (some might call them friendships) between individual chimpanzees and gorillas that last for years and occur in a variety of social contexts, including play (Sanz et al. 2022).
Parasite-Host Interactions
Primates are hosts for a variety of parasites, which are organisms that live in or on another organism (the host). Parasites come in many forms and pose varying levels of danger to the host. Blood parasites cause diseases like yellow fever and malaria. Skin parasites include fleas and ticks, which feed on the host’s blood, and botflies, which lay eggs in the host’s flesh. Bot fly larvae feed on the host’s flesh as they develop and eventually (if not removed) break through the skin at maturity. Gut parasites, like tapeworms, get into the intestines and feed off of the food that is being digested by the host. Because most primates live in groups (see the “Primate Societies” section of this chapter), the tendency for social transmission of parasites, or the transfer of parasites from one individual to another, is high. Primates have evolved mechanisms to avoid parasite infection, including switching sleeping and feeding sites so as to avoid parasites. Mandrills (Mandrillus sphinx) have been shown to avoid grooming infected conspecifics as well as to avoid their feces, which smell different than the feces of individuals who are not infected with parasites (Poirotte et al. 2017). Other primates, including chimpanzees, appear to self-medicate when infected with parasites by ingesting plants that have antiparasitic properties (Krief et al. 2005).
Human-Primate Interactions
Humans are part of many primate communities and our relationship with our closest relatives is often complicated. In some areas, humans hunt primates for their meat or as trophies, or so they can sell the infants as pets. As the human population increases in size, our demand for natural resources, like wood to build houses or land on which to grow food, also increases, often at the expense of pristine primate (and other animal) habitat. As their natural habitat shrinks, primates search for food in areas occupied by humans and may be shot as crop-raiding pests. While deforestation, hunting, and the pet trade are examples of ways in which humans negatively affect the lives of primates, some human-primate interactions are beneficial. In some parts of the world primates are central to ecotourism, which focuses on nature-based attractions to educate tourists and uses economically and ecologically sustainable practices. Perhaps one of the greatest success stories of ecotourism involves the mountain gorillas of Rwanda (see Figure 7.4b). After internal conflict plagued Rwanda during the 1990s, the Virunga Mountains area developed gorilla-based tourism to aid in socioeconomic development and to bring stability to the region. This process not only helped to increase mountain gorilla populations but was also able to generate enough income to cover the operation costs of three national parks and provide income and other benefits to people living in the area (Maekawa et al. 2013). You can learn more about human-primate interactions in Appendix B: Primate Conservation.
Primate Societies
Unlike many other animals, primates are highly social and many live in stable groups consisting of adult males and females, even outside the breeding season, when females are receptive and available for mating because they are not pregnant or nursing. Indeed, sociality, or the tendency to form social groups, is a key behavioral adaptation of the order primates (see Chapter 5). This has led primatologists to ask two questions: “Why do primates live in groups?” and “What types of groups do primates live in?”
Why Do Primates Live in Groups?
Primates live in groups when the benefits of doing so exceed the costs. Although there are many potential benefits to group living, enhanced feeding competition and predator avoidance are important benefits for many group living primates. When primates feed on high-quality, scarce food (like fruit), larger groups are more successful in competition with other groups. For example, in a long-term study of vervets in Kenya’s Amboseli National Park, larger vervet groups had larger and better home ranges, which is the area in which a group regularly moves around as it performs its daily activities, including searching for food and water. Females in larger groups had higher average infant and female survival rates than the smallest group. Because pregnancy and nursing are energetically expensive for females, female reproductive success, or genetic contribution to future generations (measured by the number of offspring produced), is limited by access to food. Although living in a group means females compete with members of their own group for food, the benefits of being a member of a larger vervet group outweigh the costs (Cheney and Seyfarth 1987).
However, because they contain more individuals, larger groups are more likely to attract the attention of predators compared to smaller groups. This is one of the reasons that primates who rely on crypsis, or the ability to avoid detection by others, including predators, are often solitary (the term used to describe individuals who do not live together with other members of their species) and nocturnal, or active at night. If an animal is already hard to see because it is active at night, then moving quietly in small groups is a good strategy to avoid detection by predators. The slow loris (Nycticebus coucang) of Southeast Asia is a good example of this strategy (Figure 7.9a). Nocturnal and solitary, the slow loris moves slowly and quietly as its primary strategy to avoid detection (Wiens and Zitzmann 2003). In contrast, primates who live in large groups and are diurnal, or active during the day (like gelada baboons [Theropithecus gelada]; Figure 7.9b) cannot avoid detection by predators. Instead, group-living primates rely on behaviors that alert others to the presence of danger and/or deter predators, including shared vigilance (watchful behavior to detect potential danger), mobbing (the act of cooperatively attacking or harassing a predator), and alarm calling (vocalizations emitted by social animals in response to danger). We will discuss alarm calls in the Communication section.

What Types of Groups Do Primates Live In?
Primates vary with regard to the types of groups in which they live. A social system describes a set of social interactions and behaviors that is typical for a species. The components that make up a species’ social system include:
- Group size, which refers to the number of individuals that typically live together. Primate group size can be highly variable, ranging from one or a few individuals, to a few dozen, upward to several hundred individuals.
- Group composition describes group membership in terms of age class (e.g., adult, juvenile, infant) and sex. In some primates, groups consist of a mother and her dependent offspring while in others, one adult male lives long-term with one adult female and their dependent offspring. In other species, one or more adult males live with multiple females and their offspring.
- A species’ mating system refers to which male(s) and female(s) mate. The terms that describe a mating system (e.g., polygyny, in which one male mates with multiple females) are sometimes used to describe a primate species’ social system, but a mating system is one component of the species’ social system. For example, two species might both have polygynous mating systems, but in one species, the group is composed of one male and multiple females, while members of the other species live as solitary individuals.
- Ranging behavior refers to the way in which animals move about their environment. Most primate species have a home range, where they perform their daily activities. Some primates defend a territory which is the part of the home range that the group actively guards in an attempt to keep out conspecifics.
- Dispersal patterns describe which sex moves to a new group to reproduce. In most primate species, males disperse because the benefits of dispersal, including increased access to mates and reduced competition from other males, outweigh the costs of migrating into a new group, which often comes with aggression from current group members. For many female primates, the opposite is true: females usually benefit from remaining philopatric, or in the group of their birth. This allows them to maintain strong alliances with female relatives, which helps them compete successfully against other groups for food. In solitary species, offspring of both sexes leave their mother’s home range and become solitary. If this did not happen, the species would not be solitary. Even though both sexes disperse in solitary species, males usually disperse farther than females.
- Social interactions describe the ways in which individuals interact with members of their own and other groups of conspecifics. Affiliative (i.e., friendly or nonaggressive) behaviors include grooming (picking through the fur of another individual), playing, or coalitions (temporary alliances between individuals). Agonistic (i.e., aggressive) behaviors include fighting over food or fighting over access to mates. In groups that contain multiple adult individuals of the same sex, it is common to have a dominance hierarchy, or a group of individuals that can be ranked according to their relative amount of power over others in the hierarchy. Initially, dominance hierarchies are established through the outcome of conflicts. Individuals who lose conflicts with others are subordinate (or low rank) to those who win them. Those who win conflicts are dominant (or high rank). Dominant individuals gain access to resources, like food or mates, before subordinates. Once a hierarchy is established, agonism decreases because everyone “knows their place.”
The main types of primate social systems are as follows: solitary; single-male, single-female; single-male, multi-female; multi-male, multi-female; fission-fusion; and multi-male, single-female. These types are discussed below.
Solitary
Recall that the term solitary is used to describe species in which individuals do not live or travel together with other members of the same species, except for mothers and unweaned offspring. Males typically occupy a large home range or territory that overlaps the home ranges of multiple females, with whom they mate (Figure 7.10a). Because one male mates with multiple females, the mating system of solitary primates is polygyny. Social interactions between adults are limited but because some males do not get to mate, competition between males is intense. When males compete physically, they benefit from large body size and weaponry. The result is sexual dimorphism, when males and females look different from one another. Both males and females disperse, although males move farther from their mother than females. The nocturnal West African potto (Perodicticus potto; Figure 7.10b) is solitary. Bornean orangutans, which are diurnal, are also solitary.
Single-Male, Single-Female
Primate species in which an adult male and adult female live together with their dependent offspring have a single-male, single-female social system, sometimes referred to as a “family,” with group sizes between two and five individuals. The adult male and adult female engage in behaviors that strengthen their social relationship, or pair bond, including mutual grooming and resting together. The pair defend a territory (Figure 7.11a) and keep same-sex individuals away from their mate. The adult male and adult female mate with each other, so the mating system is monogamy, although mating outside the pair bond may occur. Species with monogamous mating systems are usually sexually monomorphic (males and females look similar) because competition for mates is relaxed since most males are able to obtain a mate. Males are usually confident that they are the father of their mate’s infant, so they help with offspring care by carrying the infant when it is not nursing. Once offspring are sexually mature, both males and females disperse. As with solitary species, males disperse farther from their parents than females. Bolivian Gray titi monkeys (Plecturocebus donacophilus) are an example of a species that has a single-male, single-female social system. One of their signature behaviors is tail twining, when two individuals sit with their tails wrapped around each other (Figure 7.11b). This behavior reinforces the social bond among family members and is especially common between the adult male and female. Gibbons (Hylobates) and owl monkeys (Aotus) also live in single-male, single-female groups.
Single-Male, Multi-Female
Single-male, multi-female groups consist of one adult male living with multiple adult females and their dependent offspring (Figure 7.12a ) . These groups can range from as few as five or ten individuals to as many as 50. Female social relationships are governed by the female dominance hierarchy. Females are usually philopatric and males disperse. Males who are unable to join a group of females may join a bachelor group with other males. Because a single male mates with multiple females, the mating system is polygyny. Species that form single-male, multi-female groups may or may not defend a territory, but the resident male, who lives with a group of females, is aggressive toward other males, who may try to take over the group and become the new resident male. Competition between males to be the resident male of a group is intense, and these species usually display sexual dimorphism, with males being larger than females and possessing large canines. Hanuman langurs (Semnopithecus entellus) of India form single-male, multi-female groups (Figure 7.12b). When a new male takes over a group of females and ousts the former resident male, he may commit infanticide, or kill the unweaned infants. This is especially likely if the new resident male has not yet mated with any of the females and thus cannot be the infants’ father. This causes the females, who were nursing, to become sexually receptive sooner, increasing the new resident male’s chances of producing offspring (Sharma, Ram, and Raipurohit 2010). Gorillas, patas monkeys, and golden snub-nosed monkeys (Rhinopithecus roxellana) also live in single-male, multi-female groups.

Multi-Male, Multi-Female
Multi-male, multi-female groups consist of multiple adult males living with multiple adult females and their dependent offspring. Although there is more than one adult male, there are more adult females than adult males in the group (Figure 7.13a). Multi-male, multi-female groups can range in size from about ten to as many as 500 individuals. They occupy a home range but may or may not defend a territory. In groups that contain multiple males and multiple females, it is not possible for one male to monopolize all the matings, so the mating system is polygamy, in which multiple males mate with multiple females. However, this does not mean that all males have an equal opportunity to mate with all females. In multi-male, multi-female groups, both males and females form a dominance hierarchy. The male dominance hierarchy determines their access to females for mating in much the same way that a female dominance hierarchy determines a female’s access to food. Because their place in the hierarchy can affect their reproductive success, males compete with each other, but because it is rare for males to be excluded from mating altogether, the level of competition and degree of sexual dimorphism are less extreme than what we see in polygynous species. Usually, females are philopatric and males disperse. Vervet monkeys (Figure 7.13b), ring-tailed lemurs (Lemur catta), white-faced capuchins (Cebus capucinus), and black-capped squirrel monkeys (Saimiri boliviensis) live in multi-male, multi-female groups.
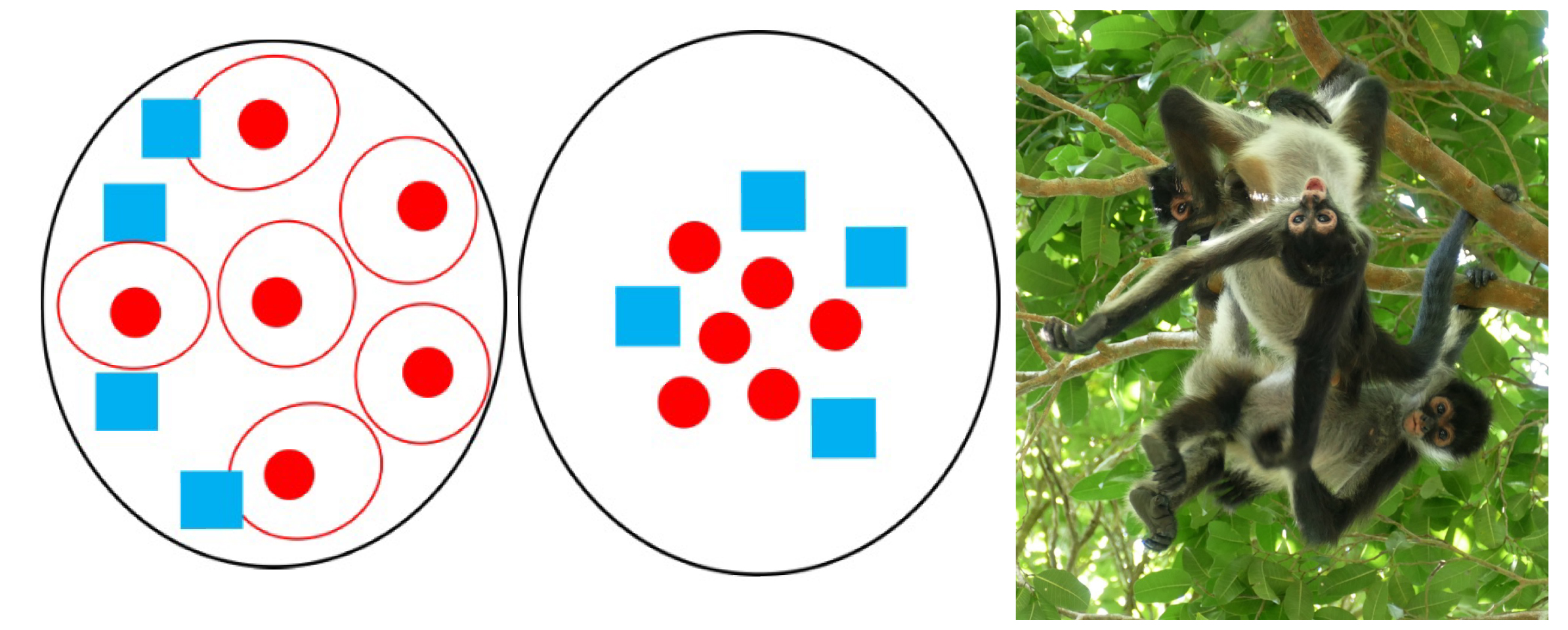
Fission-Fusion
Fission-fusion is a fluid social system in which the size and composition of the social group changes, with groups splitting (fission) or merging (fusion) depending on food availability (Pinacho-Guendulain and Ramos-Fernández 2017). When key resources are scarce, individuals spread out (fission) and move and feed individually or in small subgroups (Figure 7.14a). When key food resources are plentiful, individuals come together (fusion) and individuals travel and feed as a more cohesive group (Figure 7.14a). Fission-fusion social structure is believed to reduce feeding competition when resources are scarce. Because group composition changes over time, species with fission-fusion social systems are referred to as a community. Communities consist of multiple adult males, multiple adult females, and offspring, and group size varies but typically ranges from ten to a few dozen individuals. Females typically disperse and males are philopatric. Thus, community males are related and display unusual forms of cooperation. The mating system associated with fission-fusion is polygamy. Because males are not excluded from mating, competition for mates is relaxed and sexual dimorphism is moderate (males are slightly larger than females). Geoffroy’s spider monkeys (Ateles geoffroyi) (Figure 7.14b) and chimpanzees both have fission-fusion social system.
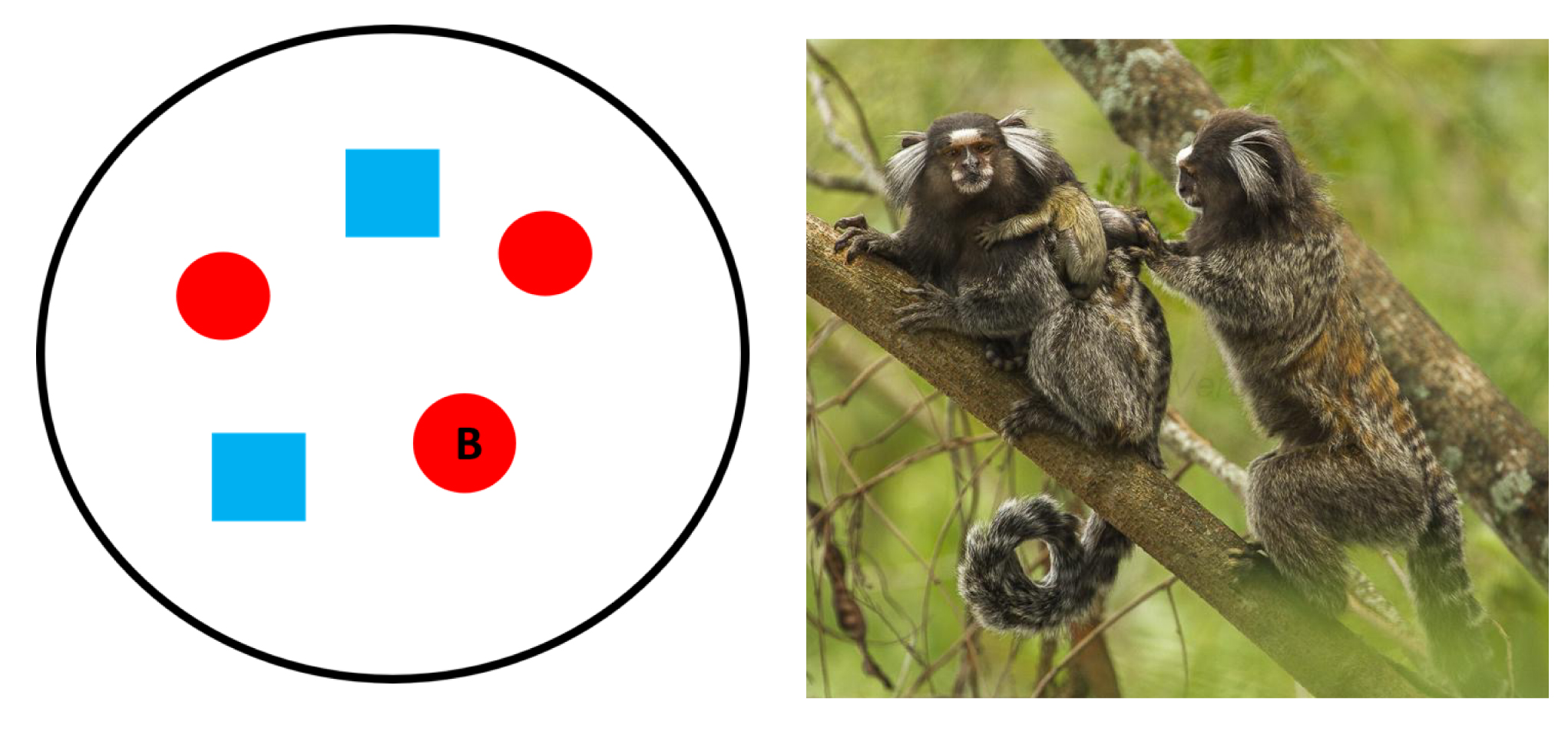
Multi-Male, Single-Female
In multi-male, single-female groups, two or more males live with one breeding female, her dependent offspring, and non-breeding females (Figure 7.15a). This type of social system is found in the callitrichids, the primate family that includes marmosets (Callithrix; Figure 7.15b) and tamarins (Saguinus) of Central and South America. Their groups rarely exceed 15 individuals, and each group actively defends their territory from conspecifics. Although more than one adult female may live in the group, the mating system is polyandry because there is only one breeding female who mates with all of the adult males. This is achieved through reproductive suppression, which involves the breeding female preventing other females from reproducing through physiological and/or behavioral means (Digby, Ferrari, and Salzman 2011). This limits the opportunities for other females in the group to become pregnant. Instead, these females, and the males in the group, help raise the breeding female’s offspring. This is referred to as cooperative breeding and usually takes the form of carrying infants, grooming them, and protecting them from danger (de Oliveira Terceiro and Burkart 2019). Because reproductive opportunities for female tamarins and marmosets are limited, they are very competitive, and females are slightly larger than males, which helps them compete for the breeding spot in a group.
Reproductive Strategies
Reproductive strategies have evolved to maximize individual reproductive success. These strategies can be divided into those that deal with offspring production and care (parental investment) and those that maximize mating success (sexual selection). Because the reproductive physiology of male and female primates differs, males and females differ with regard to parental investment and sexual selection strategies. Female strategies focus on obtaining the food necessary to sustain a pregnancy and choosing the best male(s) to father offspring. Male strategies focus on gaining access to receptive females.
Parental Investment

Biologically speaking, parental investment is any time or energy a parent devotes to the current offspring that enhances its survival (and eventual reproductive success) at the expense of the parent’s ability to invest in the next offspring (Trivers 1972). Female primates invest more heavily in offspring than males. Even before conception, females produce energy-containing eggs, and they will be responsible for sustaining a fertilized egg until it implants in the uterus. After that, they invest in pregnancy and lactation (Figure 7.16). Because all of this investment requires a lot of energy, female primates can only produce one offspring (or litter) at a time. A species’ interbirth interval, or the typical length of time between one birth and the next, is determined by the length of time necessary to maximize each offspring’s survival without jeopardizing the female’s ability to produce the greatest number of offspring possible. If a female invests too little (i.e., weans an offspring too early), she may give birth to many offspring, but very few (if any) of them will survive. If she invests too much (i.e., nurses an offspring even after it could be weaned), she ensures the survival of that individual offspring but will not be able to produce very many during her lifetime. To maximize her reproductive success, a female must invest just long enough to ensure the greatest number of offspring survive to reproduce. We often think of maternal care as an innate (or natural), instinctive behavior. Yet this is not the case. The “Special Topic: Is Maternal Behavior Innate?” dispels the myth that maternal behavior is solely instinctual and explains how female primates learn to be good mothers.
Sexual Selection
Sexual selection, or selection for traits that maximize mating success, comes in two forms. Intrasexual selection is selection for traits that enhance the ability of members of one sex to compete amongst themselves (“intrasexual” = within one sex). Intersexual selection is selection for traits that enhance the ability of one sex to attract the other (“intersexual” = between the sexes).
Intrasexual selection most often operates on males. In the wild, adult females are either pregnant or lactating for most of their adult lives. So, in a given population, there are usually more males available and willing to mate than there are females. The result? Females are a scarce resource over which males compete. Intrasexual selection favors traits that help a male win fights with other males. In primates, these traits include large body size (Figure 7.17a) and large canines (Figure 7.17b). Because females don’t possess these same traits, males and females of some species look different; that is, they are sexually dimorphic (Figure 7.17a).

Intersexual selection also tends to operate on males, selecting traits that make a male more attractive to females. Females, in turn, choose among potential fathers. Because female primates invest more in offspring production and care than males (see the “Parental Investment” section, above), it is more costly for them if the offspring dies before maturity or reaches maturity but does not reproduce. Thus, it benefits a female primate to be choosy and try to pick the healthiest male as a mate. Males must display traits that tell a female why she should choose him, and not another male, as her mate.
What traits are female primates looking for? In humans, women may look for a mate who can provide important resources, such as food, paternal care, or protection. This is rare in other primates, though, since most females do not need males to provide resources. More commonly, female primates obtain genetic benefits for their offspring from choosing one male over another. Often the specific criteria by which females select mates is unknown. However, if a female chooses a healthy (as indicated by traits like a plush coat, bright coloration, or large body size) or older male, she may obtain genes for her offspring that code for health or long life. If a male’s rank is determined by competitive ability that has a genetic component, females who choose males who win fights may acquire these genes (and qualities) for their offspring. Females in some species appear to prefer new immigrants, sometimes even “sneaking” copulations with males who are not established members of their groups. Such a preference may provide their offspring with novel genes and increase genetic variation (for more about the importance of genetic variation, see Chapter 4). Female choice is often more subtle than male-male competition, so it can be more difficult to study. However, as more research is conducted, we continue to improve our understanding of the ways that female primates exert their choice.
Special Topic: Is Maternal Behavior Innate?
Zoos almost always have nurseries where infants are cared for by zookeepers if their mothers will not care for them (Figure 7.18). These exhibits are among the most popular because the babies are so cute and so much fun to watch. And the caretaking positions in zoo nurseries are often among the most coveted by zoo personnel for the same reasons. But if maternal behavior is instinctive, why do zoo nurseries even exist? The answer is that in many species, including primates, maternal behavior is not purely instinctual; it is dependent on social learning (behavior learned by observing and imitating others), as well.

Captive female primates, including gorillas and chimpanzees, who have not had the opportunity to observe their mother or other females care for infants do not know how to care for their own offspring. Although it is preferred that the primate mother care for her own infant, there are cases when she will not and humans must step in to ensure the offspring survives. When hand-rearing by humans is necessary, the infant is returned to the group as soon as possible in the hopes that it will learn species-typical behavior from its mother and other conspecifics. Observations such as these indicate that maternal behavior is learned, not innate, and that maternal care is critically important to the social and psychological development of young primates.
Communication
In its most basic form, communication occurs when one individual (the sender) emits a signal that conveys information, which is detected by another individual (the receiver). We have discussed several aspects of primate sociality in this chapter, all of which require the communication of information between individuals. But exactly how does a female chimpanzee communicate her sexual availability? How does a vervet monkey communicate the approach of a leopard or that a python is nearby? How do solitary, nocturnal primates, like the slow loris, communicate information about themselves to conspecifics? Primate communication comes in four forms: vocal, visual, olfactory, and tactile. Species vary in their reliance on each.
Vocal Communication
Primates use sound to communicate danger or threats, to claim and maintain a territory, or make contact with other group members. Alarm calls are given in response to predators. In some cases, alarm calls are used to alert members of the group to the presence of a predator so they can take evasive action. In other cases, they are directed at the predator itself, signaling that it has been detected. You can learn more about alarm calls as forms of vocal communication in the highlight box in this chapter entitled “Dig Deeper: Alarm Calls: Signals to Friends or Foes?.”
Loud calls are designed to travel great distances and are used in territorial defense by many primate species including indris (Indri indri), orangutans, gibbons, and howler monkeys (Alouatta). In dense forest, where visual communication can be difficult, loud calls can be useful in signaling to conspecifics that a group or individual occupies a specific area. Howler monkeys are named for their loud calls, or “roars,” which can be heard one kilometer or more away (Schön Ybarra 1986). Howler monkey roars may act to maintain distance between neighboring groups or keep extragroup males from entering the home range (Schön Ybarra 1986).
Other vocalizations are intended to communicate with individuals in one’s own group. These include vocalizations given as part of threat displays or dominance interactions, as well as contact calls that provide information about one’s location to other group members. Chacma baboons (Papio ursinus) have a rich repertoire of vocalizations for communicating with other group members (Fischer et al. 2008). Adult males give specific vocalizations during threat displays and physical confrontations. Subordinates “screech” when retreating from a dominant individual, signaling submission. Since baboons rely on membership in their group for finding food and detecting predators, a baboon separated from his group will vocalize in an attempt to regain contact. Young baboons emit their own contact calls when separated from their mothers.
Visual Communication

Visual communication, which involves signals that can be seen, is an important component of nonhuman primate behavior, alone or in combination with other forms of communication. Piloerection, or raising one’s hair or fur, is used in aggressive interactions to make an individual appear larger than it actually is. Female macaques (Macaca), baboons (Papio), and chimpanzees, signal sexual receptivity through changes in the size, shape, and, often, color of their hindquarters, called a sexual swelling (Figure 7.19a). The sexual swelling reaches its maximum size at ovulation. When females are not receptive, either because they are pregnant or are nursing, they do not display a sexual swelling (Figure 7.19b). Thus, the presence or absence of a sexual swelling signals a female’s reproductive state.
Monkeys and apes use diverse facial expressions in visual communication. Showing your teeth in a “smile” sends a signal of friendship in humans. Displaying teeth in this way is a sign of anxiety or fear in primates. That male mandrill you see “yawning” at your local zoo is actually displaying his teeth to signal tension or to threaten a rival (Figure 7.20a). In addition to showing their canines, male gelada baboons use “lip flips,” in which the gums and teeth are exposed by flipping the upper lip up over the nostrils (Figure 7.20b), and “raised eyelids,” in which the pale eyelids are exposed by pulling the scalp back as threatening gestures (Aich, Moos-Heilen, and Zimmerman 1990). Submissive males respond by fleeing or presenting their hindquarters.



Primates also communicate through color. In female and male mandrills, facial coloration provides information about an individual’s health, competitive ability, and reproductive state to conspecifics (Figure 7.21; Setchell et al. 2008; Setchell, Wickings, and Knapp 2006). Variation in facial coloration among monkeys of Central and South America ranges from very simple (Figure 7.22a) to complex (Figure 7.22b). Species living with larger numbers of other primate species have evolved more complex facial coloration patterns, suggesting that this trait evolved as a form of species recognition, or the ability to differentiate conspecifics from members of other species (Santana, Lynch Alfaro, and Alfaro 2012).
Olfactory Communication
All primates use scent to communicate. Females secrete chemicals from their anogenital region (the area of the anus and genitals) that provide males with information about their reproductive state. In some species, like macaques and chimpanzees, this olfactory signal is enhanced by a sexual swelling, as discussed above. Olfactory communication, or communicating through scent, is particularly important for monkeys of Central and South America, lemurs, and lorises. Male and female common squirrel monkeys (Saimiri sciureus) (Figure 7.23a) engage in “urine washing,” in which an individual urinates on its hands and feet and then uses them to spread urine all over its body. Urine washing may be used to mark trails for others to follow, to control body temperature, as part of dominance displays, or to communicate reproductive state (Boinski 1992). During aggressive interactions with other males, male ring-tailed lemurs rub their tails with scent from glands on their wrists and chests. They use their “perfumed” tails in aggressive interactions with other males, who may respond by waiving their own scented tail, with physical aggression, or by fleeing (Jolly 1966). Males also waive their tails, saturated in scent, to attract females (Shirasu et al. 2020). Males use scent glands in their wrists to mark territorial boundaries (Figure 7.23b; Mertl-Millhollen 1988).

Tactile Communication
Tactile communication, or communicating through touch, is very important in all primate species. Physical contact is used to comfort and reassure, is part of courtship and mating, and is used to establish dominance and alliances. Grooming is an important and clearly enjoyable form of tactile communication for all primates (Figure 7.24). Not only does grooming serve to clean the skin and fur, removing parasites and debris, but it is an important affiliative behavior that helps reinforce social bonds, repair relationships, and cement alliances.

Dig Deeper: Alarm Calls: Signals to Friends or Foes?
Alarm calls are common among group-living primates. They often serve to notify conspecifics of potential danger, as is the case with vervet monkeys. Research has shown that: (1) vervets classify predators based on hunting style; (2) alarm calls convey information to other vervets about that hunting style; and (3) other vervets respond in ways appropriate for evading that type of predator (Seyfarth, Cheney, and Marler 1980a). When a vervet gives a “leopard” alarm call (directed at mammalian carnivores like leopards, Figure 7.25a), monkeys on the ground climb the nearest tree, while monkeys already in trees stay there or climb higher. Since most mammalian carnivores hunt on the ground, getting into, and staying in, a tree is the best option for escape. When the “snake” alarm call is given, vervets stand on their hind legs and look down at the ground (Figure 7.25b). Since snakes are not pursuit predators, locating them quickly so as to avoid them is the best strategy. Lastly, when an “eagle” alarm call is given, vervets look up or run into bushes, both of which are useful responses for avoiding hawks and eagles, which attack from above (Figure 7.25c). Vervets clearly understand the meaning of each type of alarm call, as they respond appropriately even when they do not see the actual predator (Seyfarth, Cheney, and Marler 1980b). Such semantic communication, which involves the systematic use of signals to refer to objects in the environment, was once believed to be unique to humans. It may be a precursor to the symbolic capacities of human language.
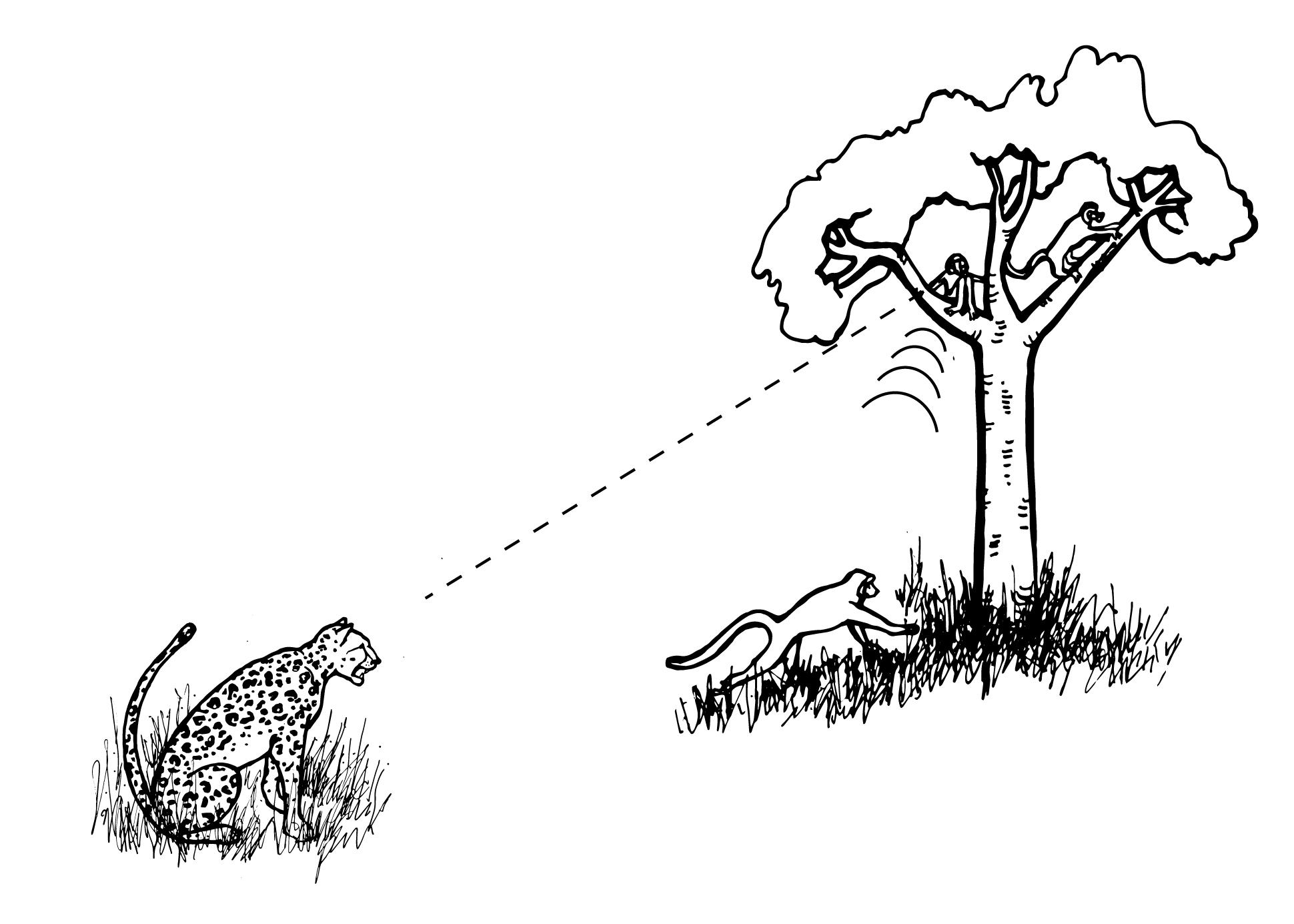
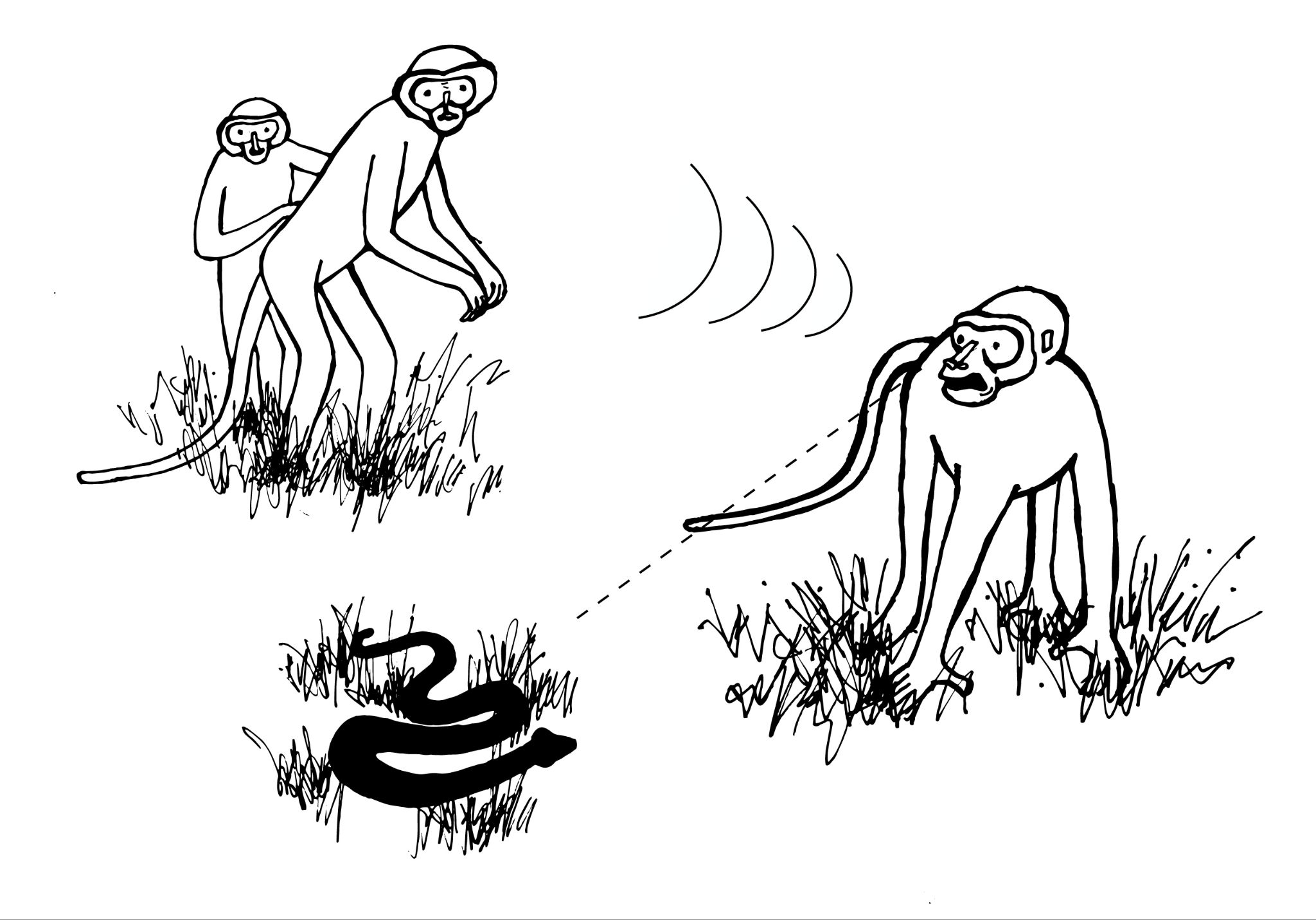
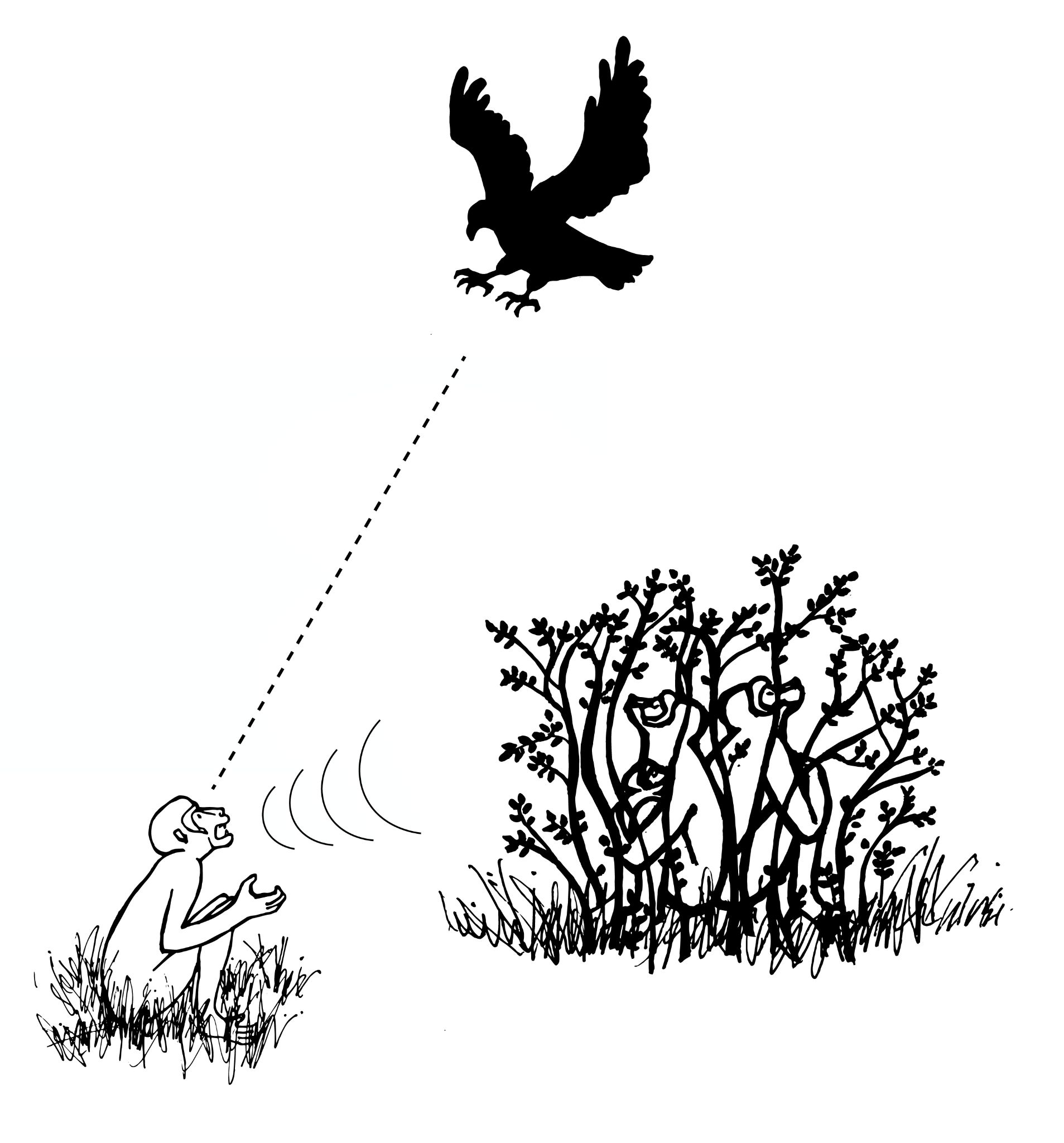
Research on other African monkeys indicates that some species use alarm calls to signal to the predator that it has been detected. Diana monkeys (Cercopithecus diana) give alarm calls to leopards (Panthera pardus) but not chimpanzees (Zuberbühler, Noë, and Seyfarth 1997). Because leopards are stealth predators, they rely on the element of surprise to sneak up on their prey (Figure 7.26a). Alarm calling at leopards appears to tell the leopard that it has been seen and therefore its chance of success will be low. Research shows leopards are more likely to stop hunting after an alarm call has been emitted. Unlike leopards, chimpanzees are pursuit predators and may even use alarm calls to locate potential prey (Figure 7.26b). With such a predator, prey are better off remaining as silent as possible so as not to alert the predator to their location (Zuberbühler et al. 1999).

The Question of Culture
It may be surprising in a chapter on nonhuman primates to see a discussion of culture. After all, culture is considered by many, including cultural anthropologists, to be a distinguishing characteristic of humans. Indeed, some anthropologists question claims of culture in primates and other animals. Definitions of animal culture focus on specific behaviors that are unique to one population. Anthropological definitions of human culture emphasize shared ideology (e.g., values, morals, beliefs) and symbols, not just behavior. Using this definition, some cultural anthropologists view primates as lacking culture because of the absence of symbolic life (e.g., religion). However, the longer we study primate groups and populations, the more insight we gain into primate behavioral variation. If we define culture as the transmission of behavior from one generation to the next through social learning, then we must view at least some of the behavioral variation we see in primates as forms of cultural tradition, or a distinctive pattern of behavior shared by multiple individuals in a social group that persists over time (Whiten 2001).
Chimpanzee Culture
Due to both their high level of intelligence and the large number of long-term studies on several different populations, chimpanzees provide the best example of cultural tradition in primates. Chimpanzees express cultural variation in multiple behavioral patterns, ranging from population-specific prey preferences and hunting strategies to tool-use techniques and social behaviors. For example, in Tanzania, chimpanzees fish for termites by stripping twigs and then poking the twigs into termite mounds. The termites react to the “invasion” by attacking the twig. The chimpanzee pulls the twig out, termites attached, and eats them. In Gambia, they use modified twigs to extract honey from holes in trees. In Fongoli, Sénégal, chimpanzees use sticks as “spears” that they stab into tree cavities to hunt for galagos (Figure 7.27). Multiple chimpanzee populations use a “hammer and anvil” to crack open nuts, but the specific techniques differ. Because the cultural traditions are so diverse and unique, if a researcher can observe enough of a chimpanzee’s behavior, it is possible to assign that individual to a specific community, much in the same way a human being can be associated with a specific culture based on his or her behavior (Whiten 2011).

How do chimpanzee cultures develop, and how does cultural transmission occur? Although we do not know for sure how chimpanzee cultural traditions develop initially, it is possible that different groups invent, either accidentally or deliberately, certain behaviors that other individuals copy. Immigration, or movement of an individual into a new group or community, is an important avenue of cultural transmission in chimpanzees, much as it is between human cultures. Immigrants (typically females) may bring cultural traditions to their new community, which residents observe and learn. Conversely, immigrants may observe and learn a cultural tradition practiced in their new community (Whiten 2011).
Cultural Transmission in Macaques
Two monkey species are well-known for behavioral variation that has been called “pre-cultural” by some primatologists: Japanese macaques and tufted capuchins (Sapajus apella). The transmission of unique foraging (the act of searching for food) behaviors through the members of a provisioned group of Japanese macaques on Koshima Island is well known (Matsuzawa 2015). In an effort to keep the monkeys nearby, researchers provided them with piles of sweet potatoes. A juvenile female named Imo spontaneously washed a muddy sweet potato in a stream. This new food-processing technique first spread among other juveniles and then gradually to older individuals. Within 30 years, it had spread across generations, and 46 of 57 monkeys in the group engaged in the behavior. Another example comes from a group living far to the north, in Shiga-Heights, Nagano Prefecture. Researchers used apples to entice Japanese macaques to the area. Within a few years, monkeys visited the area regularly and were observed playing with the water in the hot springs. Soon, they climbed into the hot springs and learned to immerse themselves to keep warm and reduce stress when not foraging (Figure 7.28; Matsuzawa 2018; Takeshita et al. 2018; recall also our discussion of hot spring use as an example of analogous traits at the beginning of this chapter). These examples share several characteristics with human culture, including invention or modification of behavior, transmission of behavior between individuals, and the persistence of the behavior across generations (McGrew 1998).

Summary
Primates are socially complex, extremely intelligent, and highly adaptable. In this chapter we discussed aspects of primate ecology, including how body size and characteristics of food affect what primates eat and how primates interact with other species in their environment. We examined why primates live in groups, the types of groups in which they are found, and the reproductive strategies used by males and females to maximize reproductive success. Like other aspects of their behavior, primate communication is varied and complex, and we discussed how primates communicate using vocal, visual, olfactory, and tactile signals. Finally, we explored the question of culture among nonhuman primates and learned that some species have cultural traditions, distinctive patterns of behavior shared by multiple individuals in a social group that persist over time. Humans and other primates are similar in many ways. Learning about principles of primate ecology and behavior can help us better understand our own behavior and the behaviors of our extinct relatives.
Review Questions
- If anthropology is the study of humans, why do some anthropologists study primates?
- How does a primate’s ecology affect their diet and interactions with other organisms?
- Why do primates live in groups and in what types of groups do they live?
- What is parental investment and sexual selection?
- What are some examples of primate communication?
- What is the evidence for cultural traditions in primates and how do primatologists think cultural transmission occurs in primates?
Key Terms
Abundance: How much food is available in a given area.
Adaptation: A trait with a function.
Affiliative: Nonaggressive social interactions and associations between individuals.
Agonistic: Conflict; aggressive interactions between individuals.
Alarm calling: Vocalizations emitted by social animals in response to danger.
Analogy: A similar trait found in different species that arose independently.
Anogenital: Relating to the anus and genitals.
Breeding season: The time of year when females are receptive to mating.
Callitrichids: The primate family that includes marmosets and tamarins.
Carnivores: Organisms whose diet consists primarily of animal tissue.
Coalition: A temporary alliance between individuals.
Community ecology: The branch of ecology that deals with the relationships and interactions between different organisms that occupy the same habitat.
Comparison: An examination of the similarities and differences between two things, such as two primate species.
Conspecifics: Members of the same species.
Cooperative breeding: When individuals other than the mother and father help raise the offspring.
Crypsis: The ability to avoid detection by other organisms, such as predators.
Cultural tradition: A distinctive pattern of behavior shared by multiple individuals in a social group, which persists over time and is acquired through social learning.
Culture: The transmission of behavior from one generation to the next through observation and imitation.
Decolonize: Understanding and highlighting the theory and research of non-Western individuals and perspectives.
Descendant: A species that comes after the ancestor species.
Direct competition: Competition that involves physical interaction between individuals, such as fighting.
Dispersal: To leave one’s group or area. This may or may not involve joining another group.
Distribution: How food is spread out.
Diurnal: Active during the day.
Dominance hierarchy: The ranked organization of individuals established by the outcome of aggressive-submissive interactions.
Dominant: Being of high rank.
Ecology: The relationship between organisms and their physical surroundings.
Ecotourism: A form of tourism that focuses on nature-based attractions to provide learning opportunities and that uses economically and ecologically sustainable practices.
Ethology: The study of animal behavior.
Fission-fusion: Societies in which group composition is flexible, such as chimpanzee and spider monkey societies. Individuals may break up into smaller feeding groups (fission) and combine into larger groups (fusion).
Fitness: An individual’s ability to survive and reproduce relative to other members of the same species.
Folivores: Organisms whose diet consists primarily of leaves.
Foraging: The act of searching for food.
Frugivores: Organisms whose diet consists primarily of fruit.
Grooming: Picking through the fur of another individual for cleaning or bonding purposes.
Heterospecifics: Members of different species.
Holism: The idea that the parts of a system interconnect and interact to make up the whole.
Home range: The area that a group or individual uses over a given period of time (often over a year).
Homology: A similar trait found in different species because it was inherited from a common ancestor.
Immigration: Movement of an individual into a new group or community.
Indirect competition: Competition that does not involve physical interaction between individuals, such as eating food before another individual arrives at the food site.
Infanticide: The killing of infants of one’s own species.
Innate: Natural; as in behavior that comes naturally.
Insectivores: Organisms whose diets consist primarily of insects.
Interbirth interval: The typical length of time between one birth and the next for a species.
Intersexual selection: The selection for traits that enhance the ability of the members of one sex to attract the attention of the other.
Intrasexual selection: Selection for traits that enhance the ability of members of one sex to compete amongst themselves.
Mating system: A way of describing which male(s) and female(s) mate.
Metabolism: The chemical changes that take place in an organism that turn nutrients into energy.
Mobbing: Cooperatively attacking or harassing a predator.
Monogamy: A mating system in which one male mates with one female.
Multi-male, multi-female: A group that consists of multiple adult males, multiple adult females, and their dependent offspring.
Multi-male, single-female: A group that consists of two or more adult males, one breeding female, their dependent offspring, and non-breeding females.
Mutualistic/mutualism: When different species work together, with each benefiting from the interaction.
Niche: The role of a species in its environment; how it meets its needs for food, shelter, etc.
Nocturnal: Active at night.
Olfactory communication: Conveying information through scent.
Omnivores: Organisms whose diet consists of plant and animal matter.
Pair bond: A strong, long-term relationship between two individuals.
Parasite: An organism that lives in or on another organism.
Parental investment: Any time or energy a parent devotes to the current offspring that enhances its survival (and eventual reproductive success) at the expense of the parent’s ability to invest in the next offspring.
Philopatric: Remaining in the group of one’s birth.
Piloerection: Raising one’s hair or fur in an effort to look bigger.
Polyandry: A mating system in which multiple males mate with a single breeding female.
Polygamy: A mating system in which multiple males mate with multiple females.
Polygyny: A mating system in which one male mates with multiple females.
Polyspecific association: An association between two or more different species that involves behavioral changes in at least one of them to maintain the association.
Primate community: All primate species that occur in an area.
Primatologist: A scientist who studies primate behavior and/or ecology.
Primatology: The scientific field that studies primate behavior and/or ecology.
Ranging behavior: Refers to the way in which animals move about their environment.
Receptive: A term used to describe females who are ready for sexual reproduction (i.e., not pregnant or nursing).
Reproductive success: An individual’s genetic contribution to future generations, often measured through the number of offspring produced.
Reproductive suppression: The prevention or inhibition of reproduction of healthy adults.
Resident male: Term that describes the male who lives with a group of females.
Seed dispersal: The process by which seeds move away from the plant that produced them in preparation for germination and becoming a new plant.
Semantic communication: The systematic use of signals to refer to objects in the environment.
Sexual dimorphism: When males and females of a species have different morphological traits.
Sexual selection: The selection for traits that increase mating success. This occurs via intersexual selection and intrasexual selection.
Sexual swelling: Area of the hindquarters that change in size, shape, and often color over the course of a female’s reproductive cycle, reaching maximum size at ovulation. Occurs in many primate species that live in Africa and Asia.
Sexually monomorphic: When males and females of a species have similar morphological traits.
Single-male, multi-female: A group that consists of one adult male, multiple adult female, and their dependent offspring.
Single-male, single-female: A group that consists of one adult male, one adult female, and their dependent offspring.
Social learning: The idea that new behaviors can be acquired by observing and imitating others.
Social system: A way of describing the typical number of males and females of all age classes that live together.
Social transmission: Transfer of something from one individual to another; this can include parasites, information, or cultural traditions.
Sociality: The tendency to form social groups.
Solitary: Living alone.
Species recognition: The ability to differentiate conspecifics from members of other species.
Subordinate: Being of low rank.
Tactile communication: Conveying information through touch.
Territory: A home range whose boundary is defended from intrusion by conspecifics.
Vertebrates: The group of animals characterized by an internal spinal column or backbone. This includes fish, amphibians, reptiles, birds, and mammals.
Vigilance: Watchful behavior used to detect potential danger, usually in the form of predators or potential competitors.
Visual communication: Conveying information through signals that can be seen.
Vocal communication: Conveying information through signals that can be heard.
For Further Exploration
Goodall, Jane. 1971. In the Shadow of Man. Boston: Houghton Mifflin.
Rowe, Noel, and Marc Myers, eds. 2016. All the World’s Primates. Charleston, RI: Pogonias Press.
Strier, Karen B. 2017. Primate Behavioral Ecology. 5th ed. New York: Routledge.
Primate Info Net is an information service of the National Primate Research Center at the University of Wisconsin, Madison. It includes Primate Factsheets, primate news and publications, a list of primate-related jobs, and an international directory of primatology, among other information.
Primate Specialist Group is a collection of scientists and conservationists who work in dozens of African, Asian, and Latin American nations to promote research on primate conservation.
Short videos of some primate behaviors discussed in this chapter:
- Watch vervet monkeys respond to different types of predators: BBC One. n.d. “Vervet Monkey’s Escape Plans – Talk to the Animals: Episode 2 Preview.” Accessed December 16, 2022. https://www.youtube.com/watch?v=q8ZG8Dpc8mM.
- Watch male gelada baboons use the lip flip in competition with other males: Smithsonian Channel, June 9, 2017. “Why These Vegetarian Monkeys Have Sharp Predator Teeth.” Accessed July 25, 2019. https://www.youtube.com/watch?time_continue=145&v=aC6iYj_EBjY.
- Watch (and listen to!) howler monkeys “roar”: Science News. N.d. “Hear a Male Howler Monkey Roar.” Accessed November 21, 2022. https://www.youtube.com/watch?v=PYar0dkZ6v8.
- Watch Japanese macaques using natural hot springs: National Geographic. N.d. “Meditative Snow Monkeys Hang Out in Hot Springs.” Accessed July 25, 2019. https://www.youtube.com/watch?v=Aat9O85ynsI.
- Watch chimpanzees make and use tools: National Geographic. n.d. “Chimps and Tools.” Accessed July 25, 2019. https://www.youtube.com/watch?v=o2TBicMRLtA.
References
Aich, H., R. Moos-Heilen, and E. Zimmermann. 1990. “Vocalizations of Adult Gelada Baboons (Theropithecus gelada): Acoustic Structure and Behavioural Context.” Folia Primatologica 55 (3–4): 109–132.
Bell, Sarah A. 2017. “Galdikas, Birute.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 445–446. Malden, MA: John Wiley & Sons.
Boinski, S. 1992. “Olfactory Communication among Costa Rican Squirrel Monkeys: A Field Study.” Folia Primatologica 59 (3): 127–136.
Cheney, D. L., and R. M. Seyfarth. 1987. “The Influence of Intergroup Competition on the Survival and Reproduction of Female Vervet Monkeys.” Behavioral Ecology and Sociobiology 21 (6): 375–386.
de Oliveira Terceiro, Francisco Edvaldo, and Judith M. Burkart. 2019. “Cooperative Breeding.” In Encyclopedia of Animal Cognition and Behavior, edited by Jennifer Vonk and Todd Shackelford, 1–6. Edinburg, Scotland: Springer Cham.
Digby, Leslie J., Stephen F. Ferrari, and Wendy Saltzman. 2011. “Callitrichines: The Role of Competition in Cooperatively Breeding Species.” In Primates in Perspective, edited by Christina J. Campbell, AugustÍn Fuentes, Katherine C. MacKinnon, Simon K. Bearder, and Rebecca M. Stumpf, 91–10. 2nd edition. New York: Oxford University Press.
Fischer, Julia, Kurt Hammerschmidt, Dorothy L. Cheney, and Robert M. Seyfarth. 2008. “Acoustic Features of Female Chacma Baboon Barks.” Ethology 107 (1): 33–54.
Jolly, Alison. 1966. Lemur Behavior: A Madagascar Field Study. Chicago: University of Chicago Press.
Krief, Sabrina, Claude Marcel Hladik, and Claudie Haxaire. 2005. “Ethnomedicinal and Bioactive Properties of Plants Ingested by Wild Chimpanzees in Uganda.” Journal of Ethnopharmacology 110 (1–3): 1–15.
Maekawa, Mkio, Annette Lanjouw, Eugène Rutagarama, and Doublas Sharp. 2013. “Mountain Gorilla Tourism Generating Wealth and Peace in Post-Conflict Rwanda.” Natural Resources Forum 37 (2): 127–137.
Matsuzawa, Tetsuro. 2015. “Sweet-Potato Washing Revisited: 50th Anniversary of the Primates Article.” Primates 56: 285–287.
Matsuzawa, Tetsuro. 2018. “Hot-Spring Bathing of Wild Monkeys in Shiga-Heights: Origin and Propagation of a Cultural Behavior.” Primates 59: 209–213.
McGrew, W. C. 1998. “Culture in Nonhuman Primates?” Annual Review of Anthropology 27: 301–328.
Mertl-Millhollen, Anne S. 1988. “Olfactory Demarcation of Territorial but Not Home Range Boundaries by Lemur catta.” Folia Primatologica 50 (3–4): 175–187.
Pinacho-Guendulain, B., and G. Ramos-Fernández. 2017. “Influence of Fruit Availability on the Fission-Fusion Dynamics of Spider Monkeys (Ateles geoffroyi).” International Journal of Primatology 38: 466–484.
Poirotte, Clémence, François Massol, Anaïs Herbert, Eric Willaume, Pacelle M. Bomo, Peter M. Kappeler, and Marie J. E. Charpentier. 2017. “Mandrills Use Olfaction to Socially Avoid Parasitized Conspicifics.” Science Advances 3 (4): e160172.
Rodrigues, Michelle. 2019. “It’s Time to Stop Lionizing Dian Fossey as a Conservation Hero.” Lady Science website, September 20. Accessed December 14, 2022. https://www.ladyscience.com/ideas/time-to-stop-lionizing-dian-fossey-conservation.
Samuni, Liran, Anna Preis, Tobias Deschner, Catherine Crockford, and Roman M. Wittig. 2018. “Reward of Labor Coordination and Hunting Success in Wild Chimpanzees.” Communications Biology 1: 138.
Santana, Sharlene E., Jessica Lynch Alfaro, and Michael E. Alfaro. 2012. “Adaptive Evolution of Facial Colour Patterns in Neotropical Primates.” Proceedings of the Royal Society B: Biological Sciences 279 (1736): 2204–2211.
Sanz, Crickette M., David Strait, Crepin Eyana Ayina, Jean Marie Massamba, Thierry Fabrice Ebombi, Severin Ndassoba Kialiema, Delon Ngoteni, et al. 2022. “Interspecific Interactions Between Sympatric Apes.” iScience 25 (10): 105059.
Schön Ybarra, M. A. 1986. “Loud Calls of Adult Male Red Howling Monkeys (Alouatta seniculus).” Folia Primatologica 47 (4): 204–216.
Setchell, Joanna M., Tessa Smith, E. Jean Wickings, and Leslie A. Knapp. 2008. “Social Correlates of Testosterone and Ornamentation in Male Mandrills.” Hormones and Behavior 54 (3): 365–372.
Setchell, Joanna M., E. Jean Wickings, and Leslie A. Knapp. 2006. “Signal Content of Red Facial Coloration in Female Mandrills (Mandrillus sphinx).” Proceedings of the Royal Society B: Biological Sciences 273 (1599): 2395–2400.
Seyfarth, R. M., D. L. Cheney, and P. Marler. 1980a. “Monkey Responses to Three Different Alarm Calls: Evidence of Predator Classification and Semantic Communication.” Science 210 (4471): 801–803.
Seyfarth, Robert M., Dorothy L. Cheney, and Peter Marler. 1980b. “Vervet Monkey Alarm Calls: Semantic Communication in a Free-Ranging Primate.” Animal Behaviour 28 (4): 1070–1094.
Sharma, Goutam, Chan Ram, and Lal Singh Rajpurohit. 2010. “A Case Study of Infantcide After Resident Male Replacement in Semnopithecus entellus around Jodhpur (India).” Proceeding of the Zoological Society 63 (2): 93–98.
Shirasu, Mika, Satomi Ito, Akihiro Itoigawa, Takashi Hayakawa, Kodzue Kinoshita, Isao Munechika, Hiroo Imai, and Kazushige Touhara. 2020. “Key Male Glandular Odorants Attracting Female Ring-Tailed Lemurs.” Current Biology 30 (11): 2131–2138.
Stanford, Craig B. 2017. “Goodall, Jane.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 471–472. Malden, MA: John Wiley & Sons.
Stewart, Kelly. 2017. “Fossey, Dian.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 432–433. Malden, MA: John Wiley & Sons.
Takeshita, Rafaela S.C., Fred B. Bercovitch, Kodzue Kinoshita, and Michael A. Huffman. 2018. “Beneficial Effect of Hot Spring Bathing on Stress Levels in Japanese Macaques.” Primates 59 (3): 215–225.
Trivers, Robert L. 1972. “Parental Investment and Sexual Selection.” In Sexual Selection and the Descent of Man, 1871–1971, edited by Bernard Campbell, 136–179. Chicago: Aldine.
Whiten, Andrew. 2011. “The Scope of Culture in Chimpanzees, Humans and Ancestral Apes.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 366 (1567): 997–1007.
Wiens, Frank, and Annette Zitzmann. 2003. “Social Structure of the Solitary Slow Loris Nycticebus coucang (Lorisidae).” Journal of Zoology 261 (1): 35–46.
Zuberbühler, Klaus, David Jenny, and Redouan Bshary. 1999. “The Predator Deterrence Function of Primate Alarm Calls.” Ethology 105 (6): 477–490.
Zuberbühler, Klaus, Ronald Noë, and Robert M. Seyfarth. 1997. “Diana Monkey Long-Distance Calls: Messages for Conspecifics and Predators.” Animal Behaviour 53 (3): 589–604.
Acknowledgments
The author is grateful to the editors for the opportunity to contribute to this open-source textbook. She thanks Dr. Stephanie Etting for her encouragement and support during the revision of this chapter. Her suggestions, along with comments made by two anonymous reviewers on an earlier draft of this chapter, improved the final version considerably. Finally, she thanks all the primatologists who came before her, especially her advisor, Lynne A. Isbell, for their tireless efforts to understand the behavior and ecology of the living primates. Without their work, this chapter would not have been possible.
Kerryn Warren, Ph.D., Grad Coach International
Lindsay Hunter, M.A., University of Iowa
Navashni Naidoo, M.Sc., University of Cape Town
Silindokuhle Mavuso, M.Sc., University of Witwatersrand
Student contributors to this chapter: Angela Durastanti, Bryce Muller, Gabriel Barr, Maisie Babbington-Bolduc
This chapter is a revision from "Chapter 9: Early Hominins" by Kerryn Warren, K. Lindsay Hunter, Navashni Naidoo, Silindokuhle Mavuso, Kimberleigh Tommy, Rosa Moll, and Nomawethu Hlazo. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Understand what is meant by “derived” and “ancestral” traits and why this is relevant for understanding early hominin evolution.
- Understand changing paleoclimates and paleoenvironments as potential factors influencing early hominin adaptations.
- Describe the anatomical changes associated with bipedalism and dentition in early hominins, as well as their implications..
- Describe early hominin genera and species, including their currently understood dates and geographic expanses.
- Describe the earliest stone tool techno-complexes and their impact on the transition from early hominins to our genus.
Defining Hominins
It is through our study of our hominin ancestors and relatives that we are exposed to a world of “might have beens”: of other paths not taken by our species, other ways of being human. But to better understand these different evolutionary trajectories, we must first define the terms we are using. If an imaginary line were drawn between ourselves and our closest relatives, the great apes, bipedalism (or habitually walking upright on two feet) is where that line would be. Hominin, then, means everyone on “our” side of the line: humans and all of our extinct bipedal ancestors and relatives since our divergence from the last common ancestor (LCA) we share with chimpanzees.
Historic interpretations of our evolution, prior to our finding of early hominin fossils, varied. Debates in the mid-1800s regarding hominin origins focused on two key issues:
- Where did we evolve?
- Which traits evolved first?
Within this conversation, naturalists and early paleoanthropologists (people who study human evolution) speculated about which human traits came first. These included the evolution of a big brain (encephalization), the evolution of the way in which we move about on two legs (bipedalism), and the evolution of our flat faces and small teeth (indications of dietary change). Original hypotheses suggested that, in order to be motivated to change diet and move about in a bipedal fashion, the large brain needed to have evolved first, as is seen in the fossil species mentioned above.
However, we now know that bipedal locomotion is one of the first things that evolved in our lineage, with early relatives having more apelike dentition and small brain sizes. While brain size expansion is seen primarily in our genus, Homo, earlier hominin brain sizes were highly variable between and within taxa, from 300 cc (cranial capacity, cm3), estimated in Ardipithecus, to 550 cc, estimated in Paranthropus boisei. The lower estimates are well within the range of variation of nonhuman extant great apes. In addition, body size variability also plays a role in the interpretation of whether brain size could be considered large or small for a particular species or specimen. In this chapter, we will tease out the details of early hominin evolution in terms of morphology (i.e. the study of the form, size, or shape of things; in this case, skeletal parts).
We also know that early human evolution occurred in a very complicated fashion. There were multiple species (multiple genera) that featured diversity in their diets and locomotion. Specimens have been found all along the East African Rift System (EARS); that is, in Ethiopia, Kenya, Tanzania, and Malawi; see Figure 9.1), in limestone caves in South Africa, and in Chad. Dates of these early relatives range from around 7 million years ago (mya) to around 1 mya, overlapping temporally with members of our genus, Homo.

Yet there is still so much to understand. Modern debates now look at the relatedness of these species to us and to one another, and they consider which of these species were able to make and use tools. As a result, every site discovery in the patchy hominin fossil record tells us more about our evolution. In addition, recent scientific techniques (not available even ten years ago) provide new insights into the diets, environments, and lifestyles of these ancient relatives.
In the past, taxonomy was primarily based on morphology. Today it is tied to known relationships based on molecular phylogeny (e.g., based on DNA) or a combination of the two. This is complicated when applied to living taxa, but becomes much more difficult when we try to categorize ancestor-descendant relationships for long-extinct species whose molecular information is no longer preserved. We therefore find ourselves falling back on morphological comparisons, often of teeth and partially fossilized skeletal material.
It is here that we turn to the related concepts of cladistics and phylogenetics. Cladistics groups organisms according to their last common ancestors based on shared derived traits. In the case of early hominins, these are often morphological traits that differ from those seen in earlier populations. These new or modified traits provide evidence of evolutionary relationships, and organisms with the same derived traits are grouped in the same clade (Figure 9.2). For example, if we use feathers as a trait, we can group pigeons and ostriches into the clade of birds. In this chapter, we will examine the grouping of the Robust Australopithecines, whose cranial and dental features differ from those of earlier hominins, and therefore are considered derived.
Dig Deeper: Problems Defining Hominin Species
It is worth noting that species designations for early hominin specimens are often highly contested. This is due to the fragmentary nature of the fossil record, the large timescale (millions of years) with which paleoanthropologists need to work, and the difficulty in evaluating whether morphological differences and similarities are due to meaningful phylogenetic or biological differences or subtle differences/variation in niche occupation or time. In other words, do morphological differences really indicate different species? How would classifying species in the paleoanthropological record compare with classifying living species today, for whom we can sequence genomes and observe lifestyles?
There are also broader philosophical differences among researchers when it comes to paleo-species designations. Some scientists, known as “lumpers,” argue that large variability is expected among multiple populations in a given species over time. These researchers will therefore prefer to “lump” specimens of subtle differences into single taxa. Others, known as “splitters,” argue that species variability can be measured and that even subtle differences can imply differences in niche occupation that are extreme enough to mirror modern species differences. In general, splitters would consider geographic differences among populations as meaning that a species is polytypic. This is worth keeping in mind when learning about why species designations may be contested.
This further plays a role in evaluating ancestry. Debates over which species “gave rise” to which continue to this day. It is common to try to create “lineages” of species to determine when one species evolved into another over time. We refer to these as chronospecies (Figure 9.3). Constructed hominin phylogenetic trees are routinely variable, changing with new specimen discoveries, new techniques for evaluating and comparing species, and, some have argued, nationalist or biased interpretations of the record. More recently, some researchers have shifted away from “treelike” models of ancestry toward more nuanced metaphors such as the “braided stream,” where some levels of interbreeding among species and populations are seen as natural processes of evolution.
Finally, it is worth considering the process of fossil discovery and publication. Some fossils are easily diagnostic to a species level and allow for easy and accurate interpretation. Some, however, are more controversial. This could be because they do not easily preserve or are incomplete, making it difficult to compare and place within a specific species (e.g., a fossil of a patella or knee bone). Researchers often need to make several important claims when announcing or publishing a find: a secure date (if possible), clear association with other finds, and an adequate comparison among multiple species (both extant and fossil). Therefore, it is not uncommon that an important find was made years before it is scientifically published.
Paleoenvironment and Hominin Evolution
There is no doubt that one of the major selective pressures in hominin evolution is the environment. Large-scale changes in global and regional climate, as well as alterations to the environment, are thought to be linked to all hominin diversification, dispersal, and extinction (Maslin et al. 2014). Environmental reconstructions often use modern analogues. Let us take, for instance, the hippopotamus. It is an animal that thrives in environments that have abundant water to keep its skin cool and moist. If the environment for some reason becomes drier, it is expected that hippopotamus populations will reduce. If a drier environment becomes wetter, it is possible that hippopotamus populations may be attracted to the new environment and thrive. Such instances have occurred multiple times in the past, and the bones of some fauna (i.e., animals, like the hippopotamus) that are sensitive to these changes give us insights into these events.
Yet reconstructing a paleoenvironment relies on a range of techniques, which vary depending on whether research interests focus on local changes or more global environmental changes/reconstructions. For local environments (such as a single site or region), comparing the faunal assemblages (collections of fossils of animals found at a site) with animals found in certain modern environments allows us to determine if past environments mirror current ones in the region. Changes in the faunal assemblages, as well as when they occur and how they occur, tell us about past environmental changes. Other techniques are also useful in this regard. Chemical analyses, for instance, can reveal the diets of individual fauna, providing clues as to the relative wetness or dryness of their environment (e.g., nitrogen isotopes; Kingston and Harrison 2007).
Global climatic changes in the distant past, which fluctuated between being colder and drier and warmer and wetter on average, would have global implications for environmental change (Figure 9.4). These can be studied by comparing marine core and terrestrial soil data across multiple sites. These techniques are based on chemical analysis, such as examination of the nitrogen and oxygen isotopes in shells and sediments. Similarly, analyzing pollen grains shows which kinds of flora survived in an environment at a specific time period. There are multiple lines of evidence that allow us to visualize global climate trends over millions of years (although it should be noted that the direction and extent of these changes could differ by geographic region).
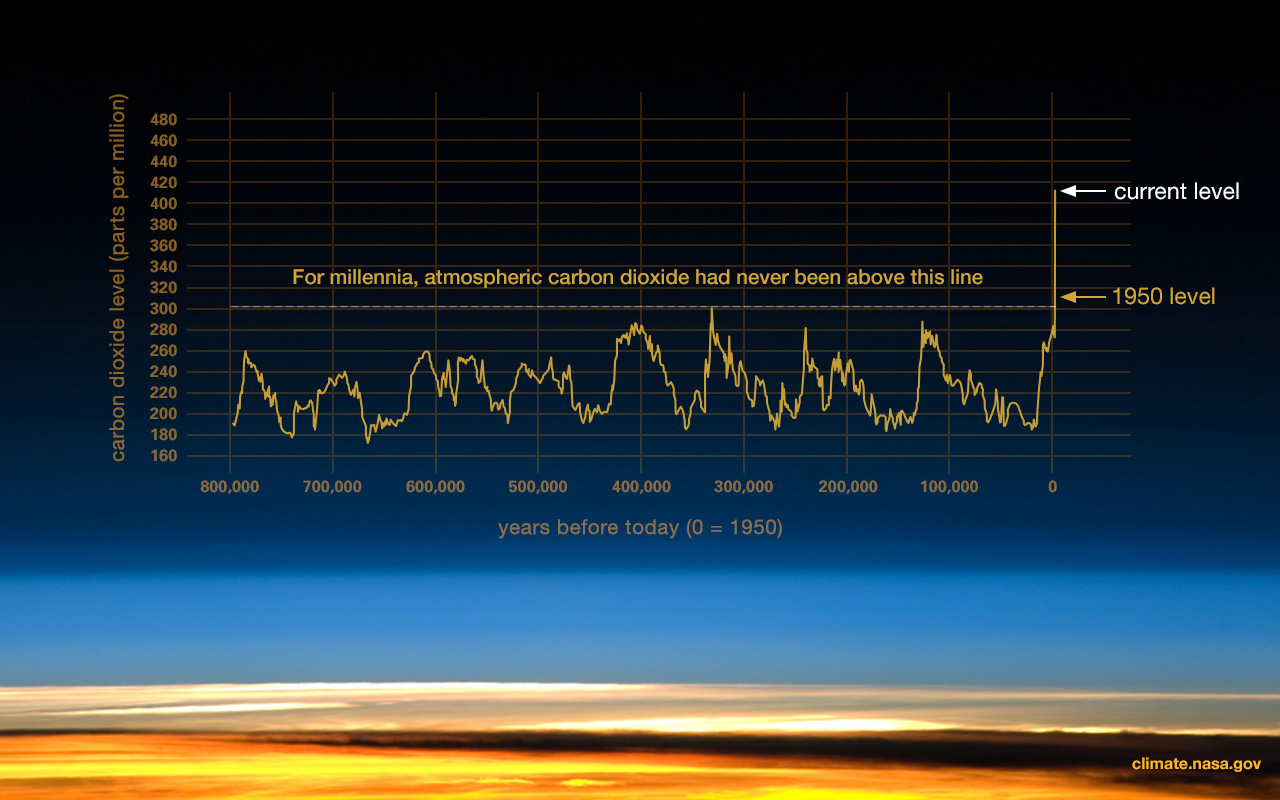
Both local and global climatic/environmental changes have been used to understand factors affecting our evolution (DeHeinzelin et al. 1999; Kingston 2007). Environmental change acts as an important factor regarding the onset of several important hominin traits seen in early hominins and discussed in this chapter. Namely, the environment has been interpreted as the following:
- the driving force behind the evolution of bipedalism,
- the reason for change and variation in early hominin diets, and
- the diversification of multiple early hominin species.
There are numerous hypotheses regarding how climate has driven and continues to drive human evolution. Here, we will focus on just three popular hypotheses.
Savannah Hypothesis (or Aridity Hypothesis)
The hypothesis: This popular theory suggests that the expansion of the savannah (or less densely forested, drier environments) forced early hominins from an arboreal lifestyle (one living in trees) to a terrestrial one where bipedalism was a more efficient form of locomotion (Figure 9.5). It was first proposed by Darwin (1871) and supported by anthropologists like Raymond Dart (1925). However, this idea was supported by little fossil or paleoenvironmental evidence and was later refined as the Aridity Hypothesis. This hypothesis states that the long-term aridification and, thereby, expansion of savannah biomes were drivers in diversification in early hominin evolution (deMenocal 2004; deMenocal and Bloemendal 1995). It advocates for periods of accelerated aridification leading to early hominin speciation events.

The evidence: While early bipedal hominins are often associated with wetter, more closed environments (i.e., not the Savannah Hypothesis), both marine and terrestrial records seem to support general cooling, drying conditions, with isotopic records indicating an increase in grasslands (i.e., colder and wetter climatic conditions) between 8 mya and 6 mya across the African continent (Cerling et al. 2011). This can be contrasted with later climatic changes derived from aeolian dust records (sediments transported to the site of interest by wind), which demonstrate increases in seasonal rainfall between 3 mya and 2.6 mya, 1.8 mya and 1.6 mya, and 1.2 mya and 0.8 mya (deMenocal 2004; deMenocal and Bloemendal 1995).
Interpretation(s): Despite a relatively scarce early hominin record, it is clear that two important factors occur around the time period in which we see increasing aridity. The first factor is the diversification of taxa, where high morphological variation between specimens has led to the naming of multiple hominin genera and species. The second factor is the observation that the earliest hominin fossils appear to have traits associated with bipedalism and are dated to around the drying period (as based on isotopic records). Some have argued that it is more accurately a combination of bipedalism and arboreal locomotion, which will be discussed later. However, the local environments in which these early specimens are found (as based on the faunal assemblages) do not appear to have been dry.
Turnover Pulse Hypothesis
The hypothesis: In 1985, paleontologist Elisabeth Vbra noticed that in periods of extreme and rapid climate change, ungulates (hoofed mammals of various kinds) that had generalized diets fared better than those with specialized diets (Vrba 1988, 1998). Specialist eaters faced extinction at greater rates than their generalist counterparts because they were unable to adapt to new environments (Vrba 2000). Thus, periods with extreme climate change would be associated with high faunal turnover: that is, the extinction of many species and the speciation, diversification, and migration of many others to occupy various niches.
The evidence: The onset of the Quaternary Ice Age, between 2.5 mya and 3 mya, brought extreme global, cyclical interglacial and glacial periods (warmer, wetter periods with less ice at the poles, and colder, drier periods with more ice near the poles). Faunal evidence from the Turkana basin in East Africa indicates multiple instances of faunal turnover and extinction events, in which global climatic change resulted in changes from closed/forested to open/grassier habitats at single sites (Behrensmeyer et al. 1997; Bobe and Behrensmeyer 2004). Similarly, work in the Cape Floristic Belt of South Africa shows that extreme changes in climate play a role in extinction and migration in ungulates. While this theory was originally developed for ungulates, its proponents have argued that it can be applied to hominins as well. However, the link between climate and speciation is only vaguely understood (Faith and Behrensmeyer 2013).
Interpretation(s): While the evidence of rapid faunal turnover among ungulates during this time period appears clear, there is still some debate around its usefulness as applied to the paleoanthropological record. Specialist hominin species do appear to exist for long periods of time during this time period, yet it is also true that Homo, a generalist genus with a varied and adaptable diet, ultimately survives the majority of these fluctuations, and the specialists appear to go extinct.
Variability Selection Hypothesis
The hypothesis: This hypothesis was first articulated by paleoanthropologist Richard Potts (1998). It links the high amount of climatic variability over the last 7 million years to both behavioral and morphological changes. Unlike previous notions, this hypothesis states that hominin evolution does not respond to habitat-specific changes or to specific aridity or moisture trends. Instead, long-term environmental unpredictability over time and space influenced morphological and behavioral adaptations that would help hominins survive, regardless of environmental context (Potts 1998, 2013). The Variability Selection Hypothesis states that hominin groups would experience varying degrees of natural selection due to continually changing environments and potential group isolation. This would allow certain groups to develop genetic combinations that would increase their ability to survive in shifting environments. These populations would then have a genetic advantage over others that were forced into habitat-specific adaptations (Potts 2013).
The evidence: The evidence for this theory is similar to that for the Turnover Pulse Hypothesis: large climatic variability and higher survivability of generalists versus specialists. However, this hypothesis accommodates for larger time-scales of extinction and survival events.
Interpretation(s): In this way, the Variability Selection Hypothesis allows for a more flexible interpretation of the evolution of bipedalism in hominins and a more fluid interpretation of the Turnover Pulse Hypothesis, where species turnover is meant to be more rapid. In some ways, this hypothesis accommodates both environmental data and our interpretations of an evolution toward greater variability among species and the survivability of generalists.
Derived Adaptations: Bipedalism
The unique form of locomotion exhibited by modern humans, called obligate bipedalism, is important in distinguishing our species from the extant (living) great apes. The ability to walk habitually upright is thus considered one of the defining attributes of the hominin lineage. We also differ from other animals that walk bipedally (such as kangaroos) in that we do not have a tail to balance us as we move.
The origin of bipedalism in hominins has been debated in paleoanthropology, but at present there are two main theories:
- early hominins initially lived in trees, but increasingly started living on the ground, so we were a product of an arboreal last common ancestor (LCA) or,
- our LCA was a terrestrial quadrupedal knuckle-walking species, more similar to extant chimpanzees.
Most research supports the first theory of an arboreal LCA based on skeletal morphology of early hominin genera that demonstrate adaptations for climbing but not for knuckle-walking. This would mean that both humans and chimpanzees can be considered “derived” in terms of locomotion since chimpanzees would have independently evolved knuckle-walking.
There are many current ideas regarding selective pressures that would lead to early hominins adapting upright posture and locomotion. Many of these selective pressures, as we have seen in the previous section, coincide with a shift in environmental conditions, supported by paleoenvironmental data. In general, however, it appears that, like extant great apes, early hominins thrived in forested regions with dense tree coverage, which would indicate an arboreal lifestyle. As the environmental conditions changed and a savannah/grassland environment became more widespread, the tree cover would become less dense, scattered, and sparse such that bipedalism would become more important.
There are several proposed selective pressures for bipedalism:
- Energy conservation: Modern bipedal humans conserve more energy than extant chimpanzees, which are predominantly knuckle-walking quadrupeds when walking over land. While chimpanzees, for instance, are faster than humans terrestrially, they expend large amounts of energy being so. Adaptations to bipedalism include “stacking” the majority of the weight of the body over a small area around the center of gravity (i.e., the head is above the chest, which is above the pelvis, which is over the knees, which are above the feet). This reduces the amount of muscle needed to be engaged during locomotion to “pull us up” and allows us to travel longer distances expending far less energy.
- Thermoregulation: Less surface area (i.e., only the head and shoulders) is exposed to direct sunlight during the hottest parts of the day (i.e., midday). This means that the body has less need to employ additional “cooling” mechanisms such as sweating, which additionally means less water loss.
- Bipedalism (Freeing of Hands): This method of locomotion freed up our ancestors’ hands such that they could more easily gather food and carry tools or infants. This further enabled the use of hands for more specialized adaptations associated with the manufacturing and use of tools.
These selective pressures are not mutually exclusive. Bipedality could have evolved from a combination of these selective pressures, in ways that increased the chances of early hominin survival.
Skeletal Adaptations for Bipedalism
Humans have highly specialized adaptations to facilitate obligate bipedalism (Figure 9.6). Many of these adaptations occur within the soft tissue of the body (e.g., muscles and tendons). However, when analyzing the paleoanthropological record for evidence of the emergence of bipedalism, all that remains is the fossilized bone. Interpretations of locomotion are therefore often based on comparative analyses between fossil remains and the skeletons of extant primates with known locomotor behaviors. These adaptations occur throughout the skeleton and are summarized in Figure 9.7.
The majority of these adaptations occur in the postcranium and are outlined in Figure 9.7. In general, these adaptations allow for greater stability and strength in the lower limb, by allowing for more shock absorption, for a larger surface area for muscle attachment, and for the “stacking” of the skeleton directly over the center of gravity to reduce energy needed to be kept upright. These adaptations often mean less flexibility in areas such as the knee and foot.
However, these adaptations come at a cost. Evolving from a nonobligate bipedal ancestor means that the adaptations we have are evolutionary compromises. For instance, the valgus knee (angle at the knee) is an essential adaptation to balance the body weight above the ankle during bipedal locomotion. However, the strain and shock absorption at an angled knee eventually takes its toll. For example, runners often experience joint pain. Similarly, the long neck of the femur absorbs stress and accommodates for a larger pelvis, but it is a weak point, resulting in hip replacements being commonplace among the elderly, especially in cases where the bone additionally weakens through osteoporosis. Finally, the S-shaped curve in our spine allows us to stand upright, relative to the more curved C-shaped spine of an LCA. Yet the weaknesses in the curves can lead to pinching of nerves and back pain. Since many of these problems primarily are only seen in old age, they can potentially be seen as an evolutionary compromise.
Despite relatively few postcranial fragments, the fossil record in early hominins indicates a complex pattern of emergence of bipedalism. Key features, such as a more anteriorly placed foramen magnum, are argued to be seen even in the earliest discovered hominins, indicating an upright posture (Dart 1925). Some early species appear to have a mix of ancestral (arboreal) and derived (bipedal) traits, which indicates a mixed locomotion and a more mosaic evolution of the trait. Some early hominins appear to, for instance, have bowl-shaped pelvises (hip bones) and angled femurs suitable for bipedalism but also have retained an opposable hallux (big toe) or curved fingers and longer arms (for arboreal locomotion). These mixed morphologies may indicate that earlier hominins were not fully obligate bipeds and thus thrived in mosaic environments. Yet the associations between postcranial and the more diagnostic cranial fossils and bones are not always clear, muddying our understanding of the specific species to which fossils belong (Grine et al. 2022).
It is also worth noting that, while not directly related to bipedalism per se, other postcranial adaptations are evident in the hominin fossil record from some of the earlier hominins. For instance, the hand and finger morphologies of many of the earliest hominins indicate adaptations consistent with arboreality. These include longer hands, more curved metacarpals and phalanges (long bones in the hand and fingers, respectively), and a shorter, relatively weaker thumb. This allows for gripping onto curved surfaces during locomotion. The earliest hominins appear to have mixed morphologies for both bipedalism and arborealism. However, among Australopiths (members of the genus, Australopithecus), there are indications for greater reliance on bipedalism as the primary form of locomotion. Similarly, adaptations consistent with tool manufacture (shorter fingers and a longer, more robust thumb, in contrast to the features associated with arborealism) have been argued to appear before the genus Homo.
| Region | Feature | Obligate Biped (H. sapiens) | Nonobligate Biped |
| Cranium | Position of the foramen magnum | Positioned inferiorly (immediately under the cranium) so that the head rests on top of the vertebral column for balance and support (head is perpendicular to the ground). | Posteriorly positioned (to the back of the cranium). Head is positioned parallel to the ground. |
| Post
cranium |
Body proportions | Shorter upper limb (not used for locomotion). | Longer upper limbs (used for locomotion). |
| Post
cranium |
Spinal curvature | S-curve due to pressure exerted on the spine from bipedalism (lumbar lordosis). | C-curve. |
| Post
cranium |
Vertebrae | Robust lumbar (lower-back) vertebrae (for shock absorbance and weight bearing). Lower back is more flexible than that of apes as the hips and trunk swivel when walking (weight transmission). | Gracile lumbar vertebrae compared to those of modern humans. |
| Post
cranium |
Pelvis | Shorter, broader, bowl-shaped pelvis (for support); very robust. Broad sacrum with large sacroiliac joint surfaces. | Longer, flatter, elongated ilia; more narrow and gracile; narrower sacrum; relatively smaller sacroiliac joint surface. |
| Post
cranium |
Lower limb | In general, longer, more robust lower limbs and more stable, larger joints.
|
In general, smaller, more gracile limbs with more flexible joints.
|
| Post
cranium |
Foot | Rigid, robust foot, without a midtarsal break.
Nonopposable and large, robust big toe (for push off while walking) and large heel for shock absorbance. |
Flexible foot, midtarsal break present (which allows primates to lift their heels independently from their feet), opposable big toe for grasping. |
Special Topic: Fear of Snakes — A Cultural or Biological Adaptation?

It is suggested that primates have three major predators: raptors, felines, and snakes; however, many studies show that of these carnivores, snakes were one of the first that mammals had to contend with alongside dinosaurs, as felines and raptors evolved at a much slower pace than their reptilian competition. Herpetologists trace the evolution of constricting snakes to about 100 million years ago, and by the time mammals arrived around 75 million years ago, constrictors were already well established as a formidable threat (Greene, 2017). Both co-existed for millennia and each sustained selective pressures requiring them to evolve specific traits to survive. When venomous snakes eventually emerged 55 to 65 million years ago, they posed yet an additional threat to proto-primates as they required less distance for the predator to kill (2017). Alongside camouflage and silent movement techniques, it was the development of the snake’s hollow fangs through which to deliver venom that was most transformative to primate evolution. As such, primates evolved their pre-conscious attention, and visual acuity to cope with this new threat; therefore, while snakes were adapting morphologically to feed themselves, they were unwittingly teaching proto-primates valuable lessons in predator detection and reacting appropriately in order to survive.
In a 2009 Harvard University study, Lynne A. Isbell hypothesizes that envenoming snakes are linked to being directly responsible for the origins of the evolving complex brains and superior visual capacity in the lineage of anthropoids leading to humans (Isbell, 2009). Forward-facing eyes for binocular vision, depth perception, enhanced visual acuity, stereoscopic and trichromatic colour vision, all traits necessary for snake detection; and the quick motor responses from the primate’s fight, flight, or freeze defence mechanism to circumvent a snake’s squeeze or bite. Numerous laboratory studies show that humans and primates both sense and visually detect snakes more rapidly than other threatening stimuli (Van Le et al., 2013). These experiments show that snakes elicited the strongest, fastest responses (Van Le et al., 2013). This is known as ‘Snake Detection Theory’ and is the evolution of the primate’s complex brain, visual acuity, and rapid motor responses towards snakes in its environment that are the adaptations needed to live successfully as arboreal beings. It is not fortuitous then, that primates that never coexisted with venomous snakes, such as lemurs in Madagascar, have less visual acuity, better olfaction and smaller brains. Within Isbell’s work, a collaborative study by a group of neuroscientists tested this hypothesis and found that, indeed, there is higher neural firing and activity in multiple areas of the primate brain, notably in the pulvinar, a region responsible for visual attention and oculomotor behaviour (Isbell, L., 2009).

Today, the fear of snakes is widespread in humans, often shown through avoidance and disgust. A study in The Journal of Ethnobiology and Ethnomedicine notes that snakes are over-hunted and excluded from conservation efforts worldwide (Ceríaco, 2012). While cultural factors shape our sentiments, instinct also plays a role—such as the developed avoidance behaviors toward threats like snakes. This blend of instinct and cultural influence is not only seen in behavior but also deeply embedded in the stories we tell. Many cultures depict mythological snakes as harbingers of death or chaos. In the Bible, Satan becomes a snake to tempt Eve. Norse mythology features Jörmungandr, the world serpent who signals the apocalypse. Egyptian myth tells of Apophis, who battles the sun god Ra nightly. Though sources vary, these myths consistently portray snakes as threats. As such, the widespread fear of snakes may reflect both evolutionary and cultural influences. Understood as an adaptive response inherited from primate ancestors—who developed avoidance behaviors toward potentially dangerous stimuli—and reinforced through myths and religious narratives, the enduring presence of snakes as potent figures of fear across human societies and primate groups highlights the complex intertwining of instinct and cultural meaning in shaping human behavior.
Early Hominins: Sahelanthropus and Orrorin
We see evidence for bipedalism in some of the earliest fossil hominins, dated from within our estimates of our divergence from chimpanzees. These hominins, however, also indicate evidence for arboreal locomotion.
The earliest dated hominin find (between 6 mya and 7 mya, based on radiometric dating of volcanic tufts) has been argued to come from Chad and is named Sahelanthropus tchadensis (Figure 9.8; Brunet et al. 1995). The initial discovery was made in 2001 by Ahounta Djimdoumalbaye and announced in Nature in 2002 by a team led by French paleontologist Michel Brunet. The find has a small cranial capacity (360 cc) and smaller canines than those in extant great apes, though they are larger and pointier than those in humans. This might imply that, over evolutionary time, the need for display and dominance among males has reduced, as has our sexual dimorphism. A short cranial base and a foramen magnum that is more humanlike in positioning have been argued to indicate upright walking.
Initially, the inclusion of Sahelanthropus in the hominin family was debated by researchers, since the evidence for bipedalism is based on cranial evidence alone, which is not as convincing as postcranial evidence. Yet, a femur (thigh bone) and ulnae (upper arm bones) thought to belong to Sahelanthropus was discovered in 2001 (although not published until 2022). These bones may support the idea that the hominin was in fact a terrestrial biped with arboreal capabilities and behaviors (Daver et al. 2022).
Orrorin tugenensis (Orrorin meaning “original man”), dated to between 6 mya and 5.7 mya, was discovered near Tugen Hills in Kenya in 2000. Smaller cheek teeth (molars and premolars) than those in even more recent hominins, thick enamel, and reduced, but apelike, canines characterize this species. This is the first species that clearly indicates adaptations for bipedal locomotion, with fragmentary leg, arm, and finger bones having been found but few cranial remains. One of the most important elements discovered was a proximal femur, BAR 1002'00. The femur is the thigh bone, and the proximal part is that which articulates with the pelvis; this is very important for studying posture and locomotion. This femur indicates that Ororrin was bipedal, and recent studies suggest that it walked in a similar way to later Pliocene hominins. Some have argued that features of the finger bones suggest potential tool-making capabilities, although many researchers argue that these features are also consistent with climbing.
Early Hominins: The Genus Ardipithecus
Another genus, Ardipithecus, is argued to be represented by at least two species: Ardipithecus (Ar.) ramidus and Ar. kadabba.
Ardipithecus ramidus (“ramid” means root in the Afar language) is currently the best-known of the earliest hominins (Figure 9.9). Unlike Sahelanthropus and Orrorin, this species has a large sample size of over 110 specimens from Aramis alone. Dated to 4.4 mya, Ar. ramidus was found in Ethiopia (in the Middle Awash region and in Gona). This species was announced in 1994 by American palaeoanthropologist Tim White, based on a partial female skeleton nicknamed “Ardi” (ARA-VP-6/500; White et al. 1994). Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, she had an opposable big toe (hallux), similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. A small brain (300 cc to 350 cc), midfacial projection, and slight prognathism show retained ancestral cranial features, but the cheek bones are less flared and robust than in later hominins.
Ardipithecus kadabba (the species name means “oldest ancestor” in the Afar language) is known from localities on the western margin of the Middle Awash region, the same locality where Ar. ramidus has been found. Specimens include mandibular fragments and isolated teeth as well as a few postcranial elements from the Asa Koma (5.5 mya to 5.77 mya) and Kuseralee Members (5.2 mya), well-dated and understood (but temporally separate) volcanic layers in East Africa. This species was discovered in 1997 by paleoanthropologist Dr. Yohannes Haile-Selassie. Originally these specimens were referred to as a subspecies of Ar. ramidus. In 2002, six teeth were discovered at Asa Koma and the dental-wear patterns confirmed that this was a distinct species, named Ar. kadabba, in 2004. One of the postcranial remains recovered included a 5.2 million-year-old toe bone that demonstrated features that are associated with toeing off (pushing off the ground with the big toe leaving last) during walking, a characteristic unique to bipedal walkers. However, the toe bone was found in the Kuseralee Member, and therefore some doubt has been cast by researchers about its association with the teeth from the Asa Koma Member.
Derived Adaptations: Early Hominin Dention
The Importance of Teeth
Teeth are abundant in the fossil record, primarily because they are already highly mineralized as they are forming, far more so than even bone. Because of this, teeth preserve readily. And, because they preserve readily, they are well-studied and better understood than many skeletal elements. In the sparse hominin (and primate) fossil record, teeth are, in some cases, all we have.
Teeth also reveal a lot about the individual from whom they came. We can tell what they evolved to eat, to which other species they may be closely related, and even, to some extent, the level of sexual dimorphism, or general variability, within a given species. This is powerful information that can be contained in a single tooth. With a little more observation, the wearing patterns on a tooth can tell us about the diet of the individual in the weeks leading up to its death. Furthermore, the way in which a tooth is formed, and the timing of formation, can reveal information about changes in diet (or even mobility) over infancy and childhood, using isotopic analyses. When it comes to our earliest hominin relatives, this information is vital for understanding how they lived.
The purpose of comparing different hominin species is to better understand the functional morphology as it applies to dentition. In this, we mean that the morphology of the teeth or masticatory system (which includes jaws) can reveal something about the way in which they were used and, therefore, the kinds of foods these hominins ate. When comparing the features of hominin groups, it is worth considering modern analogues (i.e., animals with which to compare) to make more appropriate assumptions about diet. In this way, hominin dentition is often compared with that of chimpanzees and gorillas (our close ape relatives), as well as with that of modern humans.
The most divergent group, however, is humans. Humans around the world have incredibly varied diets. Among hunter-gatherers, it can vary from a honey- and plant-rich diet, as seen in the Hadza in Tanzania, to a diet almost entirely reliant on animal fat and protein, as seen in Inuits in polar regions of the world. We are therefore considered generalists, more general than the largely frugivorous (fruit-eating) chimpanzee or the folivorous (foliage-eating) gorilla, as discussed in Chapter 5.
One way in which all humans are similar is our reliance on the processing of our food. We cut up and tear meat with tools using our hands, instead of using our front teeth (incisors and canines). We smash and grind up hard seeds, instead of crushing them with our hind teeth (molars). This means that, unlike our ape relatives, we can rely more on developing tools to navigate our complex and varied diets. (We could say) Our brain, therefore, is our primary masticatory organ. Evolutionarily, our teeth have reduced in size and our faces are flatter, or more orthognathic, partially in response to our increased reliance on our hands and brain to process food. Similarly, a reduction in teeth and a more generalist dental morphology could also indicate an increase in softer and more variable foods, such as the inclusion of more meat. The link has been made between some of the earliest evidence for stone tool manufacture, the earliest members of our genus, and the features that we associate with these specimens.
General Dental Trends in Early Hominins
Several trends are visible in the dentition of early hominins. However, all tend to have the same dental formula. The dental formula tells us how many of each tooth type are present in each quadrant of the mouth. Going from the front of the mouth, this includes the square, flat incisors; the pointy canines; the small, flatter premolars; and the larger hind molars. In many primates, from Old World monkeys to great apes, the typical dental formula is 2:1:2:3. This means that if we divide the mouth into quadrants, each has two incisors, one canine, two premolars, and three molars. The eight teeth per quadrant total 32 teeth in all (although some humans have fewer teeth due to the absence of their wisdom teeth, or third molars).

The morphology of the individual teeth is where we see the most change. Among primates, large incisors are associated with food procurement or preparation (such as biting small fruits), while small incisors indicate a diet that may contain small seeds or leaves (where the preparation is primarily in the back of the mouth). Most hominins have relatively large, flat, vertically aligned incisors that occlude (touch) relatively well, forming a “bite.” This differs from, for instance, the orangutan, whose teeth stick out (i.e., are procumbent).
While the teeth are often aligned with diet, the canines may be misleading in that regard. We tend to associate pointy, large canines with the ripping required for meat, and the reduction (or, in some animals, the absence) of canines as indicative of herbivorous diets. In humans, our canines are often a similar size to our incisors and therefore considered incisiform (Figure 9.10). However, our closest relatives all have very long, pointy canines, particularly on their upper dentition. This is true even for the gorilla, which lives almost exclusively on plants. The canines in these instances reveal more about social structure and sexual dimorphism than diet, as large canines often signal dominance.
Early on in human evolution, we see a reduction in canine size. Sahelanthropus tchadensis and Orrorin tugenensis both have smaller canines than those in extant great apes, yet the canines are still larger and pointier than those in humans or more recent hominins. In Ardipithecus ramidus, there is no obvious difference between male and female canine size, yet they are still slightly larger and pointier than in modern humans. This implies a less sexually dimorphic social structure in the earlier hominins relative to modern-day chimpanzees and gorillas.
Along with a reduction in canine size is the reduction or elimination of a canine diastema: a gap between the teeth on the mandible that allows room for elongated teeth on the maxilla to “fit” in the mouth. Absence of a diastema is an excellent indication of a reduction in canine size. In animals with large canines (such as baboons), there is also often a honing P3, where the first premolar (also known as P3 for evolutionary reasons) is triangular in shape, “sharpened” by the extended canine from the upper dentition. This is also seen in some early hominins: Ardipithecus, for example, has small canines that are almost the same height as its incisors, although still larger than those in recent hominins.
The hind dentition, such as the bicuspid (two cusped) premolars or the much larger molars, are also highly indicative of a generalist diet in hominins. Among the earliest hominins, the molars are larger than we see in our genus, increasing in size to the back of the mouth and angled in such a way from the much smaller anterior dentition as to give these hominins a parabolic (V-shaped) dental arch. This differs from our living relatives and some early hominins, such as Sahelanthropus, whose molars and premolars are relatively parallel between the left and right sides of the mouth, creating a U-shape.
Among more recent early hominins, the molars are larger than those in the earliest hominins and far larger than those in our own genus, Homo. Large, short molars with thick enamel allowed our early cousins to grind fibrous, coarse foods, such as sedges, which require plenty of chewing. This is further evidenced in the low cusps, or ridges, on the teeth, which are ideal for chewing. In our genus, the hind dentition is far smaller than in these early hominins. Our teeth also have medium-size cusps, which allow for both efficient grinding and tearing/shearing meats.
Understanding the dental morphology has allowed researchers to extrapolate very specific behaviors of early hominins. It is worth noting that while teeth preserve well and are abundant, a slew of other morphological traits additionally provide evidence for many of these hypotheses. Yet there are some traits that are ambiguous. For instance, while there are definitely high levels of sexual dimorphism in Au. afarensis, discussed in the next section, the canine teeth are reduced in size, implying that while canines may be useful indicators for sexual dimorphism, it is also worth considering other evidence.
Special Topic: Contested Species
Many named species are highly debated and argued to have specimens associated with a more variable Au. afarensis or Au. anamensis species. Sometimes these specimens are dated to times when, or found in places in which, there are “gaps” in the palaeoanthropological record. These are argued to represent chronospecies or variants of Au. afarensis. However, it is possible that, with more discoveries, the distinct species types will hold.
Australopithecus bahrelghazali is dated to within the time period of Au. afarensis (3.6 mya; Brunet et al. 1995) and was the first Australopithecine to be discovered in Chad in central Africa. Researchers argue that the holotype, whom discoverers have named “Abel,” falls under the range of variation of Au. afarensis and therefore that A. bahrelghazali does not fall into a new species (Lebatard et al. 2008). If “Abel” is a member of Au. afarensis, the geographic range of the species would be greatly extended.
On a different note, Australopithecus deyiremada (meaning “close relative” in the Ethiopian language of Afar) is dated to 3.5 mya to 3.3 mya and is based on fossil mandible bones discovered in 2011 in Woranso-Mille (in the Afar region of Ethiopia) by Yohannes Haile-Selassie, an Ethiopian paleoanthropologist (Haile-Selassie et al. 2019). The discovery indicated, in contrast to Au. afarensis, smaller teeth with thicker enamel (potentially suggesting a harder diet) as well as a larger mandible and more projecting cheekbones. This find may be evidence that more than one closely related hominin species occupied the same region at the same temporal period (Haile-Selassie et al. 2015; Spoor 2015) or that other Au. afarensis specimens have been incorrectly designated. However, others have argued that this species has been prematurely identified and that more evidence is needed before splitting the taxa, since the variation appears subtle and may be due to slightly different niche occupations between populations over time.
Australopithecus garhi is another species found in the Middle Awash region of Ethiopia. It is currently dated to 2.5 mya (younger than Au. afarensis). Researchers have suggested it fills in a much-needed temporal “gap” between hominin finds in the region, with some anatomical differences, such as a relatively large cranial capacity (450 cc) and larger hind dentition than seen in other gracile Australopithecines. Similarly, the species has been argued to have longer hind limbs than Au. afarensis, although it was still able to move arboreally (Asfaw et al. 1999). However, this species is not well documented or understood and is based on only several fossil specimens. More astonishingly, crude stone tools resembling Oldowan (which will be described later) have been found in association with Au. garhi. While lacking some of the features of the Oldowan, this is one of the earliest technologies found in direct association with a hominin.
Kenyanthopus platyops (the name “platyops” refers to its flatter-faced appearance) is a highly contested genus/species designation of a specimen (KNM-WT 40000) from Lake Turkana in Kenya, discovered by Maeve Leakey in 1999 (Figure 9.11). Dated to between 3.5 mya and 3.2 mya, some have suggested this specimen is an Australopithecus, perhaps even Au. afarensis (with a brain size which is difficult to determine, yet appears small), while still others have placed this specimen in Homo (small dentition and flat-orthognathic face). While taxonomic placing of this species is quite divided, the discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis (Leakey et al. 2001). Some researchers have additionally associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this specimen.

The Genus Australopithecus
The Australopithecines are a diverse group of hominins, comprising various species. Australopithecus is the given group or genus name. It stems from the Latin word Australo, meaning “southern,” and the Greek word pithecus, meaning “ape.” Within this section, we will outline these differing species’ geological and temporal distributions across Africa, unique derived and/or shared traits, and importance in the fossil record.
Between 3 mya and 1 mya, there seems to be differences in dietary strategy between different species of hominins designated as Australopithecines. A pattern of larger posterior dentition (even relative to the incisors and canines in the front of the mouth), thick enamel, and cranial evidence for extremely large chewing muscles is far more pronounced in a group known as the robust australopithecines. This pattern is extremely relative to their earlier contemporaries or predecessors, the gracile australopithecines, and is certainly larger than those seen in early Homo, which emerged during this time. This pattern of incredibly large hind dentition (and very small anterior dentition) has led people to refer to robust australopithecines as megadont hominins (Figure 9.12).
Because of these differences, this section has been divided into “gracile” and “robust” Australopithecines, highlighting the morphological differences between the two groups (which many researchers have designated as separate genera: Australopithecus and Paranthropus, respectively) and then focusing on the individual species. It is worth noting, however, that not all researchers accept these clades as biologically or genetically distinct, with some researchers insisting that the relative gracile and robust features found in these species are due to parallel evolutionary events toward similar dietary niches.
Despite this genus’ ancestral traits and small cranial capacity, all members show evidence of bipedal locomotion. It is generally accepted that Australopithecus species display varying degrees of arborealism along with bipedality.
Gracile Australopithecines
This section describes individual species from across Africa. These species are called “gracile australopithecines” because of their smaller and less robust features compared to the divergent “robust” group. Numerous Australopithecine species have been named, but some are only based on a handful of fossil finds, whose designations are controversial.
East African Australopithecines
East African Australopithecines are found throughout the EARS, and they include the earliest species associated with this genus. Numerous fossil-yielding sites, such as Olduvai, Turkana, and Laetoli, have excellent, datable stratigraphy, owing to the layers of volcanic tufts that have accumulated over millions of years. These tufts may be dated using absolute dating techniques, such as Potassium-Argon dating (described in Chapter 7). This means that it is possible to know a relatively refined date for any fossil if the context of that find is known. Similarly, comparisons between the faunal assemblages of these stratigraphic layers have allowed researchers to chronologically identify environmental changes.
The earliest known Australopithecine is dated to 4.2 mya to 3.8 mya. Australopithecus anamensis (after “Anam,” meaning “lake” from the Turkana region in Kenya; Leakey et al. 1995; Patterson and Howells 1967) is currently found from sites in the Turkana region (Kenya) and Middle Awash (Ethiopia; Figure 9.13). Recently, a 2019 find from Ethiopia, named MRD, after Miro Dora where it was found, was discovered by an Ethiopian herder named Ali Bereino. It is one of the most complete cranial finds of this species (Ward et al. 1999). A small brain size (370 cc), relatively large canines, projecting cheekbones, and earholes show more ancestral features as compared to those of more recent Australopithecines. The most important element discovered with this species is a fragment of a tibia (shinbone), which demonstrates features associated with weight transfer during bipedal walking. Similarly, the earliest found hominin femur belongs to this species. Ancestral traits in the upper limb (such as the humerus) indicate some retained arboreal locomotion.
Some researchers suggest that Au. anamensis is an intermediate form of the chronospecies that becomes Au. afarensis, evolving from Ar. ramidus. However, this is debated, with other researchers suggesting morphological similarities and affinities with more recent species instead. Almost 100 specimens, representing over 20 individuals, have been found to date (Leakey et al. 1995; McHenry 2009; Ward et al. 1999).
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. Au. afarensis (which means “from the Afar region”) is dated to between 2.9 mya and 3.9 mya and is found in sites all along the EARS system, in Tanzania, Kenya, and Ethiopia (Figure 9.14). The most famous individual from this species is a partial female skeleton discovered in Hadar (Ethiopia), later nicknamed “Lucy,” after the Beatles’ song “Lucy in the Sky with Diamonds,” which was played in celebration of the find (Johanson et al. 1978; Kimbel and Delezene 2009). This skeleton was found in 1974 by Donald Johanson and dates to approximately 3.2 mya. In addition, in 2002 a juvenile of the species was found by Zeresenay Alemseged and given the name “Selam” (meaning “peace,” DIK 1-1), though it is popularly known as “Lucy’s Child” or as the “Dikika Child” (Alemseged et al. 2006). Similarly, the “Laetoli Footprints” (discussed in Chapter 7; Hay and Leakey 1982; Leakey and Hay 1979) have drawn much attention.

The canines and molars of Au. afarensis are reduced relative to great apes but are larger than those found in modern humans (indicative of a generalist diet); in addition, Au. afarensis has a prognathic face (the face below the eyes juts anteriorly) and robust facial features that indicate relatively strong chewing musculature (compared with Homo) but which are less extreme than in Paranthropus. Despite a reduction in canine size in this species, large overall size variation indicates high levels of sexual dimorphism.
Skeletal evidence indicates that this species was bipedal, as its pelvis and lower limb demonstrate a humanlike femoral neck, valgus knee, and bowl-shaped hip (Figure 9.15). Further evidence of bipedalism is seen in the Laetoli Footprints, which are associated with Au. afarensis (Chapter 7).
Although not found in direct association with stone tools, potential evidence for cut marks on bones, found at Dikika, and dated to 3.39 mya indicates a possible temporal/ geographic overlap between meat eating, tool use, and this species. However, this evidence is fiercely debated. Others have associated the cut marks with the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species.
South African Australopithecines
Since the discovery of the Taung Child, there have been numerous Australopithecine discoveries from the region known as “The Cradle of Humankind,” which was recently given UNESCO World Heritage Site status as “The Fossil Hominid Sites of South Africa.” The limestone caves found in the Cradle allow for the excellent preservation of fossils. Past animals navigating the landscape and falling into cave openings, or caves used as dens by carnivores, led to the accumulation of deposits over millions of years. Many of the hominin fossils, encased in breccia (hard, calcareous sedimentary rock), are recently exposed from limestone quarries mined in the previous century. This means that extracting fossils requires excellent and detailed exposed work, often by a team of skilled technicians.
While these sites have historically been difficult to date, with mixed assemblages accumulated over large time periods, advances in techniques such as uranium-series dating have allowed for greater accuracy. Historically, the excellent faunal record from East Africa has been used to compare sites based on relative dating, whereby environmental and faunal changes and extinction events allow us to know which hominin finds are relatively younger or older than others.
The discovery of the Taung Child in 1924 (discussed in the Special Topic box “The Taung Child” below) shifted the focus of palaeoanthropological research from Europe to Africa, although acceptance of this shift was slow (Broom 1947; Dart 1925). The species to which it is assigned, Australopithecus africanus (name meaning “Southern Ape of Africa”), is currently dated to between 3.3 mya and 2.1 mya (Pickering and Kramers 2010), with discoveries from Sterkfontein, Taung, Makapansgat, and Gladysvale in South Africa (Figure 9.16). A relatively large brain (400 cc to 500 cc), small canines without an associated diastema, and more rounded cranium and smaller teeth than Au. afarensis indicate some derived traits. Similarly, the postcranial remains (in particular, the pelvis) indicate bipedalism. However, the sloping face and curved phalanges (indicative of retained arboreal locomotor abilities) show some ancestral features. Although not in direct association with stone tools, a 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities.

Another famous Au. africanus skull (the skull of “Mrs. Ples”) was previously attributed to Plesianthropus transvaalensis, meaning “near human from the Transvaal,” the old name for Gauteng Province, South Africa (Broom 1947, 1950). The name was shortened by contemporary journalists to “Ples” (Figure 9.17). Due to the prevailing mores of the time, the assumed female found herself married, at least in name, and has become widely known as “Mrs. Ples.” It was later reassigned to Au. africanus and is now argued by some to be a young male rather than an adult female cranium (Thackeray 2000, Thackeray et al. 2002).
In 2008, nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger, noted a clavicle bone in some leftover mining breccia in the Malapa Fossil Site (South Africa). After rigorous studies, the species, Australopithecus sediba (meaning “fountain” or “wellspring” in the South African language of Sesotho), was named in 2010 (Figure 9.18; Berger et al. 2010). The first type specimen belongs to a juvenile male, Karabo (MH1), but the species is known from at least six partial skeletons, from infants through adults. These specimens are currently dated to 1.97 mya (Dirks et al. 2010). The discoverers have argued that Au. sediba shows mosaic features between Au. africanus and the genus, Homo, which potentially indicates a transitional species, although this is heavily debated. These features include a small brain size (Australopithecus-like; 420 cc to 450 cc) but gracile mandible and small teeth (Homo-like). Similarly, the postcranial skeletons are also said to have mosaic features: scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. Some researchers have argued that Au. sediba shows a modern hand morphology (shorter fingers and a longer thumb), indicating that adaptations to tool manufacture and use may be present in this species.
Another famous Australopithecine find from South Africa is that of the nearly complete skeleton now known as “Little Foot” (Clarke 1998, 2013). Little Foot (StW 573) is potentially the earliest dated South African hominin fossil, dating to 3.7 mya, based on radiostopic techniques, although some argue that it is younger than 3 mya (Pickering and Kramers 2010). The name is jokingly in contrast to the cryptid species “bigfoot” and is named because the initial discovery of four ankle bones indicated bipedality. Little Foot was discovered by Ron Clarke in 1994, when he came across the ankle bones while sorting through monkey fossils in the University of Witwatersrand collections (Clarke and Tobias 1995). He asked Stephen Motsumi and Nkwane Molefe to identify the known records of the fossils, which allowed them to find the rest of the specimen within just days of searching the Sterkfontein Caves’ Silberberg Grotto.
The discoverers of Little Foot insist that other fossil finds, previously identified as Au. Africanus, be placed in this new species based on shared ancestral traits with older East African Australopithecines (Clarke and Kuman 2019). These include features such as a relatively large brain size (408 cc), robust zygomatic arch, and a flatter midface. Furthermore, the discoverers have argued that the heavy anterior dental wear patterns, relatively large anterior dentition, and smaller hind dentition of this specimen more closely resemble that of Au. anamensis or Au. afarensis. It has thus been placed in the species Australopithecus prometheus. This species name refers to a previously defunct taxon named by Raymond Dart. The species designation was, through analyzing Little Foot, revived by Ron Clarke, who insists that many other fossil hominin specimens have prematurely been placed into Au. africanus. Others say that it is more likely that Au. africanus is a more variable species and not representative of two distinct species.
Paranthropus “Robust” Australopithecines
In the robust australopithecines, the specialized nature of the teeth and masticatory system, such as flaring zygomatic arches (cheekbones), accommodate very large temporalis (chewing) muscles. These features also include a large, broad, dish-shaped face and and a large mandible with extremely large posterior dentition (referred to as megadonts) and hyper-thick enamel (Kimbel 2015; Lee-Thorp 2011; Wood 2010). Research has revolved around the shared adaptations of these “robust” australopithecines, linking their morphologies to a diet of hard and/or tough foods (Brain 1967; Rak 1988). Some argued that the diet of the robust australopithecines was so specific that any change in environment would have accelerated their extinction. The generalist nature of the teeth of the gracile australopithecines, and of early Homo, would have made them more capable of adapting to environmental change. However, some have suggested that the features of the robust australopithecines might have developed as an effective response to what are known as fallback foods in hard times rather than indicating a lack of adaptability.
There are currently three widely accepted robust australopithecus or, Paranthropus, species: P. aethiopicus, which has more ancestral traits, and P. boisei and P. robustus, which are more derived in their features (Strait et al. 1997; Wood and Schroer 2017). These three species have been grouped together by a majority of scholars as a single genus as they share more derived features (are more closely related to each other; or, in other words, are monophyletic) than the other australopithecines (Grine 1988; Hlazo 2015; Strait et al. 1997; Wood 2010 ). While researchers have mostly agreed to use the umbrella term Paranthropus, there are those who disagree (Constantino and Wood 2004, 2007; Wood 2010).
As a collective, this genus spans 2.7 mya to 1.0 mya, although the dates of the individual species differ. The earliest of the Paranthropus species, Paranthropus aethiopicus, is dated to between 2.7 mya and 2.3 mya and currently found in Tanzania, Kenya, and Ethiopia in the EARS system (Figure 9.19; Constantino and Wood 2007; Hlazo 2015; Kimbel 2015; Walker et al. 1986; White 1988). It is well known because of one specimen known as the “Black Skull” (KNM–WT 17000), so called because of the mineral manganese that stained it black during fossilization (Kimbel 2015). As with all robust Australopithecines, P. aethiopicus has the shared derived traits of large, flat premolars and molars; large, flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle); a sagittal crest (ridge on the top of the skull) for increased muscle attachment of the chewing muscles to the skull; and a robust mandible and supraorbital torus (brow ridge). However, only a few teeth have been found. A proximal tibia indicates bipedality and similar body size to Au. afarensis. In recent years, researchers have discovered and assigned a proximal tibia and juvenile cranium (L.338y-6) to the species (Wood and Boyle 2016).
First attributed as Zinjanthropus boisei (with the first discovery going by the nickname “Zinj” or sometimes “Nutcracker Man”), Paranthropus boisei was discovered in 1959 by Mary Leakey (see Figure 9.20 and 9.21; Hay 1990; Leakey 1959). This “robust” australopith species is distributed across countries in East Africa at sites such as Kenya (Koobi Fora, West Turkana, and Chesowanja), Malawi (Malema-Chiwondo), Tanzania (Olduvai Gorge and Peninj), and Ethiopia (Omo River Basin and Konso). The hypodigm, sample of fossils whose features define the group, has been found by researchers to date to roughly 2.4 mya to 1.4 mya. Due to the nature of its exaggerated, larger, and more robust features, P. boisei has been termed hyper-robust—that is, even more heavily built than other robust species, with very large, flat posterior dentition (Kimbel 2015). Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). Another famous specimen from this species is the Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu.

Paranthropus robustus was the first taxon to be discovered within the genus in Kromdraai B by a schoolboy named Gert Terblanche; subsequent fossil discoveries were made by researcher Robert Broom in 1938 (Figure 9.22; Broom 1938a, 1938b, 1950), with the holotype specimen TM 1517 (Broom 1938a, 1938b, 1950; Hlazo 2018). Paranthropus robustus dates approximately from 2.0 mya to 1 mya and is the only taxon from the genus to be discovered in South Africa. Several of these fossils are fragmentary in nature, distorted, and not well preserved because they have been recovered from quarry breccia using explosives. P. robustus features are neither as “hyper-robust” as P. boisei nor as ancestral as P. aethiopicus; instead, they have been described as being less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring; Rak 1983; Walker and Leakey 1988). Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick-enameled dentition.
Comparisons between Gracile and Robust Australopiths
Comparisons between gracile and robust australopithecines may indicate different phylogenetic groupings or parallel evolution in several species. In general, the robust australopithecines have large temporalis (chewing) muscles, as indicated by flaring zygomatic arches, sagittal crests, and robust mandibles (jawbones). Their hind dentition is large (megadont), with low cusps and thick enamel. Within the gracile australopithecines, researchers have debated the relatedness of the species, or even whether these species should be lumped together to represent more variable or polytypic species. Often researchers will attempt to draw chronospecific trajectories, with one taxon said to evolve into another over time.
Special Topic: The Taung Child
The well-known fossil of a juvenile Australopithecine, the “Taung Child,” was the first early hominin evidence ever discovered and was the first to demonstrate our common human heritage in Africa (Figure 9.23; Dart 1925). The tiny facial skeleton and natural endocast were discovered in 1924 by a local quarryman in the North West Province in South Africa and were painstakingly removed from the surrounding cement-like breccia by Raymond Dart using his wife’s knitting needles. When first shared with the scientific community in 1925, it was discounted as being nothing more than a young monkey of some kind. Prevailing biases of the time made it too difficult to contemplate that this small-brained hominin could have anything to do with our own history. The fact that it was discovered in Africa simply served to strengthen this bias.
Early Tool Use and Technology
Early Stone Age Technology (ESA)
The Early Stone Age (ESA) marks the beginning of recognizable technology made by our human ancestors. Stone-tool (or lithic) technology is defined by the fracturing of rocks and the manufacture of tools through a process called knapping. The Stone Age lasted for more than 3 million years and is broken up into chronological periods called the Early (ESA), Middle (MSA), and Later Stone Ages (LSA). Each period is further broken up into a different techno-complex, a term encompassing multiple assemblages (collections of artifacts) that share similar traits in terms of artifact production and morphology. The ESA spanned the largest technological time period of human innovation from over 3 million years ago to around 300,000 years ago and is associated almost entirely with hominin species prior to modern Homo sapiens. As the ESA advanced, stone tool makers (known as knappers) began to change the ways they detached flakes and eventually were able to shape artifacts into functional tools. These advances in technology go together with the developments in human evolution and cognition, dispersal of populations across the African continent and the world, and climatic changes.
In order to understand the ESA, it is important to consider that not all assemblages are exactly the same within each techno-complex: one can have multiple phases and traditions at different sites (Lombard et al. 2012). However, there is an overarching commonality between them. Within stone tool assemblages, both flakes or cores (the rocks from which flakes are removed) are used as tools. Large Cutting Tools (LCTs) are tools that are shaped to have functional edges. It is important to note that the information presented here is a small fraction of what is known about the ESA, and there are ongoing debates and discoveries within archaeology.
Currently, the oldest-known stone tools, which form the techno-complex the Lomekwian, date to 3.3 mya (Harmand et al. 2015; Toth 1985). They were found at a site called Lomekwi 3 in Kenya. This techno-complex is the most recently defined and pushed back the oldest-known date for lithic technology. There is only one known site thus far and, due to the age of the site, it is associated with species prior to Homo, such as Kenyanthropus platyops. Flakes were produced through indirect percussion, whereby the knappers held a rock and hit it against another rock resting on the ground. The pieces are very chunky and do not display the same fracture patterns seen in later techno-complexes. Lomekwian knappers likely aimed to get a sharp-edged piece on a flake, which would have been functional, although the specific function is currently unknown.
Stone tool use, however, is not only understood through the direct discovery of the tools. Cut marks on fossilized animal bones may illuminate the functionality of stone tools. In one controversial study in 2010, researchers argued that cut marks on a pair of animal bones from Dikika (Ethiopia), dated to 3.4 mya, were from stone tools. The discoverers suggested that they be more securely associated, temporally, with Au. afarensis. However, others have noted that these marks are consistent with teeth marks from crocodiles and other carnivores.
The Oldowan techno-complex is far more established in the scientific literature (Leakey 1971). It is called the Oldowan because it was originally discovered in Olduvai Gorge, Tanzania, but the oldest assemblage is from Gona in Ethiopia, dated to 2.6 mya (Semaw 2000). The techno-complex is defined as a core and flake industry. Like the Lomekwian, there was an aim to get sharp-edged flakes, but this was achieved through a different production method. Knappers were able to actively hold or manipulate the core being knapped, which they could directly hit using a hammerstone. This technique is known as free-hand percussion, and it demonstrates an understanding of fracture mechanics. It has long been argued that the Oldowan hominins were skillful in tool manufacture.
Because Oldowan knapping requires skill, earlier researchers have attributed these tools to members of our genus, Homo. However, some have argued that these tools are in more direct association with hominins in the genera described in this chapter (Figure 9.24).
Invisible Tool Manufacture and Use
The vast majority of our understanding of these early hominins comes from fossils and reconstructed paleoenvironments. It is only from 3 mya when we can start “looking into their minds” and lifestyles by analyzing their manufacture and use of stone tools. However, the vast majority of tool use in primates (and, one can argue, in humans) is not with durable materials like stone. All of our extant great ape relatives have been observed using sticks, leaves, and other materials for some secondary purpose (to wade across rivers, to “fish” for termites, or to absorb water for drinking). It is possible that the majority of early hominin tool use and manufacture may be invisible to us because of this preservation bias.
Summary
The fossil record of our earliest hominin relatives has allowed paleoanthropologists to unpack some of the mysteries of our evolution. We now know that traits associated with bipedalism evolved before other “human-like” traits, even though the first hominins were still very capable of arboreal locomotion. We also know that, for much of this time, hominin taxa were diverse in the way they looked and what they ate, and they were widely distributed across the African continent. And we know that the environments in which these hominins lived underwent many changes over this time during several warming and cooling phases.
Yet this knowledge has opened up many new mysteries. We still need to better differentiate some taxa. In addition, there are ongoing debates about why certain traits evolved and what they meant for the extinction of some of our relatives (like the robust australopiths). The capabilities of these early hominins with respect to tool use and manufacture is also still uncertain.
Hominin Species Summaries
|
Hominin |
Sahelanthropus tchadensis |
|
Dates |
7 mya to 6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
The initial discovery, made in 2001. |
|
Brain size |
360 cc average |
|
Dentition |
Smaller than in extant great apes; larger and pointier than in humans. Canines worn at the tips. |
|
Cranial features |
A short cranial base and a foramen magnum (hole in which the spinal cord enters the cranium) that is more humanlike in positioning; has been argued to indicate upright walking. |
|
Postcranial features |
Currently little published postcranial material. |
|
Culture |
N/A |
|
Other |
The extent to which this hominin was bipedal is currently heavily debated. If so, it would indicate an arboreal bipedal ancestor of hominins, not a knuckle-walker like chimpanzees. |
|
Hominin |
Orrorin tugenensis |
|
Dates |
6 mya to 5.7 mya |
|
Region(s) |
Tugen Hills (Kenya) |
|
Famous discoveries |
Original discovery in 2000. |
|
Brain size |
N/A |
|
Dentition |
Smaller cheek teeth (molars and premolars) than even more recent hominins (i.e., derived), thick enamel, and reduced, but apelike, canines. |
|
Cranial features |
Not many found |
|
Postcranial features |
Fragmentary leg, arm, and finger bones have been found. Indicates bipedal locomotion. |
|
Culture |
Potential toolmaking capability based on hand morphology, but nothing found directly. |
|
Other |
This is the earliest species that clearly indicates adaptations for bipedal locomotion. |
|
Hominin |
Ardipithecus kadabba |
|
Dates |
5.2 mya to 5.8 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
Discovered by Yohannes Haile-Selassie in 1997. |
|
Brain size |
N/A |
|
Dentition |
Larger hind dentition than in modern chimpanzees. Thick enamel and larger canines than in later hominins. |
|
Cranial features |
N/A |
|
Postcranial features |
A large hallux (big toe) bone indicates a bipedal “push off.” |
|
Culture |
N/A |
|
Other |
Faunal evidence indicates a mixed grassland/woodland environment. |
|
Hominin |
Ardipithecus ramidus |
|
Dates |
4.4 mya |
|
Region(s) |
Middle Awash region and Gona (Ethiopia) |
|
Famous discoveries |
A partial female skeleton nicknamed “Ardi” (ARA-VP-6/500) (found in 1994). |
|
Brain size |
300 cc to 350 cc |
|
Dentition |
Little differences between the canines of males and females (small sexual dimorphism). |
|
Cranial features |
Midfacial projection, slightly prognathic. Cheekbones less flared and robust than in later hominins. |
|
Postcranial features |
Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, an opposable big toe similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. |
|
Culture |
None directly associated |
|
Other |
Over 110 specimens from Aramis |
|
Hominin |
Australopithecus anamensis |
|
Dates |
4.2 mya to 3.8 mya |
|
Region(s) |
Turkana region (Kenya); Middle Awash (Ethiopia) |
|
Famous discoveries |
A 2019 find from Ethiopia, named MRD. |
|
Brain size |
370 cc |
|
Dentition |
Relatively large canines compared with more recent Australopithecines. |
|
Cranial features |
Projecting cheekbones and ancestral earholes. |
|
Postcranial features |
Lower limb bones (tibia and femur) indicate bipedality; arboreal features in upper limb bones (humerus) found. |
|
Culture |
N/A |
|
Other |
Almost 100 specimens, representing over 20 individuals, have been found to date. |
|
Hominin |
Australopithecus afarensis |
|
Dates |
3.9 mya to 2.9 mya |
|
Region(s) |
Afar Region, Omo, Maka, Fejej, and Belohdelie (Ethiopia); Laetoli (Tanzania); Koobi Fora (Kenya) |
|
Famous discoveries |
Lucy (discovery: 1974), Selam (Dikika Child, discovery: 2000), Laetoli Footprints (discovery: 1976). |
|
Brain size |
380 cc to 430 cc |
|
Dentition |
Reduced canines and molars relative to great apes but larger than in modern humans. |
|
Cranial features |
Prognathic face, facial features indicate relatively strong chewing musculature (compared with Homo) but less extreme than in Paranthropus. |
|
Postcranial features |
Clear evidence for bipedalism from lower limb postcranial bones. Laetoli Footprints indicate humanlike walking. Dikika Child bones indicate retained ancestral arboreal traits in the postcrania. |
|
Culture |
None directly, but close in age and proximity to controversial cut marks at Dikika and early tools in Lomekwi. |
|
Other |
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. |
|
Hominin |
Australopithecus bahrelghazali |
|
Dates |
3.6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
“Abel,” the holotype (discovery: 1995). |
|
Brain size |
N/A |
|
Dentition |
N/A |
|
Cranial features |
N/A |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Arguably within range of variation of Au. afarensis. |
|
Hominin |
Australopithecus prometheus |
|
Dates |
3.7 mya (debated) |
|
Region(s) |
Sterkfontein (South Africa) |
|
Famous discoveries |
“Little Foot” (StW 573) (discovery: 1994) |
|
Brain size |
408 cc (Little Foot estimate) |
|
Dentition |
Heavy anterior dental wear patterns, relatively large anterior dentition and smaller hind dentition, similar to Au. afarensis. |
|
Cranial features |
Relatively larger brain size, robust zygomatic arch, and a flatter midface. |
|
Postcranial features |
The initial discovery of four ankle bones indicated bipedality. |
|
Culture |
N/A |
|
Other |
Highly debated new species designation. |
|
Hominin |
Australopithecus deyiremada |
|
Dates |
3.5 mya to 3.3 mya |
|
Region(s) |
Woranso-Mille (Afar region, Ethiopia) |
|
Famous discoveries |
First fossil mandible bones were discovered in 2011 in the Afar region of Ethiopia by Yohannes Haile-Selassie. |
|
Brain size |
N/A |
|
Dentition |
Smaller teeth with thicker enamel than seen in Au. afarensis, with a potentially hardier diet. |
|
Cranial features |
Larger mandible and more projecting cheekbones than in Au. afarensis. |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Contested species designation; arguably a member of Au. afarensis. |
|
Hominin |
Kenyanthopus platyops |
|
Dates |
3.5 mya to 3.2 mya |
|
Region(s) |
Lake Turkana (Kenya) |
|
Famous discoveries |
KNM–WT 40000 (discovered 1999) |
|
Brain size |
Difficult to determine but appears within the range of Australopithecus afarensis. |
|
Dentition |
Small molars/dentition (Homo-like characteristic) |
|
Cranial features |
Flatter (i.e., orthognathic) face |
|
Postcranial features |
N/A |
|
Culture |
Some have associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species/specimen. |
|
Other |
Taxonomic placing of this species is quite divided. The discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis. |
|
Hominin |
Australopithecus africanus |
|
Dates |
3.3 mya to 2.1 mya |
|
Region(s) |
Sterkfontein, Taung, Makapansgat, Gladysvale (South Africa) |
|
Famous discoveries |
Taung Child (discovery in 1994), “Mrs. Ples” (discover in 1947), Little Foot (arguable; discovery in 1994). |
|
Brain size |
400 cc to 500 cc |
|
Dentition |
Smaller teeth (derived) relative to Au. afarensis. Small canines with no diastema. |
|
Cranial features |
A rounder skull compared with Au. afarensis in East Africa. A sloping face (ancestral). |
|
Postcranial features |
Similar postcranial evidence for bipedal locomotion (derived pelvis) with retained arboreal locomotion, e.g., curved phalanges (fingers), as seen in Au. afarensis. |
|
Culture |
None with direct evidence. |
|
Other |
A 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities. |
|
Hominin |
Australopithecus garhi |
|
Dates |
2.5 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
N/A |
|
Brain size |
450 cc |
|
Dentition |
Larger hind dentition than seen in other gracile Australopithecines. |
|
Cranial features |
N/A |
|
Postcranial features |
A femur of a fragmentary partial skeleton, argued to belong to Au. garhi, indicates this species may be longer-limbed than Au. afarensis, although still able to move arboreally. |
|
Culture |
Crude stone tools resembling Oldowan (described later) have been found in association with Au. garhi. |
|
Other |
This species is not well documented or understood and is based on only a few fossil specimens. |
|
Hominin |
Paranthropus aethiopicus |
|
Dates |
2.7 mya to 2.3 mya |
|
Region(s) |
West Turkana (Kenya); Laetoli (Tanzania); Omo River Basin (Ethiopia) |
|
Famous discoveries |
The “Black Skull” (KNM–WT 17000) (discovery 1985). |
|
Brain Size |
410 cc |
|
Dentition |
P. aethiopicus has the shared derived traits of large flat premolars and molars, although few teeth have been found. |
|
Cranial features |
Large flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle), a sagittal crest for increased muscle attachment of the chewing muscles to the skull, and a robust mandible and supraorbital torus (brow ridge). |
|
Postcranial features |
A proximal tibia indicates bipedality and similar size to Au. afarensis. |
|
Culture |
N/A |
|
Other |
The “Black Skull” is so called because of the mineral manganese that stained it black during fossilization. |
|
Hominin |
Paranthropus boisei |
|
Dates |
2.4 mya to 1.4 mya |
|
Region(s) |
Koobi Fora, West Turkana, and Chesowanja (Kenya); Malema-Chiwondo (Malawi), Olduvai Gorge and Peninj (Tanzania); and Omo River basin and Konso (Ethiopia) |
|
Famous discoveries |
“Zinj,” or sometimes “Nutcracker Man” (OH5), in 1959 by Mary Leakey. The Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu. |
|
Brain size |
500 cc to 550 cc |
|
Dentition |
Very large, flat posterior dentition (largest of all hominins currently known). Much smaller anterior dentition. Very thick dental enamel. |
|
Cranial features |
Indications of very large chewing muscles (e.g., flaring zygomatic arches and a large sagittal crest). |
|
Postcranial features |
Evidence for high variability and sexual dimorphism, with estimates of males at 1.37 meters tall and females at 1.24 meters. |
|
Culture |
Richard Leakey and Bernard Wood have both suggested that P. boisei could have made and used stone tools. Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. |
|
Other |
Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). This differs from what is seen in P. robustus. |
|
Hominin |
Australopithecus sediba |
|
Dates |
1.97 mya |
|
Region(s) |
Malapa Fossil Site (South Africa) |
|
Famous discoveries |
Karabo (MH1) (discovery in 2008) |
|
Brain size |
420 cc to 450 cc |
|
Dentition |
Small dentition with Australopithecine cusp-spacing. |
|
Cranial features |
Small brain size (Australopithecus-like) but gracile mandible (Homo-like). |
|
Postcranial features |
Scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (tree climbing, particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. |
|
Culture |
None of direct association, but some have argued that a modern hand morphology (shorter fingers and a longer thumb) means that adaptations to tool manufacture and use may be present in this species. |
|
Other |
It was first discovered through a clavicle bone in 2008 by nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger. |
|
Hominin |
Paranthropus robustus |
|
Dates |
2.3 mya to 1 mya |
|
Region(s) |
Kromdraai B, Swartkrans, Gondolin, Drimolen, and Coopers Cave (South Africa) |
|
Famous discoveries |
SK48 (original skull) |
|
Brain size |
410 cc to 530 cc |
|
Dentition |
Large posterior teeth with thick enamel, consistent with other Robust Australopithecines. Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick enameled dentition. |
|
Cranial features |
P. robustus features are neither as “hyper-robust” as P. boisei or as ancestral in features as P. aethiopicus. They have been described as less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring). |
|
Postcranial features |
Reconstructions indicate sexual dimorphism. |
|
Culture |
N/A |
|
Other |
Several of these fossils are fragmentary in nature, distorted, and not well preserved, because they have been recovered from quarry breccia using explosives. |
Review Questions
- What is the difference between a “derived” versus an “ancestral” trait? Give an example of both, seen in Au. afarensis.
- Which of the paleoenvironment hypotheses have been used to describe early hominin diversity, and which have been used to describe bipedalism?
- Which anatomical features for bipedalism do we see in early hominins?
- Describe the dentition of gracile and robust australopithecines. What might these tell us about their diets?
- List the hominin species argued to be associated with stone tool technologies. Are you convinced of these associations? Why/why not?
Key Terms
Arboreal: Related to trees or woodland.
Aridification: Becoming increasingly arid or dry, as related to the climate or environment.
Aridity Hypothesis: The hypothesis that long-term aridification and expansion of savannah biomes were drivers in diversification in early hominin evolution.
Assemblage: A collection demonstrating a pattern. Often pertaining to a site or region.
Bipedalism: The locomotor ability to walk on two legs.
Breccia: Hard, calcareous sedimentary rock.
Canines: The pointy teeth just next to the incisors, in the front of the mouth.
Cheek teeth: Or hind dentition (molars and premolars).
Chronospecies: Species that are said to evolve into another species, in a linear fashion, over time.
Clade: A group of species or taxa with a shared common ancestor.
Cladistics: The field of grouping organisms into those with shared ancestry.
Context: As pertaining to palaeoanthropology, this term refers to the place where an artifact or fossil is found.
Cores: The remains of a rock that has been flaked or knapped.
Cusps: The ridges or “bumps” on the teeth.
Dental formula: A technique to describe the number of incisors, canines, premolars, and molars in each quadrant of the mouth.
Derived traits: Newly evolved traits that differ from those seen in the ancestor.
Diastema: A tooth gap between the incisors and canines.
Early Stone Age (ESA): The earliest-described archaeological period in which we start seeing stone-tool technology.
East African Rift System (EARS): This term is often used to refer to the Rift Valley, expanding from Malawi to Ethiopia. This active geological structure is responsible for much of the visibility of the paleoanthropological record in East Africa.
Enamel: The highly mineralized outer layer of the tooth.
Encephalization: Expansion of the brain.
Extant: Currently living—i.e., not extinct.
Fallback foods: Foods that may not be preferred by an animal (e.g., foods that are not nutritionally dense) but that are essential for survival in times of stress or scarcity.
Fauna: The animals of a particular region, habitat, or geological period.
Faunal assemblages: Collections of fossils of the animals found at a site.
Faunal turnover: The rate at which species go extinct and are replaced with new species.
Flake: The piece knocked off of a stone core during the manufacture of a tool, which may be used as a stone tool.
Flora: The plants of a particular region, habitat, or geological period.
Folivorous: Foliage-eating.
Foramen magnum: The large hole (foramen) at the base of the cranium, through which the spinal cord enters the skull.
Fossil: The remains or impression of an organism from the past.
Frugivorous: Fruit-eating.
Generalist: A species that can thrive in a wide variety of habitats and can have a varied diet.
Glacial: Colder, drier periods during an ice age when there is more ice trapped at the poles.
Gracile: Slender, less rugged, or pronounced features.
Hallux: The big toe.
Holotype: A single specimen from which a species or taxon is described or named.
Hominin: A primate category that includes humans and our fossil relatives since our divergence from extant great apes.
Honing P3: The mandibular premolar alongside the canine (in primates, the P3), which is angled to give space for (and sharpen) the upper canines.
Hyper-robust: Even more robust than considered normal in the Paranthropus genus.
Hypodigm: A sample (here, fossil) from which researchers extrapolate features of a population.
Incisiform: An adjective referring to a canine that appears more incisor-like in morphology.
Incisors: The teeth in the front of the mouth, used to bite off food.
Interglacial: A period of milder climate in between two glacial periods.
Isotopes: Two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons, giving them the same chemical properties but different atomic masses.
Knappers: The people who fractured rocks in order to manufacture tools.
Knapping: The fracturing of rocks for the manufacture of tools.
Large Cutting Tool (LCT): A tool that is shaped to have functional edges.
Last Common Ancestor (LCA): The hypothetical final ancestor (or ancestral population) of two or more taxa before their divergence.
Lithic: Relating to stone (here to stone tools).
Lumbar lordosis: The inward curving of the lower (lumbar) parts of the spine. The lower curve in the human S-shaped spine.
Lumpers: Researchers who prefer to lump variable specimens into a single species or taxon and who feel high levels of variation is biologically real.
Megadont: An organism with extremely large dentition compared with body size.
Metacarpals: The long bones of the hand that connect to the phalanges (finger bones).
Molars: The largest, most posterior of the hind dentition.
Monophyletic: A taxon or group of taxa descended from a common ancestor that is not shared with another taxon or group.
Morphology: The study of the form or size and shape of things; in this case, skeletal parts.
Mosaic evolution: The concept that evolutionary change does not occur homogeneously throughout the body in organisms.
Obligate bipedalism: Where the primary form of locomotion for an organism is bipedal.
Occlude: When the teeth from the maxilla come into contact with the teeth in the mandible.
Oldowan: Lower Paleolithic, the earliest stone tool culture.
Orthognathic: The face below the eyes is relatively flat and does not jut out anteriorly.
Paleoanthropologists: Researchers that study human evolution.
Paleoenvironment: An environment from a period in the Earth’s geological past.
Parabolic: Like a parabola (parabola-shaped).
Phalanges: Long bones in the hand and fingers.
Phylogenetics: The study of phylogeny.
Phylogeny: The study of the evolutionary relationships between groups of organisms.
Pliocene: A geological epoch between the Miocene and Pleistocene.
Polytypic: In reference to taxonomy, having two or more group variants capable of interacting and breeding biologically but having morphological population differences.
Postcranium: The skeleton below the cranium (head).
Premolars: The smallest of the hind teeth, behind the canines.
Procumbent: In reference to incisors, tilting forward.
Prognathic: In reference to the face, the area below the eyes juts anteriorly.
Quaternary Ice Age: The most recent geological time period, which includes the Pleistocene and Holocene Epochs and which is defined by the cyclicity of increasing and decreasing ice sheets at the poles.
Relative dating: Dating techniques that refer to a temporal sequence (i.e., older or younger than others in the reference) and do not estimate actual or absolute dates.
Robust: Rugged or exaggerated features.
Site: A place in which evidence of past societies/species/activities may be observed through archaeological or paleontological practice.
Specialist: A specialist species can thrive only in a narrow range of environmental conditions or has a limited diet.
Splitters: Researchers who prefer to split a highly variable taxon into multiple groups or species.
Taxa: Plural of taxon, a taxonomic group such as species, genus, or family.
Taxonomy: The science of grouping and classifying organisms.
Techno-complex: A term encompassing multiple assemblages that share similar traits in terms of artifact production and morphology.
Thermoregulation: Maintaining body temperature through physiologically cooling or warming the body.
Ungulates: Hoofed mammals—e.g., cows and kudu.
Volcanic tufts: Rock made from ash from volcanic eruptions in the past.
Valgus knee: The angle of the knee between the femur and tibia, which allows for weight distribution to be angled closer to the point above the center of gravity (i.e., between the feet) in bipeds.
For Further Exploration
The Smithsonian Institution website hosts descriptions of fossil species, an interactive timeline, and much more.
The Maropeng Museum website hosts a wealth of information regarding South African Fossil Bearing sites in the Cradle of Humankind.
This quick comparison between Homo naledi and Australopithecus sediba from the Perot Museum.
This explanation of the braided stream by the Perot Museum.
A collation of 3-D files for visualizing (or even 3-D printing) for homes, schools, and universities.
PBS learning materials, including videos and diagrams of the Laetoli footprints, bipedalism, and fossils.
A wealth of information from the Australian Museum website, including species descriptions, family trees, and explanations of bipedalism and diet.
References
Alemseged, Zeresenay, Fred Spoor, William H. Kimbel, René Bobe, Denis Geraads, Denné Reed, and Jonathan G. Wynn. 2006. “A Juvenile Early Hominin Skeleton from Dikika, Ethiopia.” Nature 443 (7109): 296–301.
Asfaw, Berhane, Tim White, Owen Lovejoy, Bruce Latimer, Scott Simpson, and Gen Suwa. 1999. “Australopithecus garhi: A New Species of Early Hominid from Ethiopia.” Science 284 (5414): 629–635.
Behrensmeyer, Anna K., Nancy E. Todd, Richard Potts, and Geraldine E. McBrinn. 1997. “Late Pliocene Faunal Turnover in the Turkana Basin, Kenya, and Ethiopia.” Science 278 (5343): 637–640.
Berger, Lee R., Darryl J. De Ruiter, Steven E. Churchill, Peter Schmid, Kristian J. Carlson, Paul HGM Dirks, and Job M. Kibii. 2010. “Australopithecus sediba: A New Species of Homo-like Australopith from South Africa.” Science 328 (5975): 195–204.
Bobe, René, and Anna K. Behrensmeyer. 2004. “The Expansion of Grassland Ecosystems in Africa in Relation to Mammalian Evolution and the Origin of the Genus Homo.” Palaeogeography, Palaeoclimatology, Palaeoecology 207 (3–4): 399–420.
Brain, C. K. 1967. “The Transvaal Museum's Fossil Project at Swartkrans.” South African Journal of Science 63 (9): 378–384.
Broom, R. 1938a. “More Discoveries of Australopithecus.” Nature 141 (1): 828–829.
Broom, R. 1938b. “The Pleistocene Anthropoid Apes of South Africa.” Nature 142 (3591): 377–379.
Broom, R. 1947. “Discovery of a New Skull of the South African Ape-Man, Plesianthropus.” Nature 159 (4046): 672.
Broom, R. 1950. “The Genera and Species of the South African Fossil Ape-Man.” American Journal of Physical Anthropology 8 (1): 1–14.
Brunet, Michel, Alain Beauvilain, Yves Coppens, Emile Heintz, Aladji HE Moutaye, and David Pilbeam. 1995. “The First Australopithecine 2,500 Kilometers West of the Rift Valley (Chad).” Nature 378 (6554): 275–273.
Ceríaco, L. M. (2012). Human attitudes towards herpetofauna: The influence of folklore and negative values on the conservation of amphibians and reptiles in Portugal. Journal of Ethnobiology and Ethnomedicine, 8(1). https://doi.org/10.1186/1746-4269-8-8
Cerling, Thure E., Jonathan G. Wynn, Samuel A. Andanje, Michael I. Bird, David Kimutai Korir, Naomi E. Levin, William Mace, Anthony N. Macharia, Jay Quade, and Christopher H. Remien. 2011. “Woody Cover and Hominin Environments in the Past 6 Million Years.” Nature 476, no. 7358 (2011): 51-56..
Clarke, Ronald J. 1998. “First Ever Discovery of a Well-Preserved Skull and Associated Skeleton of Australopithecus.” South African Journal of Science 94 (10): 460–463.
Clarke, Ronald J. 2013. “Australopithecus from Sterkfontein Caves, South Africa.” In The Paleobiology of Australopithecus, edited by K. E. Reed, J. G. Fleagle, and R. E. Leakey, 105–123. Netherlands: Springer.
Clarke, Ronald J., and Kathleen Kuman. 2019. “The Skull of StW 573, a 3.67 Ma Australopithecus Prometheus Skeleton from Sterkfontein Caves, South Africa.” Journal of Human Evolution 134: 102634.
Clarke, R. J., and P. V. Tobias. 1995. “Sterkfontein Member 2 Foot Bones of the Oldest South African Hominid.” Science 269 (5223): 521–524.
Constantino, P. J., and B. A. Wood. 2004. “Paranthropus Paleobiology”. In Miscelanea en Homenae a Emiliano Aguirre, volumen III: Paleoantropologia, edited by E. G. Pérez and S. R. Jara, 136–151. Alcalá de Henares: Museo Arqueologico Regional.
Constantino, P. J., and B. A. Wood. 2007. “The Evolution of Zinjanthropus boisei.” Evolutionary Anthropology: Issues, News, and Reviews 16 (2): 49–62.
Dart, Raymond A. 1925. “Australopithecus africanus, the Man-Ape of South Africa.” Nature 115: 195–199.
Darwin, Charles. 1871. The Descent of Man: And Selection in Relation to Sex. London: J. Murray.
Daver, Guillaume, F. Guy, Hassane Taïsso Mackaye, Andossa Likius, J-R. Boisserie, Abderamane Moussa, Laurent Pallas, Patrick Vignaud, and Nékoulnang D. Clarisse. 2022. "Postcranial Evidence of Late Miocene Hominin Bipedalism in Chad." Nature 609 (7925): 94–100.
Heinzelin, Jean de, J. Desmond Clark, Tim White, William Hart, Paul Renne, Giday WoldeGabriel, Yonas Beyene, and Elisabeth Vrba. 1999. “Environment and Behavior of 2.5-Million-Year-Old Bouri Hominids.” Science 284 (5414): 625–629.
DeMenocal, Peter B. D. 2004. “African Climate Change and Faunal Evolution during the Pliocene–Pleistocene.” Earth and Planetary Science Letters 220 (1–2): 3–24.
DeMenocal, Peter B. D. and J. Bloemendal, J. 1995. “Plio-Pleistocene Climatic Variability in Subtropical Africa and the Paleoenvironment of Hominid Evolution: A Combined Data-Model Approach.” In Paleoclimate and Evolution, with Emphasis on Human Origins, edited by E. S. Vrba, G. H. Denton, T. C. Partridge, and L. H. Burckle, 262–288. New Haven: Yale University Press.
Dirks, Paul HGM, Job M. Kibii, Brian F. Kuhn, Christine Steininger, Steven E. Churchill, Jan D. Kramers, Robyn Pickering, Daniel L. Farber, Anne-Sophie Mériaux, Andy I. R. Herries, Geoffrey C. P. King, And Lee R. Berger. 2010. “Geological Setting and Age of Australopithecus sediba from Southern Africa.” Science 328 (5975): 205–208.
Faith, J. Tyler, and Anna K. Behrensmeyer. 2013. “Climate Change and Faunal Turnover: Testing the Mechanics of the Turnover-Pulse Hypothesis with South African Fossil Data.” Paleobiology 39 (4): 609–627.
Greene, H. W. (2017). Primates And Snakes: 75 Million Years of Deadly Dialogue? Cornell Lab Seminar Series. Cornell University. https://academy.allaboutbirds.org/live-event/primates%20and-snakes-75-million-years-of-deadly-dialogue/
Grine, Frederick E. 1988. “New Craniodental Fossils of Paranthropus from the Swartkrans Formation and Their Significance in ‘Robust’ Australopithecine Evolution.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 223–243. New York: Aldine de Gruyter.
Grine, Frederick E., Carrie S. Mongle, John G. Fleagle, and Ashley S. Hammond. 2022. "The Taxonomic Attribution of African Hominin Postcrania from the Miocene through the Pleistocene: Associations and Assumptions." Journal of Human Evolution 173: 103255.
Haile-Selassie, Yohannes, Luis Gibert, Stephanie M. Melillo, Timothy M. Ryan, Mulugeta Alene, Alan Deino, Naomi E. Levin, Gary Scott, and Beverly Z. Saylor. 2015. “New Species from Ethiopia Further Expands Middle Pliocene Hominin Diversity.” Nature 521 (7553): 432–433.
Haile-Selassie, Yohannes, Stephanie M. Melillo, Antonino Vazzana, Stefano Benazzi, and Timothy M. Ryan. 2019. “A 3.8-Million-Year-Old Hominin Cranium from Woranso-Mille, Ethiopia.” Nature 573 (7773): 214-219.
Harmand, Sonia, Jason E. Lewis, Craig S. Feibel, Christopher J. Lepre, Sandrine Prat, Arnaud Lenoble, Xavier Boës et al. 2015. “3.3-Million-Year-Old Stone Tools from Lomekwi3, West Turkana, Kenya.” Nature 521 (7552): 310–316.
Hay, Richard L. 1990. “Olduvai Gorge: A Case History in the Interpretation of Hominid Paleoenvironments.” In East Africa: Establishment of a Geologic Framework for Paleoanthropology, edited by L. Laporte, 23–37. Boulder: Geological Society of America.
Hay, Richard L., and Mary D. Leakey. 1982. “The Fossil Footprints of Laetoli.” Scientific American 246 (2): 50–57.
Hlazo, Nomawethu. 2015. “Paranthropus: Variation in Cranial Morphology.” Honours thesis, Archaeology Department, University of Cape Town, Cape Town.
Hlazo, Nomawethu. 2018. “Variation and the Evolutionary Drivers of Diversity in the Genus Paranthropus.” Master’s thesis, Archaeology Department, University of Cape Town, Cape Town.
Isbell, L. A. (2009). The Fruit, the Tree, and the Serpent: Why We See So Well. Harvard University Press. https://doi.org/10.2307/j.ctvjnrvj0
Johanson, D. C., T. D. White, and Y. Coppens. 1978. “A New Species of the Genus Australopithecus (Primates: Hominidae) from the Pliocene of East Africa.” Kirtlandia 28: 1–14.
Kimbel, William H. 2015. “The Species and Diversity of Australopiths.” In Handbook of Paleoanthropology, 2nd ed., edited by T. Hardt, 2071–2105. Berlin: Springer.
Kimbel, William H., and Lucas K. Delezene. 2009. “‘Lucy’ Redux: A Review of Research on Australopithecus afarensis.” American Journal of Physical Anthropology 140 (S49): 2–48.
Kingston, John D. 2007. “Shifting Adaptive Landscapes: Progress and Challenges in Reconstructing Early Hominid Environments.” American Journal of Physical Anthropology 134 (S45): 20–58.
Kingston, John D., and Terry Harrison. 2007. “Isotopic Dietary Reconstructions of Pliocene Herbivores at Laetoli: Implications for Early Hominin Paleoecology.” Palaeogeography, Palaeoclimatology, Palaeoecology 243 (3–4): 272–306.
Leakey, Louis S. B. 1959. “A New Fossil Skull from Olduvai.” Nature 184 (4685): 491–493.
Leakey, Mary 1971. Olduvai Gorge, Vol. 3. Cambridge: Cambridge University Press.
Leakey, Mary D., and Richard L. Hay. 1979. “Pliocene Footprints in the Laetoli Beds at Laetoli, Northern Tanzania.” Nature 278 (5702): 317–323.
Leakey, Meave G., Craig S. Feibel, Ian McDougall, and Alan Walker. 1995. “New Four–Million-Year-Old Hominid Species from Kanapoi and Allia Bay, Kenya.” Nature 376 (6541): 565–571.
Meave G., Fred Spoor, Frank H. Brown, Patrick N. Gathogo, Christopher Kiarie, Louise N. Leakey, and Ian McDougall. 2001. “New Hominin Genus from Eastern Africa Shows Diverse Middle Pliocene Lineages.” Nature 410 (6827): 433–440.
Lebatard, Anne-Elisabeth, Didier L. Bourlès, Philippe Duringer, Marc Jolivet, Régis Braucher, Julien Carcaillet, Mathieu Schuster et al. 2008. “Cosmogenic Nuclide Dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene Hominids from Chad.” Proceedings of the National Academy of Sciences 105 (9): 3226–3231.
Lee-Thorp, Julia. 2011. “The Demise of ‘Nutcracker Man.’” Proceedings of the National Academy of Sciences 108 (23): 9319–9320.
Lombard, Marlize, L. Y. N. Wadley, Janette Deacon, Sarah Wurz, Isabelle Parsons, Moleboheng Mohapi, Joane Swart, and Peter Mitchell. 2012. “South African and Lesotho Stone Age Sequence Updated.” The South African Archaeological Bulletin 67 (195): 123–144.
Maslin, Mark A., Chris M. Brierley, Alice M. Milner, Susanne Shultz, Martin H. Trauth, and Katy E. Wilson. 2014. “East African Climate Pulses and Early Human Evolution.” Quaternary Science Reviews 101: 1–17.
McHenry, Henry M. 2009. “Human Evolution.” In Evolution: The First Four Billion Years, edited by M. Ruse and J. Travis, 256–280. Cambridge: The Belknap Press of Harvard University Press..
Patterson, Bryan, and William W. Howells. 1967. “Hominid Humeral Fragment from Early Pleistocene of Northwestern Kenya.” Science 156 (3771): 64–66.
Pickering, Robyn, and Jan D. Kramers. 2010. “Re-appraisal of the Stratigraphy and Determination of New U-Pb Dates for the Sterkfontein Hominin Site.” Journal of Human Evolution 59 (1): 70–86.
Potts, Richard. 1998. “Environmental Hypotheses of Hominin Evolution.” American Journal of Physical Anthropology 107 (S27): 93–136.
Potts, Richard. 2013. “Hominin Evolution in Settings of Strong Environmental Variability.” Quaternary Science Reviews 73: 1–13.
Rak, Yoel. 1983. The Australopithecine Face. New York: Academic Press.
Rak, Yoel. 1988. “On Variation in the Masticatory System of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by M. Ruse and J. Travis, 193–198. New York: Aldine de Gruyter.
Semaw, Sileshi. 2000. “The World’s Oldest Stone Artefacts from Gona, Ethiopia: Their Implications for Understanding Stone Technology and Patterns of Human Evolution between 2.6 Million Years Ago and 1.5 Million Years Ago.” Journal of Archaeological Science 27(12): 1197–1214.
Shipman, Pat. 2002. The Man Who Found the Missing Link: Eugene Dubois and his Lifelong Quest to Prove Darwin Right. New York: Simon & Schuster.
Spoor, Fred. 2015. “Palaeoanthropology: The Middle Pliocene Gets Crowded.” Nature 521 (7553): 432–433.
Strait, David S., Frederick E. Grine, and Marc A. Moniz. 1997. A Reappraisal of Early Hominid Phylogeny.” Journal of Human Evolution 32 (1): 17–82.
Thackeray, J. Francis. 2000. “‘Mrs. Ples’ from Sterkfontein: Small Male or Large Female?” The South African Archaeological Bulletin 55: 155–158.
Thackeray, J. Francis, José Braga, Jacques Treil, N. Niksch, and J. H. Labuschagne. 2002. “‘Mrs. Ples’ (Sts 5) from Sterkfontein: An Adolescent Male?” South African Journal of Science 98 (1–2): 21–22.
Toth, Nicholas. 1985. “The Oldowan Reassessed.” Journal of Archaeological Science 12 (2): 101–120.
Van Le, Q., Isbell, L. A., Matsumoto, J., Nguyen, M., Hori, E., Maior, R. S., Tomaz, C., Tran, A. H., Ono, T., & Nishijo, H. (2013). Pulvinar neurons reveal neurobiological evidence of past selection for rapid detection of snakes. Proceedings of the National Academy of Sciences, 110(47), 19000–19005.
Vrba, E. S. 1988. “Late Pliocene Climatic Events and Hominid Evolution.” In The Evolutionary History of the Robust Australopithecines, edited by F. E. Grine, 405–426. New York: Aldine.
Vrba, Elisabeth S. 1998. “Multiphasic Growth Models and the Evolution of Prolonged Growth Exemplified by Human Brain Evolution.” Journal of Theoretical Biology 190 (3): 227–239.
Vrba, Elisabeth S. 2000. “Major Features of Neogene Mammalian Evolution in Africa.” In Cenozoic Geology of Southern Africa, edited by T. C. Partridge and R. Maud, 277–304. Oxford: Oxford University Press.
Walker, Alan C., and Richard E. Leakey. 1988. “The Evolution of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 247–258. New York: Aldine de Gruyter.
Walker, Alan, Richard E. Leakey, John M. Harris, and Francis H. Brown. 1986. “2.5-my Australopithecus boisei from West of Lake Turkana, Kenya.” Nature 322 (6079): 517–522.
Ward, Carol, Meave Leakey, and Alan Walker. 1999. “The New Hominid Species Australopithecus anamensis.” Evolutionary Anthropology 7 (6): 197–205.
White, Tim D. 1988. “The Comparative Biology of ‘Robust’ Australopithecus: Clues from Content.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 449–483. New York: Aldine de Gruyter.
White, Tim D., Gen Suwa, and Berhane Asfaw. 1994. “Australopithecus ramidus, a New Species of Early Hominid from Aramis, Ethiopia.” Nature 371 (6495): 306–312.
Wood, Bernard. 2010. “Reconstructing Human Evolution: Achievements, Challenges, and Opportunities.” Proceedings of the National Academy of Sciences 10 (2): 8902–8909.
Wood, Bernard, and Eve K. Boyle. 2016. “Hominin Taxic Diversity: Fact or Fantasy?” Yearbook of Physical Anthropology 159 (S61): 37–78.
Wood, Bernard, and Kes Schroer. 2017. “Paranthropus: Where Do Things Stand?” In Human Paleontology and Prehistory, edited by A. Marom and E. Hovers, 95–107. New York: Springer, Cham.
Acknowledgements
All of the authors in this section are students and early career researchers in paleoanthropology and related fields in South Africa (or at least have worked in South Africa). We wish to thank everyone who supports young and diverse talent in this field and would love to further acknowledge Black, African, and female academics who have helped pave the way for us.

