5 Forces of Evolution
Andrea J. Alveshere, Ph.D., Western Illinois University
Student contributors for this chapter: Corin Laberge, Hazel Moorcroft, Isabella Michel, Julian J. Pantoja Quiroz
This chapter is a revision from “Chapter 4: Forces of Evolution” by Andrea J. Alveshere. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Outline a 21st-century perspective of the Modern Synthesis.
- Define populations and population genetics as well as the methods used to study them.
- Identify the forces of evolution and become familiar with examples of each.
- Discuss the evolutionary significance of mutation, genetic drift, gene flow, and natural selection.
- Explain how allele frequencies can be used to study evolution as it happens.
- Contrast micro- and macroevolution.
It’s hard for us, with our typical human life spans of less than 100 years, to imagine all the way back, 3.8 billion years ago, to the origins of life. Scientists still study and debate how life came into being and whether it originated on Earth or in some other region of the universe (including some scientists who believe that studying evolution can reveal the complex processes that were set in motion by God or a higher power). What we do know is that a living single-celled organism was present on Earth during the early stages of our planet’s existence. This organism had the potential to reproduce by making copies of itself, just like bacteria, many amoebae, and our own living cells today. In fact, with modern technologies, we can now trace genetic lineages, or phylogenies, and determine the relationships between all of today’s living organisms—eukaryotes (animals, plants, fungi, etc.), archaea, and bacteria—on the branches of the phylogenetic tree of life (Figure 4.1).
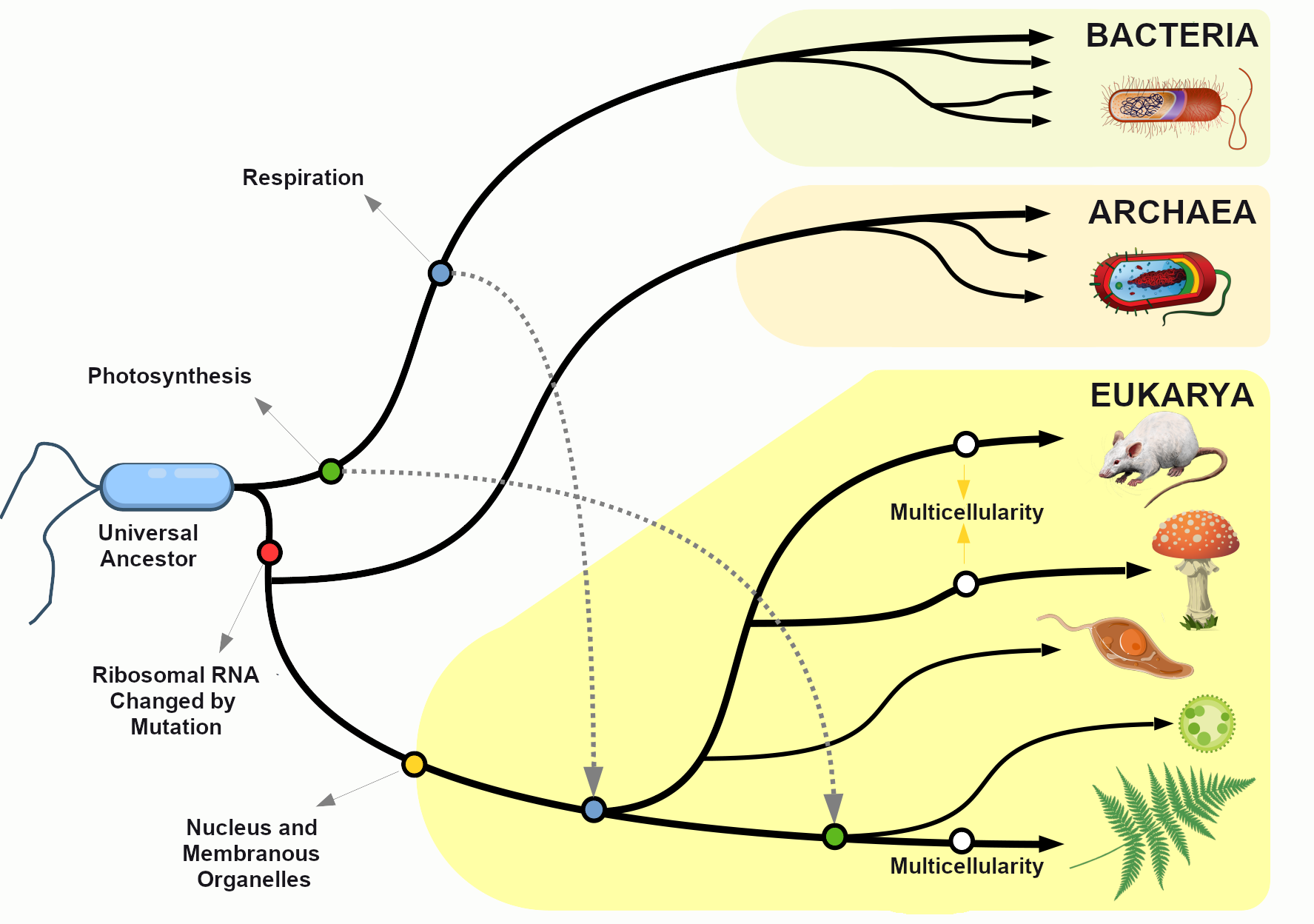
Looking at the common sequences in modern genomes, we can even make educated guesses about the likely genetic sequence of the Last Universal Common Ancestor (LUCA) of all living things. Through a wondrous series of mechanisms and events over nearly four billion years, that ancient single-celled organism gave rise to the rich diversity of species that fill the lands, seas, and skies of our planet. This chapter explores the mechanisms by which that amazing transformation occurred and considers some of the crucial scientific experiments that shaped our current understanding of the evolutionary process.
Population Genetics
Defining Populations and the Variations within Them
One of the major breakthroughs in understanding the mechanisms of evolutionary change came with the realization that evolution takes place at the level of populations, not within individuals. In the biological sciences, a population is defined as a group of individuals of the same species who are geographically near enough to one another that they can breed and produce new generations of individuals.
For the purpose of studying evolution, we recognize populations by their even smaller units: genes. Remember, a gene is the basic unit of information that encodes the proteins needed to grow and function as a living organism. Each gene can have multiple alleles, or variants—each of which may produce a slightly different protein. Each individual, for genetic inheritance purposes, carries a collection of genes that can be passed down to future generations. For this reason, in population genetics, we think of populations as gene pools, which refers to the entire collection of genetic material in a breeding community that can be passed on from one generation to the next.
For genes carried on our human chromosomes (our nuclear DNA), we inherit two copies of each, one from each parent. This means we may carry two of the same alleles (a homozygous genotype) or two different alleles (a heterozygous genotype) for each nuclear gene.
Defining Evolution
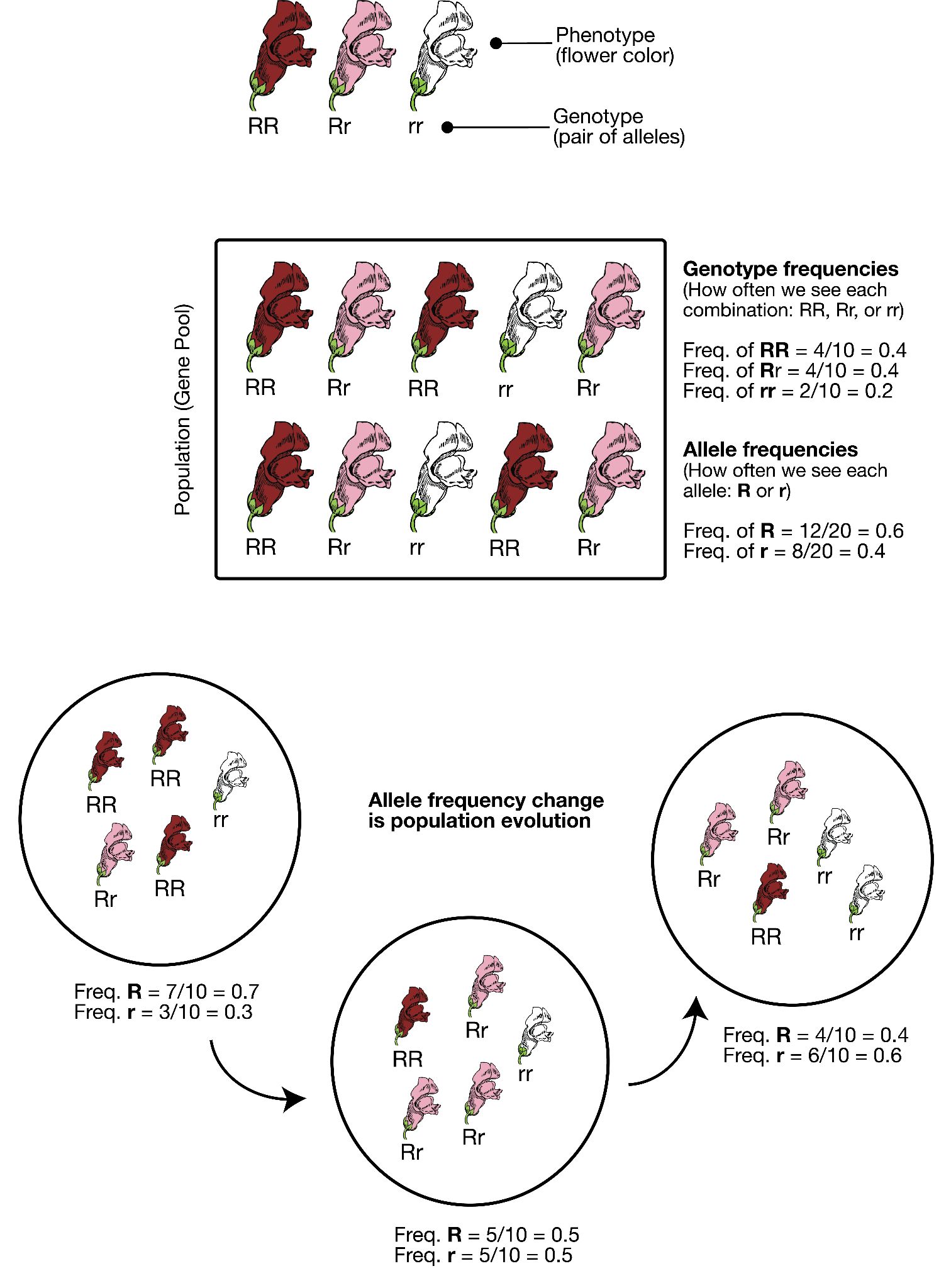
In order to understand evolution, it’s crucial to remember that evolution is always studied at the population level. Also, if a population were to stay exactly the same from one generation to the next, it would not be evolving. So evolution requires both a population of breeding individuals and some kind of a genetic change occurring within it. Thus, the simple definition of evolution is a change in the allele frequencies in a population over time. What do we mean by allele frequencies? Allele frequencies refer to the ratio, or percentage, of one allele (one variant of a gene) compared to the other alleles for that gene within the study population (Figure 4.2). By contrast, genotype frequencies are the ratios or percentages of the different homozygous and heterozygous genotypes in the population. Because we carry two alleles per genotype, the total count of alleles in a population will usually be exactly double the total count of genotypes in the same population (with the exception being rare cases in which an individual carries a different number of chromosomes than the typical two; e.g., Down syndrome results when a child carries three copies of Chromosome 21).
The Forces of Evolution
Today, we recognize that evolution takes place through a combination of mechanisms: mutation, genetic drift, gene flow, and natural selection. These mechanisms are called the “forces of evolution”; together they account for all the genotypic variation observed in the world today. Keep in mind that each of these forces was first defined and then tested—and retested—through the experimental work of the many scientists who contributed to the Modern Synthesis.
Mutation
The first force of evolution we will discuss is mutation, and for good reason: mutation is the original source of all the genetic variation found in every living thing. Imagine all the way back in time to the very first single-celled organism, floating in Earth’s primordial sea. Based on what we observe in simple, single-celled organisms today, that organism probably spent its lifetime absorbing nutrients and dividing to produce cloned copies of itself. While the numbers of individuals in that population would have grown (as long as the environment was favorable), nothing would have changed in that perfectly cloned population. There would not have been variety among the individuals. It was only through a copying error—the introduction of a mutation, or change, into the genetic code—that new alleles were introduced into the population.
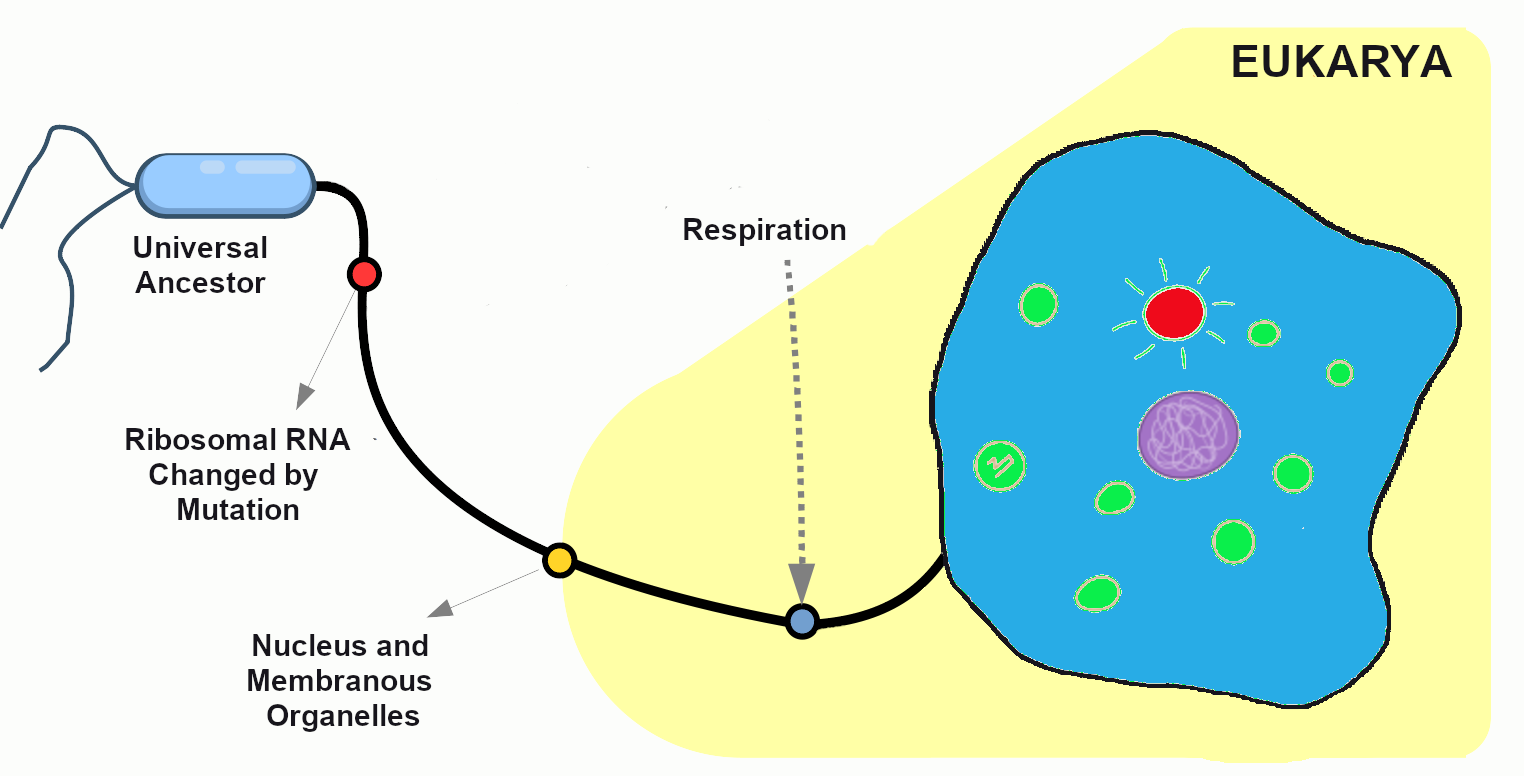
After many generations have passed in our primordial population, mutations have created distinct chromosomes. The cells are now amoeba-like, larger than many of their tiny bacterial neighbors, who have long since become their favorite source of nutrients. Without mutation to create this diversity, all living things would still be identical to LUCA, our universal ancestor (Figure 4.3).
When we think of genetic mutation, we often first think of deleterious mutations—the ones associated with negative effects such as the beginnings of cancers or heritable disorders. The fact is, though, that every genetic adaptation that has helped our ancestors survive since the dawn of life is directly due to beneficial mutations—changes in the DNA that provided some sort of advantage to a given population at a particular moment in time. For example, a beneficial mutation allowed chihuahuas and other tropical-adapted dog breeds to have much thinner fur coats than their cold-adapted cousins the northern wolves, malamutes, and huskies.
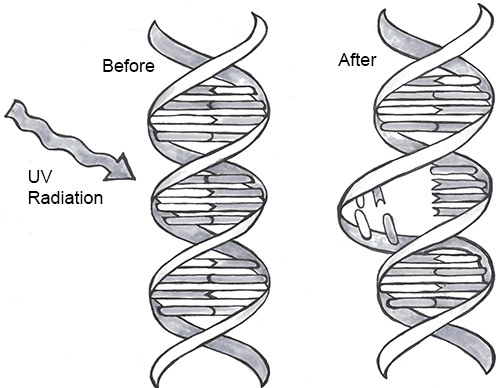
Every one of us has genetic mutations. Yes, even you. The DNA in some of your cells today differs from the original DNA that you inherited when you were a tiny, fertilized egg. Mutations occur all the time in the cells of our skin and other organs, due to chemical changes in the nucleotides. Exposure to the UV radiation in sunlight is one common cause of skin mutations. Interaction with UV light causes UV crosslinking, in which adjacent thymine bases bind with one another (Figure 4.4). Many of these mutations are detected and corrected by DNA repair mechanisms, enzymes that patrol and repair DNA in living cells, while other mutations may cause a new freckle or mole or, perhaps, an unusual hair to grow. For people with the autosomal recessive disease xeroderma pigmentosum, these repair mechanisms do not function correctly, resulting in a host of problems especially related to sun exposure, including severe sunburns, dry skin, heavy freckling, and other pigment changes.
Most of our mutations exist in somatic cells, which are the cells of our organs and other body tissues. Those will not be passed onto future generations and so will not affect the population over time. Only mutations that occur in the gametes, the reproductive cells (i.e., the sperm or egg cells), will be passed onto future generations. When a new mutation pops up at random in a family lineage, it is known as a spontaneous mutation. If the individual born with this spontaneous mutation passes it on to his offspring, those offspring receive an inherited mutation. Geneticists have identified many classes of mutations and the causes and effects of many of these.
Point Mutations
A point mutation is a single-letter (single-nucleotide) change in the genetic code resulting in the substitution of one nucleic acid base for a different one. As you learned in Chapter 3, the DNA code in each gene is translated through three-letter “words” known as codons. So depending on how the point mutation changes the “word,” the effect it will have on the protein may be major or minor or may make no difference at all.
If a mutation does not change the resulting protein, then it is called a synonymous mutation. Synonymous mutations do involve a letter (nucleic acid) change, but that change results in a codon that codes for the same “instruction” (the same amino acid or stop code) as the original codon. Mutations that do cause a change in the protein are known as nonsynonymous mutations. Nonsynonymous mutations may change the resulting protein’s amino acid sequence by altering the DNA sequence that encodes the mRNA or by changing how the mRNA is spliced prior to translation (refer to Chapter 3 for more details).
Insertions and Deletions
In addition to point mutations, another class of mutations are insertions and deletions, or indels, for short. As the name suggests, these involve the addition (insertion) or removal (deletion) of one or more coding sequence letters (nucleic acids). These typically first occur as an error in DNA replication, wherein one or more nucleotides are either duplicated or skipped in error. Entire codons or sets of codons may also be removed or added if the indel is a multiple of three nucleotides.
Frameshift mutations are types of indels that involve the insertion or deletion of any number of nucleotides that is not a multiple of three (e.g., adding one or two extra letters to the code). Because these indels are not consistent with the codon numbering, they “shift the reading frame,” causing all the codons beyond the mutation to be misread. Like point mutations, small indels can also disrupt splice sites.
Transposable elements, or transposons, are fragments of DNA that can “jump” around in the genome. There are two types of transposons: retrotransposons are transcribed from DNA into RNA and then “reverse transcribed,” to insert the copied sequence into a new location in the DNA, and DNA transposons, which do not involve RNA. DNA transposons are clipped out of the DNA sequence itself and inserted elsewhere in the genome. Because transposable elements insert themselves into existing DNA sequences, they are frequent gene disruptors. At certain times, and in certain species, it appears that transposons became very active, likely accelerating the mutation rate (and thus, the genetic variation) in those populations during the active periods.
Chromosomal Alterations
The final major category of genetic mutations are changes at the chromosome level: crossover events, nondisjunction events, and translocations. Crossover events occur when DNA is swapped between homologous chromosomes while they are paired up during meiosis I. Crossovers are thought to be so common that some DNA swapping may happen every time chromosomes go through meiosis I. Crossovers don’t necessarily introduce new alleles into a population, but they do make it possible for new combinations of alleles to exist on a single chromosome that can be passed to future generations. This also enables new combinations of alleles to be found within siblings who share the same parents. Also, if the fragments that cross over don’t break at exactly the same point, they can cause genes to be deleted from one of the homologous chromosomes and duplicated on the other.
Nondisjunction events occur when the homologous chromosomes (in meiosis I) or sister chromatids (in meiosis II and mitosis) fail to separate after pairing. The result is that both chromosomes or chromatids end up in the same daughter cell, leaving the other daughter cell without any copy of that chromosome (Figure 4.5). Most nondisjunctions at the gamete level are fatal to the embryo. The most widely known exception is Trisomy 21, or Down syndrome, which results when an embryo inherits three copies of Chromosome 21: two from one parent (due to a nondisjunction event) and one from the other (Figure 4.6). Trisomies (triple chromosome conditions) of Chromosomes 18 (Edwards syndrome) and 13 (Patau syndrome) are also known to result in live births, but the children usually have severe complications and rarely survive beyond the first year of life.
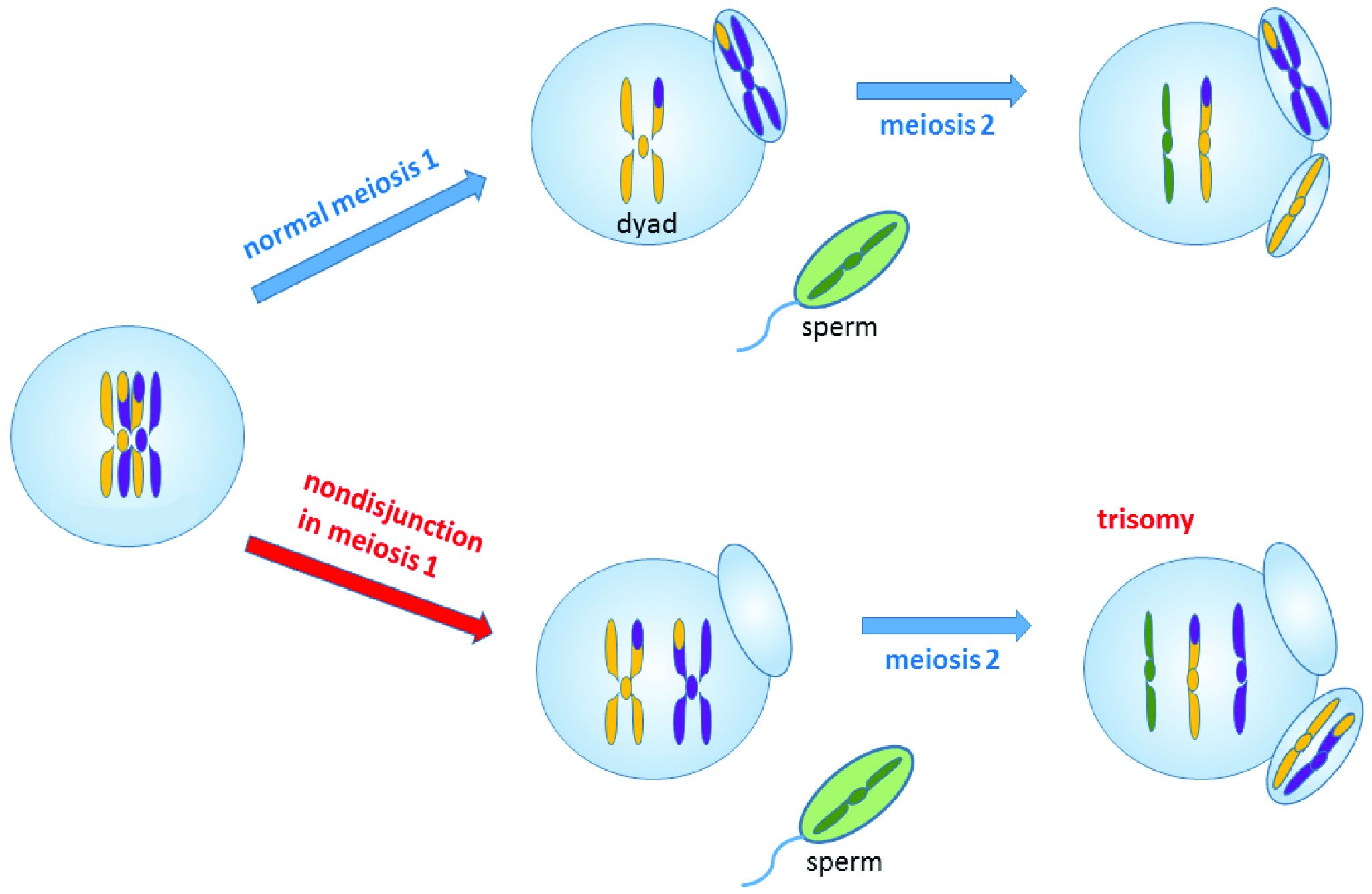

Sex chromosome trisomies (XXX, XXY, XYY) and X chromosome monosomies (inheritance of an X chromosome from one parent and no sex chromosome from the other) are also survivable and fairly common. The symptoms vary but often include atypical sexual characteristics, either at birth or at puberty, and often result in sterility. The X chromosome carries unique genes that are required for survival; therefore, Y chromosome monosomies are incompatible with life.
Chromosomal translocations involve transfers of DNA between nonhomologous chromosomes. This may involve swapping large portions of two or more chromosomes. The exchanges of DNA may be balanced or unbalanced. In balanced translocations, the genes are swapped, but no genetic information is lost. In unbalanced translocations, there is an unequal exchange of genetic material, resulting in duplication or loss of genes. Translocations result in new chromosomal structures called derivative chromosomes, because they are derived or created from two different chromosomes. Translocations are often found to be linked to cancers and can also cause infertility. Even if the translocations are balanced in the parent, the embryo often won’t survive unless the baby inherits both of that parent’s derivative chromosomes (to maintain the balance).
Genetic Drift
The second force of evolution is commonly known as genetic drift. This is an unfortunate misnomer, as this force actually involves the drifting of alleles, not genes. Genetic drift refers to random changes (“drift”) in allele frequencies from one generation to the next. The genes are remaining constant within the population; it is only the alleles of the genes that are changing in frequency. The random nature of genetic drift is a crucial point to understand: it specifically occurs when none of the variant alleles confer an advantage.
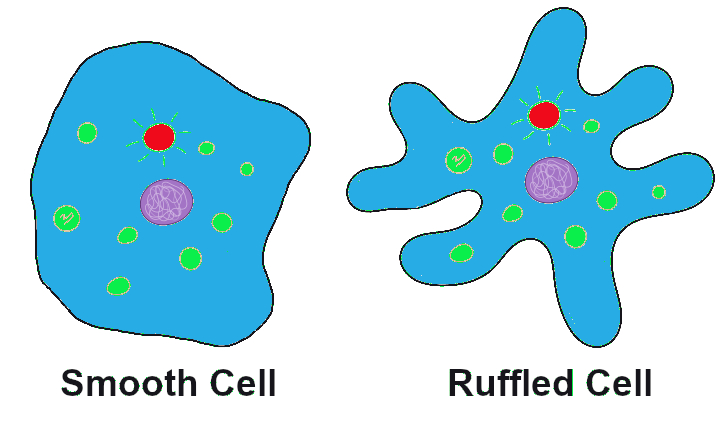
Let’s imagine far back in time, again, to that ancient population of amoeba-like cells, subsisting and occasionally dividing, in the primordial sea. A mutation occurs in one of the cells that changes the texture of the cell membrane from a relatively smooth surface to a highly ruffled one (Figure 4.7). This has absolutely no effect on the cell’s quality of life or ability to reproduce. In fact, eyes haven’t evolved yet, so no one in the world at the time would even notice the difference. The cells in the population continue to divide, and the offspring of the ruffled cell inherit the ruffled membrane. The frequency (percentage) of the ruffled allele in the population, from one generation to the next, will depend entirely on how many offspring that first ruffled cell ends up having, and the random events that might make the ruffled alleles more common or more rare (such as population bottlenecks and founder effects, which are discussed below).
Sexual Reproduction and Random Inheritance
Tracking alleles gets a bit more complicated in our primordial cells when, after a number of generations, a series of mutations have created populations that reproduce sexually. These cells now must go through an extra round of cell division (meiosis) to create haploid gametes. The combination of two gametes is now required to produce each new diploid offspring.
In the earlier population, which reproduced via asexual reproduction, a cell either carried the smooth allele or the ruffled allele. With sexual reproduction, a cell inherits one allele from each parent, so there are homozygous cells that contain two smooth alleles, homozygous cells that contain two ruffled alleles, and heterozygous cells that contain one of each allele (Figure 4.8). If the new, ruffled allele happens to be dominant (and we’ll imagine that it is), the heterozygotes will have ruffled cell phenotypes but also will have a 50/50 chance of passing on a smooth allele to each offspring. As long as neither phenotype (ruffled nor smooth) provides any advantage over the other, the variation in the population from one generation to the next will remain completely random.
In sexually reproducing populations (including humans and many other animals and plants in the world today), that 50/50 chance of inheriting one or the other allele from each parent plays a major role in the random nature of genetic drift.
Population Bottlenecks
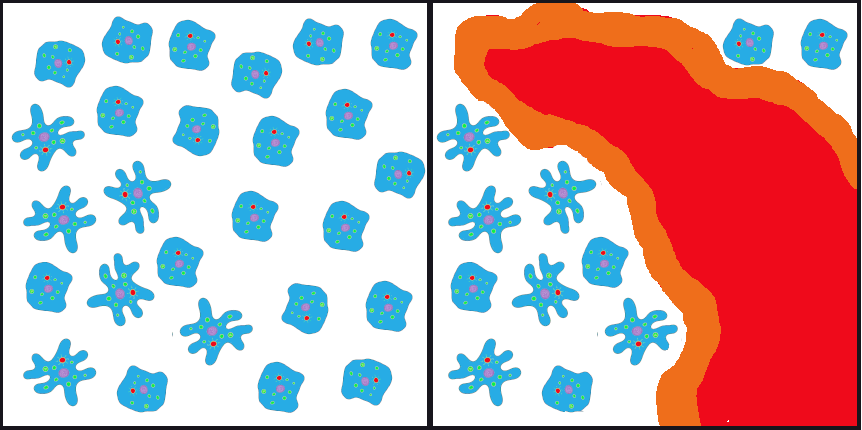
A population bottleneck occurs when the number of individuals in a population drops dramatically due to some random event. The most obvious, familiar examples are natural disasters. Tsunamis and hurricanes devastating island and coastal populations and forest fires and river floods wiping out populations in other areas are all too familiar. When a large portion of a population is randomly wiped out, the allele frequencies (i.e., the percentages of each allele) in the small population of survivors are often much different from the frequencies in the predisaster, or “parent,” population.
If such an event happened to our primordial ocean cell population—perhaps a volcanic fissure erupted in the ocean floor and only the cells that happened to be farthest from the spewing lava and boiling water survived—we might end up, by random chance, with a surviving population that had mostly ruffled alleles, in contrast to the parent population, which had only a small percentage of ruffles (Figure 4.9).
One of the most famous examples of a population bottleneck is the prehistoric disaster that led to the extinction of dinosaurs, the Cretaceous–Paleogene extinction event (often abbreviated K–Pg; previously K-T). This occurred approximately 66 million years ago. Dinosaurs and all their neighbors were going about their ordinary routines when a massive asteroid zoomed in from space and crashed into what is now the Gulf of Mexico, creating an impact so enormous that populations within hundreds of miles of the crash site were likely immediately wiped out. The skies filled with dust and debris, causing temperatures to plummet worldwide. It’s estimated that 75% of the world’s species went extinct as a result of the impact and the deep freeze that followed (Jablonski and Chaloner 1994).
The populations that emerged from the K-Pg extinction were markedly different from their pre-disaster communities. Surviving mammal populations expanded and diversified, and other new creatures appeared. The ecosystems of Earth were filled with new organisms and have never been the same (Figure 4.10).
Much more recently in geological time, during the colonial period, many human populations experienced bottlenecks as a result of the fact that imperial powers were inclined to slaughter communities who were reluctant to give up their lands and resources. This effect was especially profound in the Americas, where Indigenous populations faced the compounded effects of brutal warfare, exposure to new bacteria and viruses (against which they had no immunity), and ultimately segregation on resource-starved reservations. The populations in Europe, Asia, and Africa had experienced regular gene flow during the 10,000-year period in which most kinds of livestock were being domesticated, giving them many generations of experience building up immunity against zoonotic diseases (those that can pass from animals to humans). In contrast, the residents of the Americas had been almost completely isolated during those millennia, so all these diseases swept through the Americas in rapid succession, creating a major loss of genetic diversity in the Indigenous American population. It is estimated that between 50% and 95% of the Indigenous American populations died during the first decades after European contact, around 500 years ago (Livi-Bacci 2006).
An urgent health challenge facing humans today involves human-induced population bottlenecks that produce antibiotic-resistant bacteria. Antibiotics are medicines prescribed to treat bacterial infections. The typical prescription includes enough medicine for ten days. People often feel better much sooner than ten days and sometimes decide to quit taking the medicine ahead of schedule. This is often a big mistake. The antibiotics have quickly killed off a large percentage of the bacteria—enough to reduce the symptoms and make you feel much better. However, this has created a bacterial population bottleneck. There are usually a small number of bacteria that survive those early days. If you take the medicine as prescribed for the full ten days, it’s quite likely that there will be no bacterial survivors. If you quit early, though, the survivors—who were the members of the original population who were most resistant to the antibiotic—will begin to reproduce again. Soon the infection will be back, possibly worse than before, and now all of the bacteria are resistant to the antibiotic that you had been prescribed.
Other activities that have contributed to the rise of antibiotic-resistant bacteria include the use of antibacterial cleaning products and the inappropriate use of antibiotics as a preventative measure in livestock or to treat infections that are viral instead of bacterial (viruses do not respond to antibiotics). In 2017, the World Health Organization published a list of twelve antibiotic-resistant pathogens that are considered top priority targets for the development of new antibiotics (World Health Organization 2017).
Dig Deeper: The North American Elephant Seal: Thriving Bottleneck Populations That Still Face Genetic Defects
In 1892, the Northern Elephant Seal underwent a severe population bottleneck caused by commercial hunting, reducing the species to an estimated 20 individuals at the time. This drastic decline led to a substantial loss of genetic diversity–a common consequence of extreme population bottlenecks (Hoelzel et al., 2024 & Weber et al., 2000). While the population has since recovered to over 200,000 individuals, its genetic variability remains significantly low. Analyses of genetic markers, including allozymes, mitochondrial DNA, and microsatellites, consistently reflect this reduced diversity (Hoelzel et al., 2024). Comparative studies further underscore this loss by highlighting the higher genetic variation observed in the Southern Elephant Seal, which did not experience similar population constraints (2024).

In a 2024 study for Nature, Ecology, and Evolution, Hoelzel and colleagues sequenced 260 modern and 8 historical genomes of the northern elephant seal. This comparison revealed a decrease in average heterozygosity from 0.00142 before the bottleneck to 0.000176 in the contemporary population, confirming the decline in genetic variation (2024). Hoelzel’s mitogenome tree further illustrates this loss, revealing only two significant lineages remaining post-bottleneck, with limited diversity within each. Among the issues of diversity, the population has shown an increased number of loss-of-function (LOF) alleles, suggesting that increased inbreeding has amplified the frequency of these detrimental alleles; this reduced genetic diversity negatively affects both male and female reproductive fitness. Females who practiced repetitive inbreeding had higher LOF alleles and subsequently weaned fewer pups per year over their lifetime, while male reproductive success was linked to specific LOF loci associated with sperm production (2024). Hoelzel uses the example of “Alpha-Male M12”–known for low paternity success despite frequent copulations–which was homozygous for non-functional versions of four out of five LOF loci related to sperm function (2024, p. 688). The species’ mating system, characterized by extreme polygyny, further exacerbates the loss of genetic variation even with countless copulatory partners
Prior research published in Current Biology presents an empirical genetic assessment of this population bottleneck, highlighting its long-term genetic consequences, particularly the loss of mitochondrial diversity (Weber et al., 2000). In this research, Weber and colleagues note that random lineage sampling during the bottleneck led to the persistence of specific genetic variants by chance rather than through natural selection (2000). This research emphasizes that the loss of diversity poses potential future genetic vulnerabilities for the seals, and that further studies are crucial for understanding the full scope of these impacts on the seals’ overall fitness (2000). In 2024, the work led by Hoelzen and company provided the missing data that the previous study had left unanswered. Their previously explored findings indicate that, although the seals have recovered in numbers, their genetic resilience remains compromised, leaving the population more vulnerable to future environmental pressures, such as climate change or resource shortages (Hoelzel et al., 2024). Ultimately, while the population’s size remains stable, the genetic consequences of the bottleneck indicate that past stochastic events continue to influence the seals’ long-term fitness and adaptability.
This research indicates that the historical bottleneck continues to affect the seals’ health and fitness, despite the population’s recovery. Limited genetic diversity and the persistence of harmful alleles due to inbreeding have continued to handicap the species’ ability to thrive in environmental challenges such as climate change and resource fluctuations (2024). This emphasizes the importance of incorporating genetic factors into conservation strategies, as populations that have rebounded may still harbour long-term genetic weaknesses. Moreover, the elephant seal’s history serves as a powerful example of how human actions —such as overhunting — can have long-lasting impacts on biodiversity, reinforcing the importance of understanding human-environment interactions in ecological and conservation contexts.
Founder Effects
Founder effects occur when members of a population leave the main or “parent” group and form a new population that no longer interbreeds with the other members of the original group. Similar to survivors of a population bottleneck, the newly founded population often has allele frequencies that are different from the original group. Alleles that may have been relatively rare in the parent population can end up being very common due to the founder effect. Likewise, recessive traits that were seldom seen in the parent population may be seen frequently in the descendants of the offshoot population.
One striking example of the founder effect was first noted in the Dominican Republic in the 1970s. During a several-year period, eighteen children who had been born with female genitalia and raised as girls suddenly grew penises at puberty. This culture tended to value sons over daughters, so these transitions were generally celebrated. They labeled the condition guevedoces, which translates to “penis at twelve,” due to the average age at which this occurred. Scientists were fascinated by the phenomenon.
Genetic and hormonal studies revealed that the condition, scientifically termed 5-alpha reductase deficiency, is an autosomal recessive syndrome that manifests when a child having both X and Y sex chromosomes inherits two nonfunctional (mutated) copies of the SRD5A2 gene (Imperato-McGinley and Zhu 2002). These children develop testes internally, but the 5-alpha reductase 2 steroid, which is necessary for development of male genitals in babies, is not produced. In absence of this male hormone, the baby develops female-looking genitalia (in humans, “female” is the default infant body form, if the full set of the necessary male hormones are not produced). At puberty, however, a different set of male hormones are produced by other fully functional genes. These hormones complete the male genital development that did not happen in infancy. This condition became quite common in the Dominican Republic during the 1970s due to founder effect—that is, the mutated SRD5A2 gene happened to be much more common among the Dominican Republic’s founding population than in the parent populations. (The Dominican population derives from a mixture of Indigenous Americans [Taino] peoples, West Africans, and Western Europeans.) Five-alpha reductase syndrome has since been observed in other small, isolated populations around the world.
Founder effect is closely linked to the concept of inbreeding, which in population genetics does not necessarily mean breeding with immediate family relatives. Instead, inbreeding refers to the selection of mates exclusively from within a small, closed population—that is, from a group with limited allelic variability. This can be observed in small, physically isolated populations but also can happen when cultural practices limit mates to a small group. As with the founder effect, inbreeding increases the risk of inheriting two copies of any nonfunctional (mutant) alleles.
The Amish in the United States are a population that, due to their unique history and cultural practices, emerged from a small founding population and have tended to select mates from within their groups. The Old Order Amish population of Lancaster County, Pennsylvania, has approximately 50,000 current members, all of whom can trace their ancestry back to a group of approximately 80 individuals. This small founding population immigrated to the United States from Switzerland in the mid-1700s to escape religious persecution. Since the Amish keep to themselves and almost exclusively select mates from within their own communities, they have more recessive traits compared to their parent population.

One of the genetic conditions that has been observed much more frequently in the Lancaster County Amish population is Ellis-van Creveld syndrome, which is an autosomal recessive disorder characterized by short stature (dwarfism), polydactyly (the development of more than five digits [fingers or toes] on the hands or feet], abnormal tooth development, and heart defects (Figure 4.11). Among the general world population, Ellis-van Creveld syndrome is estimated to affect approximately 1 in 60,000 individuals; among the Old Order Amish of Lancaster County, the rate is estimated to be as high as 1 in every 200 births (D’Asdia et al. 2013).
One important insight that has come from the study of founder effects is that a limited gene pool carries a much higher risk for genetic diseases. Genetic diversity in a population greatly reduces these risks.
Gene Flow
The third force of evolution is traditionally called gene flow. As with genetic drift, this is a misnomer, because it refers to flowing alleles, not genes. (All members of the same species share the same genes; it is the alleles of those genes that may vary.) Gene flow refers to the movement of alleles from one population to another. In most cases, gene flow can be considered synonymous with migration.
Returning again to the example of our primordial cell population, let’s imagine that, after the volcanic fissure opened up in the ocean floor, wiping out the majority of the parent population, two surviving populations developed in the waters on opposite sides of the fissure. Ultimately, the lava from the fissure cooled into a large island that continued to provide a physical barrier between the populations (Figure 4.12).
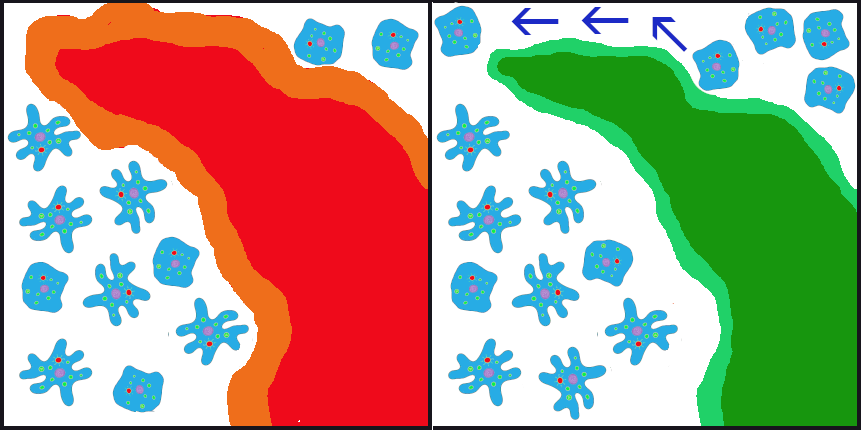
In the initial generations after the eruption, due to founder effect, isolation, and random inheritance (genetic drift), the population to the west of the islands contained a vast majority of the ruffled membrane alleles while the eastern population carried only the smooth alleles. Ocean currents in the area typically flowed from east to west, sometimes carrying cells (facilitating gene flow) from the eastern (smooth) population to the western (ruffled) population. Due to the ocean currents, it was almost impossible for any cells from the western population to be carried eastward. Thus, for inheritance purposes, the eastern (smooth) population remained isolated. In this case, the gene flow is unidirectional (going only in one direction) and unbalanced (only one population is receiving the new alleles).
Among humans, gene flow is often described as admixture. In forensic cases, anthropologists and geneticists are often asked to estimate the ancestry of unidentified human remains to help determine whether they match any missing persons’ reports. This is one of the most complicated tasks in these professions because, while “race” or “ancestry” involves simple checkboxes on a missing person’s form, among humans today there are no truly distinct genetic populations. All modern humans are members of the same fully breeding compatible species, and all human communities have experienced multiple episodes of gene flow (admixture), leading all humans today to be so genetically similar that we are all members of the same (and only surviving) human subspecies: Homo sapiens sapiens.
Gene flow between otherwise isolated nonhuman populations is often termed hybridization.. One example of this involves the hybridization and spread of Scutellata honey bees (a.k.a. “killer bees”) in the Americas. All honey bees worldwide are classified as Apis mellifera. Due to distinct adaptations to various environments around the world, there are 28 different subspecies of Apis mellifera.
During the 1950s, a Brazilian biologist named Warwick E. Kerr experimented with hybridizing African and European subspecies of honey bees to try to develop a strain that was better suited to tropical environments than the European honey bees that had long been kept by North American beekeepers. Dr. Kerr was careful to contain the reproductive queens and drones from the African subspecies, but in 1957, a visiting beekeeper accidentally released 26 queen bees of the Scutellata subspecies (Apis mellifera scutellata) from southern Africa into the Brazilian countryside. The Scutellata bees quickly interbred with local European honey bee populations. The hybridized bees exhibited a much more aggressively defensive behavior, fatally or near-fatally attacking many humans and livestock that ventured too close to their hives. The hybridized bees spread throughout South America and reached Mexico and California by 1985. By 1990, permanent colonies had been established in Texas, and by 1997, 90% of trapped bee swarms around Tucson, Arizona, were found to be Scutellata hybrids (Sanford 2006).
Another example involves the introduction of the Harlequin ladybeetle, Harmonia axyridis, native to East Asia, to other parts of the world as a “natural” form of pest control. Harlequin ladybeetles are natural predators of some of the aphids and other crop-pest insects. First introduced to North America in 1916, the “biocontrol” strains of Harlequin ladybeetles were considered to be quite successful in reducing crop pests and saving farmers substantial amounts of money. After many decades of successful use in North America, biocontrol strains of Harlequin ladybeetles were also developed in Europe and South America in the 1980s.
Over the seven decades of biocontrol use, the Harlequin ladybeetle had never shown any potential for development of wild colonies outside of its native habitat in China and Japan. New generations of beetles always had to be reared in the lab. That all changed in 1988, when a wild colony took root near New Orleans, Louisiana. Either through admixture with a native ladybeetle strain, or due to a spontaneous mutation, a new allele was clearly introduced into this population that suddenly enabled them to survive and reproduce in a wide range of environments. This population spread rapidly across the Americas and had reached Africa by 2004.
In Europe, the invasive, North American strain of Harlequin ladybeetle admixed with the European strain (Figure 4.13), causing a population explosion (Lombaert et al. 2010). Even strains specifically developed to be flightless (to curtail the spreading) produced flighted offspring after admixture with members of the North American population (Facon et al. 2011). The fast-spreading, invasive strain has quickly become a disaster, out-competing native ladybeetle populations (some to the point of extinction), causing home infestations, decimating fruit crops, and contaminating many batches of wine with their bitter flavor after being inadvertently harvested with the grapes (Pickering et al. 2004).
Natural Selection
The final force of evolution is natural selection. This is the evolutionary process that Charles Darwin first brought to light, and it is what the general public typically evokes when considering the process of evolution. Natural selection occurs when certain phenotypes confer an advantage or disadvantage in survival and/or reproductive success. The alleles associated with those phenotypes will change in frequency over time due to this selective pressure. It’s also important to note that the advantageous allele may change over time (with environmental changes) and that an allele that had previously been benign may become advantageous or detrimental. Of course, dominant, recessive, and codominant traits will be selected upon a bit differently from one another. Because natural selection acts on phenotypes rather than the alleles themselves, deleterious (disadvantageous) alleles can be retained by heterozygotes without any negative effects.
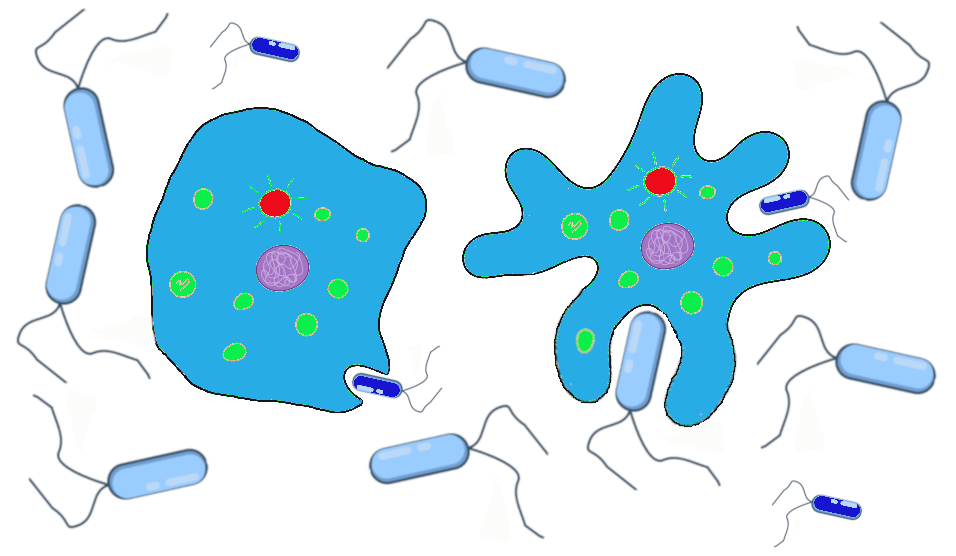
In the case of our primordial ocean cells, up until now, the texture of their cell membranes has been benign. The frequencies of smooth to ruffled alleles, and smooth to ruffled phenotypes, has changed over time, due to genetic drift and gene flow. Let’s now imagine that the Earth’s climate has cooled to a point that the waters frequently become too cold for survival of the tiny bacteria that are the dietary staples of our smooth and ruffled cell populations. The way amoeba-like cells “eat” is to stretch out the cell membrane, almost like an arm, to encapsulate, then ingest, the tiny bacteria. When the temperatures plummet, the tiny bacteria populations plummet with them. Larger bacteria, however, are better able to withstand the temperature change.
The smooth cells were well-adapted to ingesting tiny bacteria but poorly suited to encapsulating the larger bacteria. The cells with the ruffled membranes, however, are easily able to extend their ruffles to encapsulate the larger bacteria. They also find themselves able to stretch their entire membrane to a much larger size than their smooth-surfaced neighbors, allowing them to ingest more bacteria at a given time and to go for longer periods between feedings (Figure 4.14).
The smooth and ruffled traits, which had previously offered no advantage or disadvantage while food was plentiful, now are subject to natural selection. During the cold snaps, at least, the ruffled cells have a definite advantage. We can imagine that the western population that has mostly ruffled alleles will continue to do well, while the eastern population is at risk of dying out if the smaller bacteria remain scarce and no ruffled alleles are introduced.
A classic example of natural selection involves the study of an insect called the peppered moth (Biston betularia) in England during the Industrial Revolution in the 1800s. Prior to the Industrial Revolution, the peppered moth population was predominantly light in color, with dark (pepper-like) speckles on the wings. The “peppered” coloration was very similar to the appearance of the bark and lichens that grew on the local trees (Figure 4.15). This helped to camouflage the moths as they rested on a tree, making it harder for moth-eating birds to find and snack on them. There was another phenotype that popped up occasionally in the population. These individuals were heterozygotes that carried an overactive, dominant pigment allele, producing a solid black coloration. As you can imagine, the black moths were much easier for birds to spot, making this phenotype a real disadvantage.
The situation changed, however, as the Industrial Revolution took off. Large factories began spewing vast amounts of coal smoke into the air, blanketing the countryside, including the lichens and trees, in black soot. Suddenly, it was the light-colored moths that were easy for birds to spot and the black moths that held the advantage. The frequency of the dark pigment allele rose dramatically. By 1895, the black moth phenotype accounted for 98% of observed moths (Grant 1999).
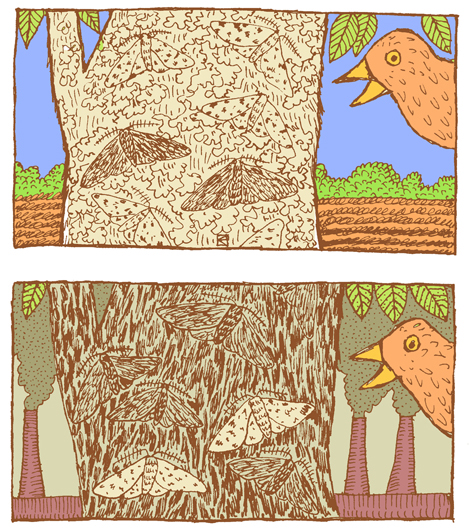
Thanks to new environmental regulations in the 1960s, the air pollution in England began to taper off. As the soot levels decreased, returning the trees to their former, lighter color, this provided the perfect opportunity to study how the peppered moth population would respond. Repeated follow-up studies documented the gradual rise in the frequency of the lighter-colored phenotype. By 2003, the maximum frequency of the dark phenotype was 50% and in most parts of England had decreased to less than 10% (Cook 2003).
Directional, Balancing/Stabilizing, and Disruptive/Diversifying Selection
Natural selection can be classified as directional, balancing/stabilizing, or disruptive/diversifying, depending on how the pressure is applied to the population (Figure 4.16).
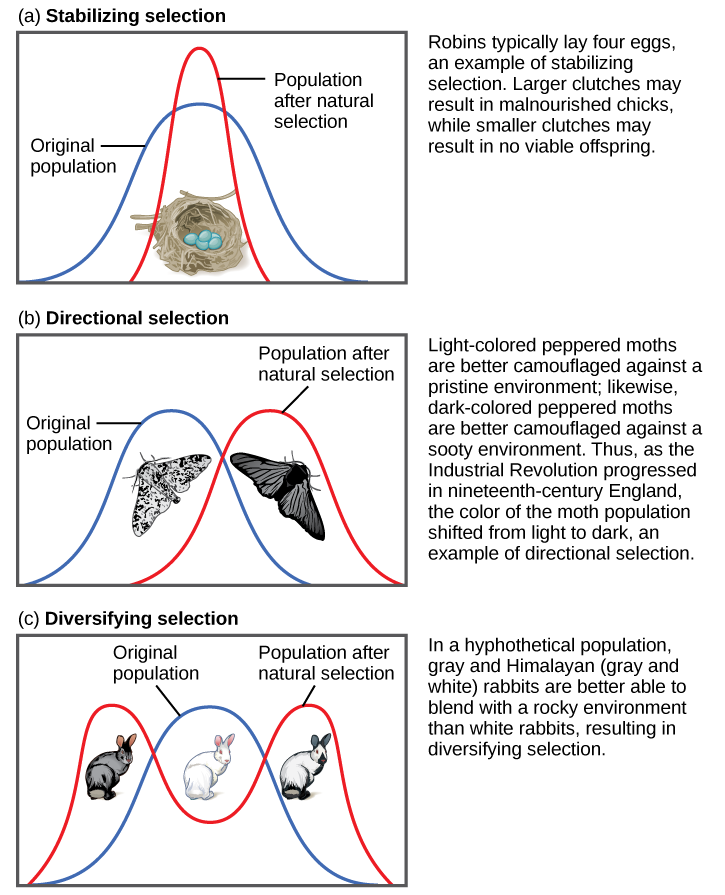
Both of the above examples of natural selection involve directional selection: the environmental pressures favor one phenotype over the other and cause the frequencies of the associated advantageous alleles (ruffled membranes, dark pigment) to gradually increase. In the case of the peppered moths, the direction shifted three times: first, it was selecting for lighter pigment; then, with the increase in pollution, the pressure switched to selection for darker pigment; finally, with reduction of the pollution, the selection pressure shifted back again to favoring light-colored moths.
Balancing selection (a.k.a. stabilizing selection) occurs when selection works against the extremes of a trait and favors the intermediate phenotype. For example, humans maintain an average birth weight that balances the need for babies to be small enough not to cause complications during pregnancy and childbirth but big enough to maintain a safe body temperature after they are born. Another example of balancing selection is found in the genetic disorder called sickle cell anemia (see “Special Topic: Sickle Cell Anemia”).
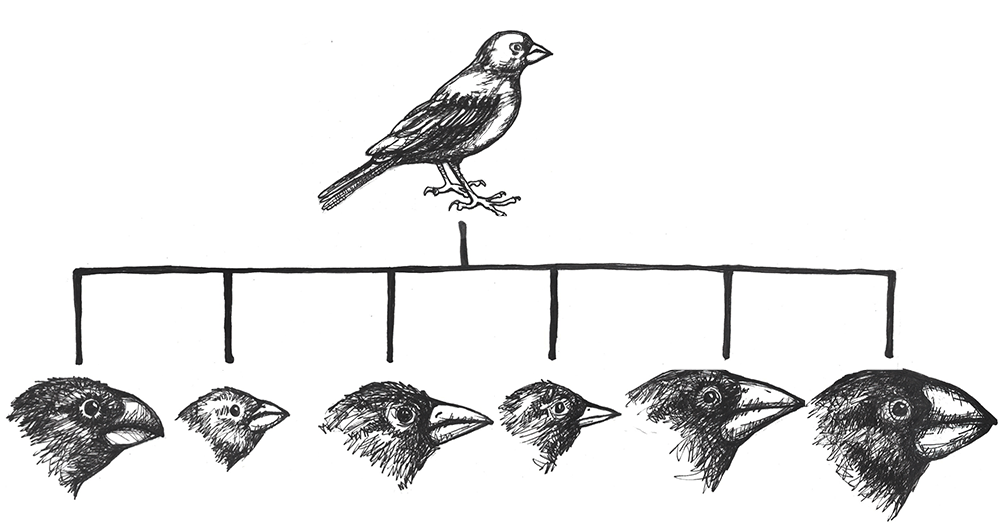
Disruptive selection (a.k.a. diversifying selection), the opposite of balancing selection, occurs when both extremes of a trait are advantageous. Since individuals with traits in the mid-range are selected against, disruptive selection can eventually lead to the population evolving into two separate species. Darwin believed that the many species of finches (small birds) found in the remote Galapagos Islands provided a clear example of disruptive selection leading to speciation. He observed that seed-eating finches either had large beaks, capable of eating very large seeds, or small beaks, capable of retrieving tiny seeds. The islands did not have many plants that produced medium-size seeds. Thus, birds with medium-size beaks would have trouble eating the very large seeds and would also have been inefficient at picking up the tiny seeds. Over time, Darwin surmised, this pressure against mid-size beaks may have led the population to divide into two separate species.
Sexual Selection
Sexual selection is an aspect of natural selection in which the selective pressure specifically affects reproductive success (the ability to successfully breed and raise offspring) rather than survival. Sexual selection favors traits that will attract a mate. Sometimes these sexually appealing traits even carry greater risks in terms of survival.
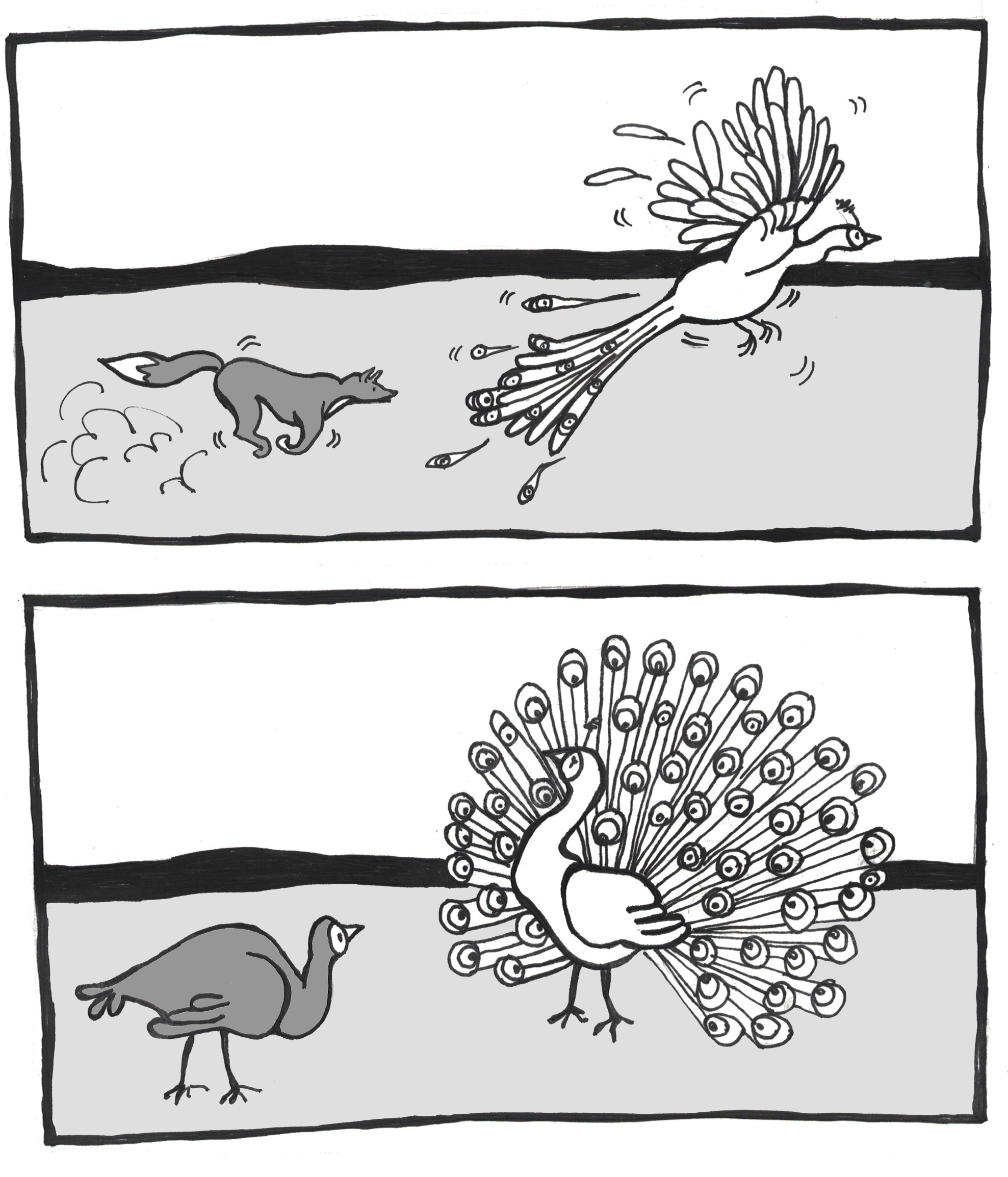
A classic example of sexual selection involves the brightly colored feathers of the peacock. The peacock is the male sex of the peafowl genera Pavo and Afropavo. During mating season, peacocks will fan their colorful tails wide and strut in front of the peahens in a grand display. The peahens will carefully observe these displays and will elect to mate with the male that they find the most appealing. Many studies have found that peahens prefer the males with the fullest, most colorful tails. While these large, showy tails provide a reproductive advantage, they can be a real burden in terms of escaping predators. The bright colors and patterns as well as the large size of the peacock tail make it difficult to hide. Once predators spot them, peacocks also struggle to fly away, with the heavy tail trailing behind and weighing them down (Figure 4.17). Some researchers have argued that the increased risk is part of the appeal for the peahens: only an especially strong, alert, and healthy peacock would be able to avoid predators while sporting such a spectacular tail.
It’s important to keep in mind that sexual selection relies on the trait being present throughout mating years. Reflecting on the NF1 genetic disorder (see “Special Topic: Neurofibromatosis Type 1 [NF1]”), given how disfiguring the symptoms can become, some might find it surprising that half of the babies born with NF1 inherited it from a parent. Given that the disorder is autosomal dominant and fully penetrant (meaning it has no unaffected carriers), it may seem surprising that sexual selection doesn’t exert more pressure against the mutated alleles. One important factor is that, while the neurofibromas typically begin to appear during puberty, they usually emerge only a few at a time and may grow very slowly. Many NF1 patients don’t experience the more severe or disfiguring symptoms until later in life, long after they have started families of their own.
Some researchers prefer to classify sexual selection separately, as a fifth force of evolution. The traits that underpin mate selection are entirely natural, of course. Research has shown that subtle traits, such as the type of pheromones (hormonal odors related to immune system alleles) someone emits and how those are perceived by the immune system genotype of the “sniffer,” may play crucial and subconscious roles in whether we find someone attractive or not (Chaix, Cao, and Donnelly 2008).
Special Topic: Neurofibromatosis Type 1 (NF1)
Neurofibromatosis Type 1, also known as NF1, is a genetic disorder that illustrates how a mutation in a single gene can affect multiple systems in the body. Surprisingly common, more people have NF1 than cystic fibrosis and muscular dystrophy combined. Even more surprising, given how common it is, is how few people have heard of it. One in every 3,000 babies is born with NF1, and this holds true for all populations worldwide (Riccardi 1992). This means that, for every 3,000 people in your community, there is likely at least one person living with this disorder. NF1 is an autosomal dominant condition, which means that everyone born with a mutation in the gene, whether inherited or spontaneous, has a 50/50 chance of passing it on to each of their own children.
The NF1 disorder results from mutation of the NF1 gene on Chromosome 17. Almost any mutation that affects the sequence of the gene’s protein product, neurofibromin, will cause the disorder. Studies of individuals with NF1 have identified over 3,000 different mutations of all kinds (including point mutations, small and large indels, and translocations). The NF1 gene is one of the largest known genes, containing at least 60 exons (protein-encoding sequences) in a span of about 300,000 nucleotides.
We know that neurofibromin plays an important role in preventing tumor growth because one of the most common symptoms of the NF1 disorder is the growth of benign (noncancerous) tumors, called neurofibromas. Neurofibromas sprout from nerve sheaths—the tissues that encase our nerves—throughout the body, usually beginning around puberty. There is no way to predict where the tumors will occur, or when or how quickly they will grow, although only about 15% turn malignant (cancerous). The two types of neurofibromas that are typically most visible are cutaneous neurofibromas, which are spherical bumps on, or just under, the surface of the skin (Figure 4.18), and plexiform neurofibromas, growths involving whole branches of nerves, often giving the appearance that the surface of the skin is “melting” (Figure 4.19).


Unfortunately, there is currently no cure for NF1. Surgical removal of neurofibromas risks paralysis, due to the high potential for nerve damage, and often results in the tumors growing back even more vigorously. This means that patients are often forced to live with disfiguring and often painful neurofibromas. People who are not familiar with NF1 often mistake neurofibromas for something contagious. This makes it especially hard for people living with NF1 to get jobs working with the public or even to enjoy spending time away from home. Raising public awareness about NF1 and its symptoms can be a great help in improving the quality of life for people living with this condition.

One of the first symptoms of NF1 in a small child is usually the appearance of café-au-lait spots, or CALS, which are flat, brown birthmark-like spots on the skin (Figure 4.20). CALS are often light brown, similar to the color of coffee with cream, which is the reason for the name, although the shade of the pigment depends on a person’s overall complexion. Some babies are born with CALS, but for others the spots appear within the first few years of life. Having six or more CALS larger than five millimeters (mm) across is a strong indicator that a child may have NF1.
Other common symptoms include the following: gliomas (tumors) of the optic nerve, which can cause vision loss; thinning of bones and failure to heal if they break (often requiring amputation); low muscle tone (poor muscle development, often delaying milestones such as sitting up, crawling, and walking); hearing loss, due to neurofibromas on auditory nerves; and learning disabilities, especially those involving spatial reasoning. Approximately 50% of people with NF1 have some type of speech and/or learning disability and often benefit greatly from early intervention services. Generalized developmental disability, however, is not common with NF1, so most people with NF1 live independently as adults. Many people with NF1 live full and successful lives, as long as their symptoms can be managed.
Based on the wide variety of symptoms, it’s clear that the neurofibromin protein plays important roles in many biochemical pathways. While everyone who has NF1 will exhibit some symptoms during their lifetime, there is a great deal of variation in the types and severity of symptoms, even between individuals from the same family who share the exact same NF1 mutation. It seems crazy that a gene with so many important functions would be so susceptible to mutation. Part of this undoubtedly has to do with its massive size—a gene with 300,000 nucleotides has ten times more nucleotides available for mutation than does a gene of 30,000 bases. This also suggests that the mutability of this gene might provide some benefits, which is a possibility that we will revisit later in this chapter.
Special Topic: Sickle Cell Anemia
Sickle cell anemia is an autosomal recessive genetic disorder that affects millions of people worldwide. It is most common in Africa, countries around the Mediterranean Sea, and eastward as far as India. Populations in the Americas that have high percentages of ancestors from these regions also have high rates of sickle cell anemia. In the United States, it’s estimated that 72,000 people live with the disease, with one in approximately 1,200 Hispanic-American babies and one in every 500 African-American babies inheriting the condition (World Health Organization 1996).
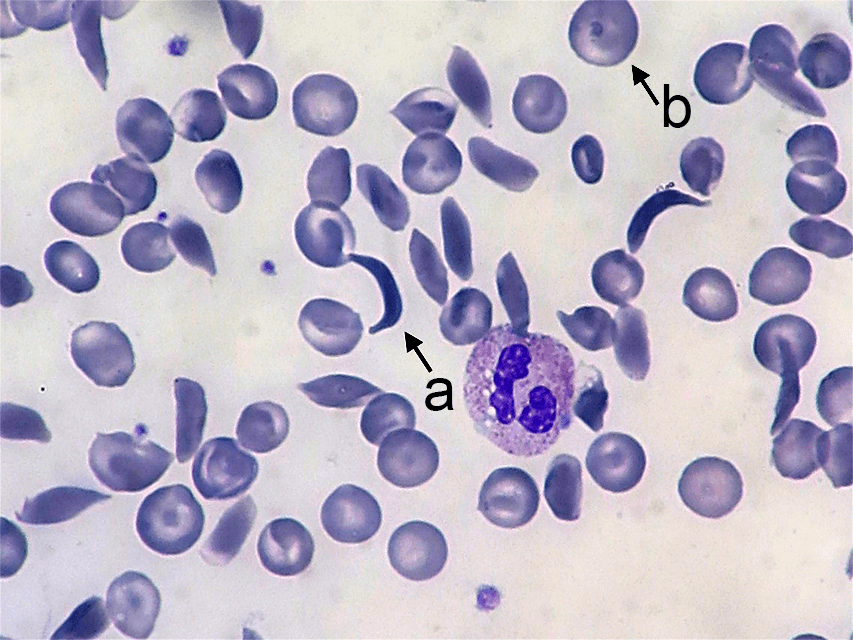
Sickle cell anemia affects the hemoglobin protein in red blood cells. Normal red blood cells are somewhat doughnut-shaped—round with a depression on both sides of the middle. They carry oxygen around the bloodstream to cells throughout the body. Red blood cells produced by the mutated form of the gene take on a stiff, sickle-like crescent shape when stressed by low oxygen or dehydration (Figure 4.21). Because of their elongated shape and the fact that they are stiff rather than flexible, they tend to form clumps in the blood vessels, inhibiting blood flow to adjacent areas of the body. This causes episodes of extreme pain and can cause serious problems in the oxygen-deprived tissues. The sickle cells also break down much more quickly than normal cells, often lasting only 20 days rather than the 120 days of normal cells. This causes an overall shortage of blood cells in the sickle cell patient, resulting in low iron (anemia) and problems associated with it such as extreme fatigue, shortness of breath, and hindrances to children’s growth and development.
The devastating effects of sickle cell anemia made its high frequency a pressing mystery. Why would an allele that is so deleterious in its homozygous form be maintained in a population at levels as high as the one in twelve African Americans estimated to carry at least one copy of the allele? The answer turned out to be one of the most interesting cases of balancing selection in the history of genetic study.
While looking for an explanation, scientists noticed that the countries with high rates of sickle cell disease also shared a high risk for another disease called malaria, which is caused by infection of the blood by a Plasmodium parasite. These parasites are carried by mosquitoes and enter the human bloodstream via a mosquito bite. Once infected, the person will experience flu-like symptoms that, if untreated, can often lead to death. Researchers discovered that many people living in these regions seemed to have a natural resistance to malaria. Further study revealed that people who carry the sickle cell allele are far less likely to experience a severe case of malaria. This would not be enough of a benefit to make the allele advantageous for the sickle cell homozygotes, who face shortened life spans due to sickle cell anemia. The real benefit of the sickle cell allele goes to the heterozygotes.
People who are heterozygous for sickle cell carry one normal allele, which produces the normal, round, red blood cells, and one sickle cell allele, which produces the sickle-shaped red blood cells. Thus, they have both the sickle and round blood cell types in their bloodstream. They produce enough of the round red blood cells to avoid the symptoms of sickle cell anemia, but they have enough sickle cells to provide protection from malaria.
When the Plasmodium parasites infect an individual, they begin to multiply in the liver, but then must infect the red blood cells to complete their reproductive cycle. When the parasites enter sickle-type cells, the cells respond by taking on the sickle shape. This prevents the parasite from circulating through the bloodstream and completing its life cycle, greatly inhibiting the severity of the infection in the sickle cell heterozygotes compared to non–-sickle cell homozygotes. See Chapter 14 for more discussion of sickle cell anemia.
Special Topic: The Real Primordial Cells—Dictyostelium Discoideum
The amoeba-like primordial cells that were used as recurring examples throughout this chapter are inspired by actual research that is truly fascinating. In 2015, Gareth Bloomfield and colleagues reported on their genomic study of the social amoeba Dictyostelium discoideum (a.k.a. “slime molds,” although technically they are amoebae, not molds). Strains of these amoebae have been grown in research laboratories for many decades and are useful in studying the mechanisms that amoeboid single-celled organisms use to ingest food and liquid. For simplification of our examples in this chapter, our amoeba-like cells remained ocean dwellers. Wild Dictyostelium discoideum, however, live in soil and feed on soil bacteria by growing ruffles in their membranes that reach out to encapsulate the bacterial cell. Laboratory strains, however, are typically raised on liquid media (agar) in Petri dishes, which is not suitable for the wild-type amoebae. It was widely known that the laboratory strains must have developed mutations in one or more genes to allow them to ingest the larger nutrient particles in the agar and larger volumes of liquid, but the genes involved were not known.
Bloomfield and colleagues performed genomic testing on both the wild and the laboratory strains of Dictyostelium discoideum. Their discovery was astounding: every one of the laboratory strains carried a mutation in the NF1 gene, the very same gene associated with Neurofibromatosis Type 1 (NF1) in humans. The antiquity of this massive, easily mutated gene is incredible. It originated in an ancestor common to both humans and these amoebae, and it has been retained in both lineages ever since. As seen in Dictyostelium discoideum, breaking the gene can be advantageous. Without a functioning copy of the neurofibromin protein, the cell membrane is able to form much-larger feeding structures, allowing the NF1 mutants to ingest larger particles and larger volumes of liquid. For these amoebae, this may provide dietary flexibility that functions somewhat like an insurance policy for times when the food supply is limited.
Dictyostelium discoideum are also interesting in that they typically reproduce asexually, but under certain conditions, one cell will convert into a “giant” cell, which encapsulates surrounding cells, transforming into one of three sexes. This cell will undergo meiosis, producing gametes that must combine with one of the other two sexes to produce viable offspring. This ability for sexual reproduction may be what allows Dictyostelium discoideum to benefit from the advantages of NF1 mutation, while also being able to restore the wild type NF1 gene in future generations.
What does this mean for humans living with NF1? Well, understanding the role of the neurofibromin protein in the membranes of simple organisms like Dictyostelium discoideum may help us to better understand how it functions and malfunctions in the sheaths of human neurons. It’s also possible that the mutability of the NF1 gene confers certain advantages to humans as well. Alleles of the NF1 gene have been found to reduce one’s risk for alcoholism (Repunte-Canonigo Vez et al. 2015), opiate addiction (Sanna et al. 2002), Type 2 diabetes (Martins et al. 2016), and hypomusicality (a lower-than-average musical aptitude; Cota et al. 2018). This research is ongoing and will be exciting to follow in the coming years.
Studying Evolution in Action
The Hardy-Weinberg Equilibrium
This chapter has introduced you to the forces of evolution, the mechanisms by which evolution occurs. How do we detect and study evolution, though, in real time, as it happens? One tool we use is the Hardy-Weinberg Equilibrium: a mathematical formula that allows estimation of the number and distribution of dominant and recessive alleles in a population. This aids in determining whether allele frequencies are changing and, if so, how quickly over time, and in favor of which allele? It’s important to note that the Hardy-Weinberg formula only gives us an estimate based on the data for a snapshot in time. We will have to calculate it again later, after various intervals, to determine if our population is evolving and in what way the allele frequencies are changing.
Calculating the Hardy-Weinberg Equilibrium
In the Hardy-Weinberg formula, p represents the frequency of the dominant allele, and q represents the frequency of the recessive allele. Remember, an allele’s frequency is the proportion, or percentage, of that allele in the population. For the purposes of Hardy-Weinberg, we give the allele percentages as decimal numbers (e.g., 42% = 0.42), with the entire population (100% of alleles) equaling 1. If we can figure out the frequency of one of the alleles in the population, then it is simple to calculate the other. Simply subtract the known frequency from 1 (the entire population): 1 – p = q and 1 – q = p.
The Hardy-Weinberg formula is p2 + 2pq + q2, where:
p2 represents the frequency of the homozygous dominant genotype;
2pq represents the frequency of the heterozygous genotype; and
q2 represents the frequency of the homozygous recessive genotype.
It is often easiest to determine q2 first, simply by counting the number of individuals with the unique, homozygous recessive phenotype (then dividing by the total individuals in the population to arrive at the “frequency”). Once we have this number, we simply need to calculate the square root of the homozygous recessive phenotype frequency. That gives us q. Remember, 1 – q equals p, so now we have the frequencies for both alleles in the population. If we needed to figure out the frequencies of heterozygotes and homozygous dominant genotypes, we’d just need to plug the p and q frequencies back into the p2 and 2pq formulas.
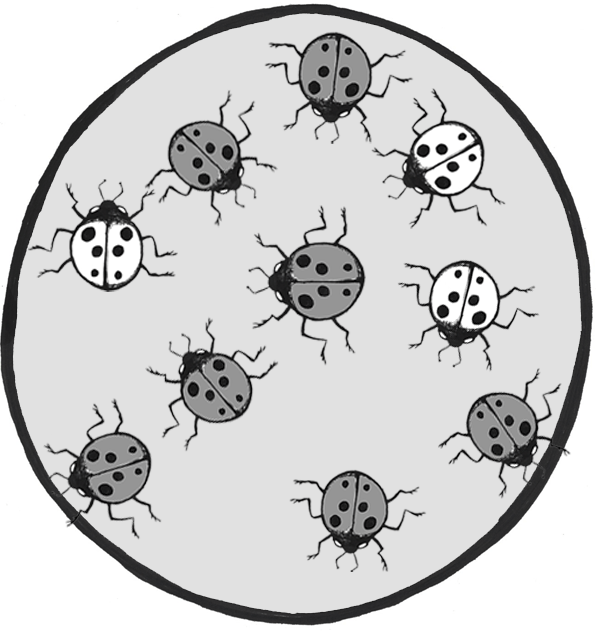
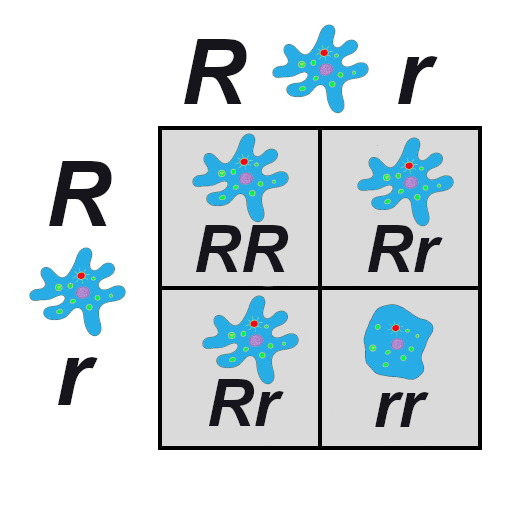
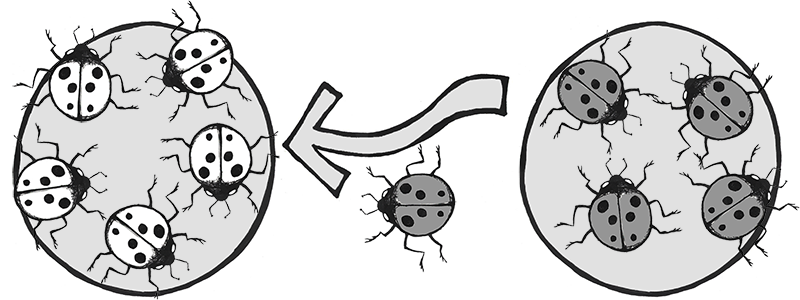
Let’s imagine we have a population of ladybeetles that carries two alleles: a dominant allele that produces red ladybeetles and a recessive allele that produces orange ladybeetles. Since red is dominant, we’ll use R to represent the red allele, and r to represent the orange allele. Our population has ten beetles, and seven are red and three are orange (Figure 4.24). Let’s calculate the number of genotypes and alleles in this population.
Of ten total beetles, we have three orange beetles3/10 = .30 (30%) frequency—and we know they are homozygous recessive (rr). So:
rr = .3; therefore, r = √.3 = .5477
R = 1 – .5477 = .4523
Using the Hardy-Weinberg formula:
1=.45232 + 2 x .4523 x .5477 +.54772 = .20 + .50 + .30 = 1
Thus, the genotype breakdown is 20% RR, 50% Rr, and 30% rr
(2 red homozygotes, 5 red heterozygotes, and 3 orange homozygotes).
Since we have 10 individuals, we know we have 20 total alleles: 4 red from the RR group, 5 red and 5 orange from the Rr group, and 6 orange from the rr group, for a grand total of 9 red and 11 orange (45% red and 55% orange, just like we estimated in the 1 – q step).
Reminder: The Hardy-Weinberg formula only gives us an estimate for a snapshot in time. We will have to calculate it again later, after various intervals, to determine if our population is evolving and in what way the allele frequencies are changing.
Interpreting Evolutionary Change: Nonrandom Mating
Once we have detected change occurring in a population, we need to consider which evolutionary processes might be the cause of the change. It is important to watch for nonrandom mating patterns, to see if they can be included or excluded as possible sources of variation in allele frequencies.
Nonrandom mating (also known as assortative mating) occurs when mate choice within a population follows a nonrandom pattern.
Positive assortative mating patterns result from a tendency for individuals to mate with others who share similar phenotypes. This often happens based on body size. Taking as an example dog breeds, it is easier for two Chihuahuas to mate and have healthy offspring than it is for a Chihuahua and a St. Bernard to do so. This is especially true if the Chihuahua is the female and would have to give birth to giant St. Bernard pups.
Negative assortative mating patterns occur when individuals tend to select mates with qualities different from their own. This is what is at work when humans choose partners whose pheromones indicate that they have different and complementary immune alleles, providing potential offspring with a better chance at a stronger immune system.
Among domestic animals, such as pets and livestock, assortative mating is often directed by humans who decide which pairs will mate to increase the chances of offspring having certain desirable traits. This is known as artificial selection.
Among humans, in addition to phenotypic traits, cultural traits such as religion and ethnicity may also influence assortative mating patterns.
Defining a Species
Species are organisms whose individuals are capable of breeding because they are biologically and behaviorally compatible to produce viable, fertile offspring. Viable offspring are those offspring that are healthy enough to survive to adulthood. Fertile offspring are able to reproduce successfully, resulting in offspring of their own. Both conditions must be met for individuals to be considered part of the same species. As you can imagine, these criteria complicate the identification of distinct species in fossilized remains of extinct populations. In those cases, we must examine how much phenotypic variation is typically found within a comparable modern-day species; we can then determine whether the fossilized remains fall within the expected range of variation for a single species.
Some species have subpopulations that are regionally distinct. These are classified as separate subspecies because they have their own unique phenotypes and are geographically isolated from one another. However, if they do happen to encounter one another, they are still capable of successful interbreeding.
There are many examples of sterile hybrids that are offspring of parents from two different species. For example, horses and donkeys can breed and have offspring together. Depending on which species is the mother and which is the father, the offspring are either called mules, or hennies. Mules and hennies can live full life spans but are not able to have offspring of their own. Likewise, tigers and lions have been known to mate and have viable offspring. Again, depending on which species is the mother and which is the father, these offspring are called either ligers or tigons. Like mules and hennies, ligers and tigons are unable to reproduce. In each of these cases, the mismatched set of chromosomes that the offspring inherit produce an adequate set of functioning genes for the hybrid offspring; however, once mixed and divided in meiosis, the gametes don’t contain the full complement of genes needed for survival in the third generation.
Micro- to Macroevolution
Microevolution refers to changes in allele frequencies within breeding populations—that is, within single species. Macroevolution describes how the similarities and differences between species, as well as the phylogenetic relationships with other taxa, lead to changes that result in the emergence of new species. Consider our example of the peppered moth that illustrated microevolution over time, via directional selection favoring the peppered allele when the trees were clean and the dark pigment allele when the trees were sooty. Imagine that environmental regulations had cleaned up the air pollution in one part of the nation, while the coal-fired factories continued to spew soot in another area. If this went on long enough, it’s possible that two distinct moth populations would eventually emerge—one containing only the peppered allele and the other only harboring the dark pigment allele.
When a single population divides into two or more separate species, it is called speciation. The changes that prevent successful breeding between individuals who descended from the same ancestral population may involve chromosomal rearrangements, changes in the ability of the sperm from one species to permeate the egg membrane of the other species, or dramatic changes in hormonal schedules or mating behaviors that prevent members from the new species from being able to effectively pair up.
There are two types of speciation: allopatric and sympatric. Allopatric speciation is caused by long-term isolation (physical separation) of subgroups of the population (Figure 4.22). Something occurs in the environment—perhaps a river changes its course and splits the group, preventing them from breeding with members on the opposite riverbank. Over many generations, new mutations and adaptations to the different environments on each side of the river may drive the two subpopulations to change so much that they can no longer produce fertile, viable offspring, even if the barrier is someday removed.
Sympatric speciation occurs when the population splits into two or more separate species while remaining located together without a physical barrier. This typically results from a new mutation that pops up among some members of the population that prevents them from successfully reproducing with anyone who does not carry the same mutation. This is seen particularly often in plants, as they have a higher frequency of chromosomal duplications.
One of the quickest rates of speciation is observed in the case of adaptive radiation. Adaptive radiation refers to the situation in which subgroups of a single species rapidly diversify and adapt to fill a variety of ecological niches. An ecological niche is a set of constraints and resources that is available in an environmental setting. Evidence for adaptive radiations is often seen after population bottlenecks. A mass disaster kills off many species, and the survivors have access to a new set of territories and resources that were either unavailable or much coveted and fought over before the disaster. The offspring of the surviving population will often split into multiple species, each of which stems from members in that first group of survivors who happened to carry alleles that were advantageous for a particular niche.
The classic example of adaptive radiation brings us back to Charles Darwin and his observations of the many species of finches on the Galapagos Islands. We are still not sure how the ancestral population of finches first arrived on that remote Pacific Island chain, but they found themselves in an environment filled with various insects, large and tiny seeds, fruit, and delicious varieties of cactus. Some members of that initial population carried alleles that gave them advantages for each of these dietary niches. In subsequent generations, others developed new mutations, some of which were beneficial. These traits were selected for, making the advantageous alleles more common among their offspring. As the finches spread from one island to the next, they would be far more likely to find mates among the birds on their new island. Birds feeding in the same area were then more likely to mate together than birds who have different diets, contributing to additional assortative mating. Together, these evolutionary mechanisms caused rapid speciation that allowed the new species to make the most of the various dietary niches (Figure 4.23).
In today’s modern world, understanding these evolutionary processes is crucial for developing immunizations and antibiotics that can keep up with the rapid mutation rate of viruses and bacteria. This is also relevant to our food supply, which relies, in large part, on the development of herbicides and pesticides that keep up with the mutation rates of pests and weeds. Viruses, bacteria, agricultural pests, and weeds have all shown great flexibility in developing alleles that make them resistant to the latest medical treatment, pesticide, or herbicide. Billion-dollar industries have specialized in trying to keep our species one step ahead of the next mutation in the pests and infectious diseases that put our survival at risk.
Review Questions
- Summarize the Modern Synthesis and provide several examples of how it is relevant to questions and problems in our world today.
- You inherit a house from a long-lost relative that contains a fancy aquarium, filled with a variety of snails. The phenotypes include large snails and small snails; red, black, and yellow snails; and solid, striped, and spotted snails. Devise a series of experiments that would help you determine how many snail species are present in your aquarium.
- Match the correct force of evolution with the correct real-world example:
a. Mutationi. 5-alpha reductase deficiency
b. Genetic Driftii. Peppered Moths
c. Gene Flowiii. Neurofibromatosis Type 1
d. Natural Selectioniv. Scutellata Honey Bees - Imagine a population of common house mice (Mus musculus). Draw a comic strip illustrating how mutation, genetic drift, gene flow, and natural selection might transform this population over several (or more) generations.
-
The many breeds of the single species of domestic dog (Canis familiaris) provide an extreme example of microevolution. Discuss why this is the case. What future scenarios can you imagine that could potentially transform the domestic dog into an example of macroevolution?
-
The ability to roll one’s tongue (lift the outer edges of the tongue to touch each other, forming a tube) is a dominant trait. In a small town of 1,500 people, 500 can roll their tongues. Use the Hardy-Weinberg formula to determine how many individuals in the town are homozygous dominant, heterozygous, and homozygous recessive.
Key Terms
5-alpha reductase deficiency: An autosomal recessive syndrome that manifests when a child having both X and Y sex chromosomes inherits two nonfunctional (mutated) copies of the SRD5A2 gene, producing a deficiency in a hormone necessary for development in infancy of typical male genitalia. These children often appear at birth to have female genitalia, but they develop a penis and other sexual characteristics when other hormones kick in during puberty.
Adaptive radiation: The situation in which subgroups of a single species rapidly diversify and adapt to fill a variety of ecological niches.
Admixture: A term often used to describe gene flow between human populations. Sometimes also used to describe gene flow between nonhuman populations.
Allele frequency: The ratio, or percentage, of one allele compared to the other alleles for that gene within the study population.
Alleles: Variant forms of genes.
Allopatric speciation: Speciation caused by long-term isolation (physical separation) of subgroups of the population.
Antibiotics: Medicines prescribed to treat bacterial infections.
Artificial selection: Human-directed assortative mating among domestic animals, such as pets and livestock, designed to increase the chances of offspring having certain desirable traits.
Asexual reproduction: Reproduction via mitosis, whereby offspring are clones of the parents.
Autosomal dominant: A phenotype produced by a gene on an autosomal chromosome that is expressed, to the exclusion of the recessive phenotype, in heterozygotes.
Autosomal recessive: A phenotype produced by a gene on an autosomal chromosome that is expressed only in individuals homozygous for the recessive allele.
Balanced translocations: Chromosomal translocations in which the genes are swapped but no genetic information is lost.
Balancing selection: A pattern of natural selection that occurs when the extremes of a trait are selected against, favoring the intermediate phenotype (a.k.a. stabilizing selection).
Beneficial mutations: Mutations that produce some sort of an advantage to the individual.
Benign: Noncancerous. Benign tumors may cause problems due to the area in which they are located (e.g., they might put pressure on a nerve or brain area), but they will not release cells that aggressively spread to other areas of the body.
Café-au-lait spots (CALS): Flat, brown birthmark-like spots on the skin, commonly associated with Neurofibromatosis Type 1.
Chromosomal translocations: The transfer of DNA between nonhomologous chromosomes.
Chromosomes: Molecules that carry collections of genes.
Codons: Three-nucleotide units of DNA that function as three-letter “words,” encoding instructions for the addition of one amino acid to a protein or indicating that the protein is complete.
Cretaceous–Paleogene extinction: A mass disaster caused by an asteroid that struck the earth approximately 66 million years ago and killed 75% of life on Earth, including all terrestrial dinosaurs. (a.k.a. K-Pg Extinction, Cretatious-Tertiary Extinction, and K-T Extinction).
Crossover events: Chromosomal alterations that occur when DNA is swapped between homologous chromosomes while they are paired up during meiosis I.
Cutaneous neurofibromas: Neurofibromas that manifest as spherical bumps on or just under the surface of the skin.
Deleterious mutation: A mutation producing negative effects to the individual such as the beginnings of cancers or heritable disorders.
Deletions: Mutations that involve the removal of one or more nucleotides from a DNA sequence.
Derivative chromosomes: New chromosomal structures resulting from translocations.
Dictyostelium discoideum: A species of social amoebae that has been widely used for laboratory research. Laboratory strains of Dictyostelium discoideum all carry mutations in the NF1 gene, which is what allows them to survive on liquid media (agar) in Petri dishes.
Directional selection: A pattern of natural selection in which one phenotype is favored over the other, causing the frequencies of the associated advantageous alleles to gradually increase.
Disruptive selection: A pattern of natural selection that occurs when both extremes of a trait are advantageous and intermediate phenotypes are selected against (a.k.a. diversifying selection).
DNA repair mechanisms: Enzymes that patrol and repair DNA in living cells.
DNA transposons: Transposons that are clipped out of the DNA sequence itself and inserted elsewhere in the genome.
Ecological niche: A set of constraints and resources that are available in an environmental setting.
Ellis-van Creveld syndrome: An autosomal recessive disorder characterized by short stature (dwarfism), polydactyly (the development of more than five digits [fingers or toes] on the hands or feet), abnormal tooth development, and heart defects. Estimated to affect approximately one in 60,000 individuals worldwide, among the Old Order Amish of Lancaster County, the rate is estimated to be as high as one in every 200 births.
Evolution: A change in the allele frequencies in a population over time.
Exons: The DNA sequences within a gene that directly encode protein sequences. After being transcribed into messenger RNA, the introns (DNA sequences within a gene that do not directly encode protein sequences) are clipped out, and the exons are pasted together prior to translation.
Fertile offspring: Offspring that can successfully reproduce, resulting in offspring of their own.
Founder effect: A type of genetic drift that occurs when members of a population leave the main or “parent” group and form a new population that no longer interbreeds with the other members of the original group.
Frameshift mutations: Types of indels that involve the insertion or deletion of any number of nucleotides that is not a multiple of three. These “shift the reading frame” and cause all codons beyond the mutation to be misread.
Gametes: The reproductive cells, produced through meiosis (a.k.a. germ cells or sperm or egg cells).
Gene: A sequence of DNA that provides coding information for the construction of proteins.
Gene flow: The movement of alleles from one population to another. This is one of the forces of evolution.
Gene pool: The entire collection of genetic material in a breeding community that can be passed on from one generation to the next.
Genetic drift: Random changes in allele frequencies within a population from one generation to the next. This is one of the forces of evolution.
Genotype: The set of alleles that an individual has for a given gene.
Genotype frequencies: The ratios or percentages of the different homozygous and heterozygous genotypes in the population.
Guevedoces: The term coined locally in the Dominican Republic for the condition scientifically known as 5-alpha reductase deficiency. The literal translation is “penis at twelve.”
Hardy-Weinberg Equilibrium: A mathematical formula (1=p2 + 2pq + q2 ) that allows estimation of the number and distribution of dominant and recessive alleles in a population.
Harlequin ladybeetle: A species of ladybeetle, native to East Asia, that was introduced to Europe and the Americas as a form of pest control. After many decades of use, one of the North American strains developed the ability to reproduce in diverse environments, causing it to spread rapidly throughout the Americas, Europe, and Africa. It has hybridized with European strains and is now a major pest in its own right.
Heterozygous genotype: A genotype comprising two different alleles.
Homozygous genotype: A genotype comprising an identical set of alleles.
Hybridization: A term often used to describe gene flow between nonhuman populations.
Inbreeding: The selection of mates exclusively from within a small, closed population.
Indels: A class of mutations that includes both insertions and deletions.
Inherited mutation: A mutation that has been passed from parent to offspring.
Insertions: Mutations that involve the addition of one or more nucleotides into a DNA sequence.
Isolation: Prevention of a population subgroup from breeding with other members of the same species due to a physical barrier or, in humans, a cultural rule.
Last Universal Common Ancestor (LUCA): The ancient organism from which all living things on Earth are descended.
Macroevolution: Changes that result in the emergence of new species, how the similarities and differences between species, as well as the phylogenetic relationships with other taxa, lead to changes that result in the emergence of new species.
Malaria: A frequently deadly mosquito-borne disease caused by infection of the blood by a Plasmodium parasite.
Malignant: Cancerous. Malignant tumors grow aggressively and their cells may metastasize (travel through the blood or lymph systems) to form new, aggressive tumors in other areas of the body.
Microevolution: Changes in allele frequencies within breeding populations—that is, within a single species.
Modern Synthesis: The integration of Darwin’s, Mendel’s, and subsequent research into a unified theory of evolution.
Monosomies: Conditions resulting from a nondisjunction event, in which a cell ends up with only one copy of a chromosome. In humans, a single X chromosome is the only survivable monosomy.
Mutation: A change in the nucleotide sequence of the genetic code. This is one of the forces of evolution.
Natural selection: An evolutionary process that occurs when certain phenotypes confer an advantage or disadvantage in survival and/or reproductive success. This is one of the forces of evolution, and it was first identified by Charles Darwin.
Negative assortative mating: A pattern that occurs when individuals tend to select mates with qualities different from their own.
Neurofibromas: Nerve sheath tumors that are common symptoms of Neurofibromatosis Type 1.
Neurofibromatosis Type 1: An autosomal dominant genetic disorder affecting one in every 3,000 people. It is caused by mutation of the NF1 gene on Chromosome 17, resulting in a defective neurofibromin protein. The disorder is characterized by neurofibromas, café-au-lait spots, and a host of other potential symptoms.
NF1: An abbreviation for Neurofibromatosis Type 1. When italicized, NF1 refers to the gene on Chromosome 17 that encodes the neurofibromin protein.
Nondisjunction events: Chromosomal abnormalities that occur when the homologous chromosomes (in meiosis I) or sister chromatids (in meiosis II and mitosis) fail to separate after pairing. The result is that both chromosomes or chromatids end up in the same daughter cell, leaving the other daughter cell without any copy of that chromosome.
Nonrandom mating: A scenario in which mate choice within a population follows a nonrandom pattern (a.k.a. assortative mating).
Nonsynonymous mutation: A point mutation that causes a change in the resulting protein.
Old Order Amish: A culturally isolated population in Lancaster County, Pennsylvania, that has approximately 50,000 current members, all of whom can trace their ancestry back to a group of approximately eighty individuals. This group has high rates of certain genetics disorders, including Ellis-van Creveld syndrome.
Origins of life: How the first living organism came into being.
Peacock: The male sex of the peafowl, famous for its large, colorful tail, which it dramatically displays to attract mates. (The female of the species is known as a peahen.)
Peppered moth: A species of moth (Biston betularia) found in England that has light and dark phenotypes. During the Industrial Revolution, when soot blackened the trees, the frequency of the previously rare dark phenotype dramatically increased, as lighter-colored moths were easier for birds to spot against the sooty trees. After environmental regulations eliminated the soot, the lighter-colored phenotype gradually became most common again.
Phenotype: The observable traits that are produced by a genotype.
Phylogenetic tree of life: A family tree of all living organisms, based on genetic relationships.
Phylogenies: Genetically determined family lineages.
Plasmodium: A genus of mosquito-borne parasite. Several Plasmodium species cause malaria when introduced to the human bloodstream via a mosquito bite.
Plexiform neurofibromas: Neurofibromas that involve whole branches of nerves, often giving the appearance that the surface of the skin is “melting.”
Point mutation: A single-letter (single-nucleotide) change in the genetic code, resulting in the substitution of one nucleic acid base for a different one.
Polymorphisms: Multiple forms of a trait; alternative phenotypes within a given species.
Population: A group of individuals who are genetically similar enough and geographically near enough to one another that they can breed and produce new generations of individuals.
Population bottleneck: A type of genetic drift that occurs when the number of individuals in a population drops dramatically due to some random event.
Positive assortative mating: A pattern that results from a tendency for individuals to mate with others who share similar phenotypes.
Retrotransposons: Transposons that are transcribed from DNA into RNA, and then are “reverse transcribed,” to insert the copied sequence into a new location in the DNA.
Scutellata honey bees: A strain of honey bees that resulted from the hybridization of African and European honey bee subspecies. These bees were accidentally released into the wild in 1957 in Brazil and have since spread throughout South and Central America and into the United States. Also known as “killer bees,” they tend to be very aggressive in defense of their hives and have caused many fatal injuries to humans and livestock.
Sexual reproduction: Reproduction via meiosis and combination of gametes. Offspring inherit genetic material from both parents.
Sexual selection: An aspect of natural selection in which the selective pressure specifically affects reproductive success (the ability to successfully breed and raise offspring).
Sickle cell anemia: An autosomal recessive genetic disorder that affects millions of people worldwide. It is most common in Africa, countries around the Mediterranean Sea, and eastward as far as India. Homozygotes for the recessive allele develop the disorder, which produce misshapen red blood cells that cause iron deficiency, painful episodes of oxygen-deprivation in localized tissues, and a host of other symptoms. In heterozygotes, though, the sickle cell allele confers a greater resistance to malaria.
Somatic cells: The cells of our organs and other body tissues (all cells except gametes) that replicate by mitosis.
Speciation: The process by which a single population divides into two or more separate species.
Species: Organisms whose individuals are capable of breeding because they are biologically and behaviorally compatible to produce viable, fertile offspring.
Spontaneous mutation: A mutation that occurs due to random chance or unintentional exposure to mutagens. In families, a spontaneous mutation is the first case, as opposed to mutations that are inherited from parents.
Subspecies: A distinct subtype of a species. Most often, this is a geographically isolated population with unique phenotypes; however, it remains biologically and behaviorally capable of interbreeding with other populations of the same species.
Sympatric speciation: When a population splits into two or more separate species while remaining located together without a physical (or cultural) barrier.
Synonymous mutation: A point mutation that does not change the resulting protein.
Transposable elements: Fragments of DNA that can “jump” around in the genome.
Transposon: Another term for “transposable element.”
Trisomies: Conditions in which three copies of the same chromosome end up in a cell, resulting from a nondisjunction event. Down syndrome, Edwards syndrome, and Patau syndrome are trisomies.
Unbalanced translocations: Chromosomal translocations in which there is an unequal exchange of genetic material, resulting in duplication or loss of genes.
UV crosslinking: A type of mutation in which adjacent thymine bases bind to one another in the presence of UV light.
Viable offspring: Offspring that are healthy enough to survive to adulthood.
Xeroderma pigmentosum: An autosomal recessive disease in which DNA repair mechanisms do not function correctly, resulting in a host of problems especially related to sun exposure, including severe sunburns, dry skin, heavy freckling, and other pigment changes.
For Further Exploration
Explore Evolution on HHMI’s Biointeractive website.
Teaching Evolution through Human Examples, Smithsonian Museum of Natural History websites.
References
Bloomfield, Gareth, David Traynor, Sophia P. Sander, Douwe M. Veltman, Justin A. Pachebat, and Robert R. Kay. 2015. “Neurofibromin Controls Macropinocytosis and Phagocytosis in Dictyostelium.” eLife 4:e04940.
Chaix, Raphaëlle, Chen Cao, and Peter Donnelly. 2008. “Is Mate Choice in Humans MHC-Dependent?” PLoS Genetics 4 (9): e1000184.
Cook, Laurence M. 2003. “The Rise and Fall of the Carbonaria Form of the Peppered Moth.” The Quarterly Review of Biology 78 (4): 399–417.
Cota, Bruno Cézar Lage, João Gabriel Marques Fonseca, Luiz Oswaldo Carneiro Rodrigues, Nilton Alves de Rezende, Pollyanna Barros Batista, Vincent Michael Riccardi, and Luciana Macedo de Resende. 2018. “Amusia and Its Electrophysiological Correlates in Neurofibromatosis Type 1.” Arquivos de Neuro-Psiquiatria 76 (5): 287–295.
D’Asdia, Maria Cecilia, Isabella Torrente, Federica Consoli, Rosangela Ferese, Monia Magliozzi, Laura Bernardini, Valentina Guida, et al. 2013. “Novel and Recurrent EVC and EVC2 Mutations in Ellis-van Creveld Syndrome and Weyers Acrofacial Dyostosis.” European Journal of Medical Genetics 56 (2): 80–87.
Dobzhansky, Theodosius. 1937. Genetics and the Origin of Species. Columbia University Biological Series. New York: Columbia University Press.
Facon, Benoît, Laurent Crespin, Anne Loiseau, Eric Lombaert, Alexandra Magro, and Arnaud Estoup. 2011. “Can Things Get Worse When an Invasive Species Hybridizes? The Harlequin Ladybird Harmonia axyridis in France as a Case Study.” Evolutionary Applications 4 (1): 71–88.
Fisher, Ronald A. 1919. “The Correlation between Relatives on the Supposition of Mendelian Inheritance.” Transactions of the Royal Society of Edinburgh 52 (2): 399–433.
Ford, E. B. 1942. Genetics for Medical Students. London: Methuen.
Ford, E. B. 1949. Mendelism and Evolution. London: Methuen.
Grant, Bruce S. 1999. “Fine-tuning the Peppered Moth Paradigm.” Evolution 53 (3): 980–984.
Haldane, J. B. S. 1924. “A Mathematical Theory of Natural and Artificial Selection (Part 1).” Transactions of the Cambridge Philosophical Society 23 (2):19–41.
Hoelzel, A. R., Gkafas, G. A., Kang, H., Sarigol, F., Le Boeuf, B., Costa, D. P., Beltran, R. S., Reiter, J., Robinson, P. W., McInerney, N., Seim, I., Sun, S., Fan, G., & Li, S. (2024). Genomics of post-bottleneck recovery in the northern elephant seal. Nature Ecology & Evolution, 8, 686–694. https://doi.org/10.1038/s41559-024-02337-4
Imperato-McGinley, J., and Y.-S. Zhu. 2002. “Androgens and Male Physiology: The Syndrome of 5 Alpha-Reductase-2 Deficiency.” Molecular and Cellular Endocrinology 198 (1-2): 51–59.
Jablonski, David, and W. G. Chaloner. 1994. “Extinctions in the Fossil Record.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 344 (1307): 11–17.
Livi-Bacci, Massimo. 2006. “The Depopulation of Hispanic America after the Conquest.” Population Development and Review 32 (2): 199–232.
Lombaert, Eric, Thomas Guillemaud, Jean-Marie Cornuet, Thibaut Malausa, Benoît Facon, and Arnaud Estoup. 2010. “Bridgehead Effect in the Worldwide Invasion of the Biocontrol Harlequin Ladybird.” PLoS ONE 5 (3): e9743.
Martins, Aline Stangherlin, Ann Kristine Jansen, Luiz Oswaldo Carneiro Rodrigues, Camila Maria Matos, Marcio Leandro Ribeiro Souza, Juliana Ferreira de Souza, Maria de Fátima Haueisen Sander Diniz, et al. 2016. “Lower Fasting Blood Glucose in Neurofibromatosis Type 1.” Endocrine Connections 5 (1): 28–33.
Pickering, Gary, James Lin, Roland Riesen, Andrew Reynolds, Ian Brindle, and George Soleas. 2004. “Influence of Harmonia axyridis on the Sensory Properties of White and Red Wine.” American Journal of Enology and Viticulture 55 (2): 153–159.
Repunte-Canonigo Vez, Melissa A. Herman, Tomoya Kawamura, Henry R. Kranzler, Richard Sherva, Joel Gelernter, Lindsay A. Farrer, Marisa Roberto, and Pietro Paolo Sanna. 2015. “NF1 Regulates Alcohol Dependence-Associated Excessive Drinking and Gamma-Aminobutyric Acid Release in the Central Amygdala in Mice and Is Associated with Alcohol Dependence in Humans.” Biological Psychiatry 77 (10): 870–879.
Riccardi, Vincent M. 1992. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore: Johns Hopkins University Press.
Sanford, Malcolm T. 2006. “The Africanized Honey Bee in the Americas: A Biological Revolution with Human Cultural Implications, Part V—Conclusion.” American Bee Journal 146 (7): 597–599.
Sanna, Pietro Paolo, Cindy Simpson, Robert Lutjens, and George Koob. 2002. “ERK Regulation in Chronic Ethanol Exposure and Withdrawal.” Brain Research 948 (1–2): 186–191.
Weber, DianaS., Stewart, B. S., Garza, J. Carlos., & Lehman, N. (2000). An empirical genetic assessment of the severity of the northern elephant seal population bottleneck. Current Biology, 10(20), 1287–1290. https://doi.org/10.1016/s0960-9822(00)00759-4
World Health Organization. 1996. “Control of Hereditary Disorders: Report of WHO Scientific meeting (1996).” WHO Technical Reports 865. Geneva: World Health Organization.
World Health Organization. 2017. “Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics.” Global Priority Pathogens List, February 27. Geneva: World Health Organization. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
Wright, Sewall. 1932. “The Roles of Mutation, Inbreeding, Crossbreeding, and Selection in Evolution.” Proceedings of the Sixth International Congress on Genetics 1 (6): 356–366.
Acknowledgment
Many thanks to Dr. Vincent M. Riccardi for sharing his vast knowledge of neurofibromatosis and for encouraging me to explore it from an anthropological perspective.
Hayley Mann, M.A., Binghamton University
Student contributors for this chapter: Emma Costa, Shima Gahima, Will Lefebvre, Audrey Chékinaël
This chapter is a revision from "Chapter 3: Molecular Biology and Genetics" by Hayley Mann, Xazmin Lowman, and Malaina Gaddis. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Explain and identify the purpose of both DNA replication and the cell cycle.
- Identify key differences between mitosis and meiosis.
- Outline the process of protein synthesis, including transcription and translation.
- Use principles of Mendelian inheritance to predict genotypes and phenotypes of future generations.
- Explain complexities surrounding patterns of genetic inheritance and polygenic traits.
- Discuss challenges to and bioethical concerns of genetic testing.
I [Hayley Mann] started my Bachelor’s degree in 2003, which was the same year the Human Genome Project released its first draft sequence. I initially declared a genetics major because I thought it sounded cool. However, upon taking an actual class, I discovered that genetics was challenging. In addition to my genetics major, I signed up for biological anthropology classes and soon learned that anthropology could bring all those molecular lessons to life. For instance, we are composed of cells, proteins, nucleic acids, carbohydrates, and lipids. Anthropologists often include these molecules in their studies to identify how humans vary; if there are meaningful differences, they propose theories to explain them. Anthropologists study biomolecules in both living and ancient individuals. Ancient biomolecules can also be found on artifacts such as stone tools and cooking vessels. Over the years, scientific techniques for studying organic molecules have improved, which has unlocked new insights into the deep human past.
Cells and Molecules
Molecules of Life
All organisms are composed of four basic types of molecules that are essential for cell structure and function: proteins, lipids, carbohydrates, and nucleic acids (Figure 3.1). Proteins are crucial for cell shape and nearly all cellular tasks, including receiving signals from outside the cell and mobilizing intra-cellular responses. Lipids are a class of organic compounds that include fats, oils, and hormones. Carbohydrates are sugar molecules and serve as energy to cells in the form of glucose. Lastly, nucleic acids, including deoxyribonucleic acid (DNA), carry genetic information about a living organism.
|
Molecule |
Definition |
Example |
|
Proteins |
Composed of one or more long chains of amino acids (i.e., basic units of protein) Often folded into complex 3D shapes that relate to function Proteins interact with other types of proteins and molecules |
Proteins come in different categories including structural (e.g., collagen, keratin, lactase, hemoglobin, cell membrane proteins), defense proteins (e.g, antibodies), enzymes (e.g., lactase), hormones (e.g., insulin), and motor proteins (e.g., actin) |
|
Lipids |
Insoluble in water due to hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail |
Fats, such as triglycerides, store energy for your body Steroid hormones (e.g., estrogen and testosterone) act as chemical messengers to communicate between cells and tissues, as well as biochemical pathways inside of the cell |
|
Carbohydrates |
Large group of organic molecules that are composed of carbon and hydrogen atoms |
Starches and sugars, including blood glucose, provide cells with energy |
|
Nucleic Acids |
Carries the genetic information of an organism |
DNA RNA |
Cells
In 1665, Robert Hooke observed slices of plant cork using a microscope. Hooke noted that the microscopic plant structures he saw resembled cella, meaning “a small room” in Latin. Approximately two centuries later, biologists recognized the cell as being the most fundamental unit of life and that all life is composed of cells. Cellular organisms can be characterized as two main cell types: prokaryotes and eukaryotes (Figure 3.2).
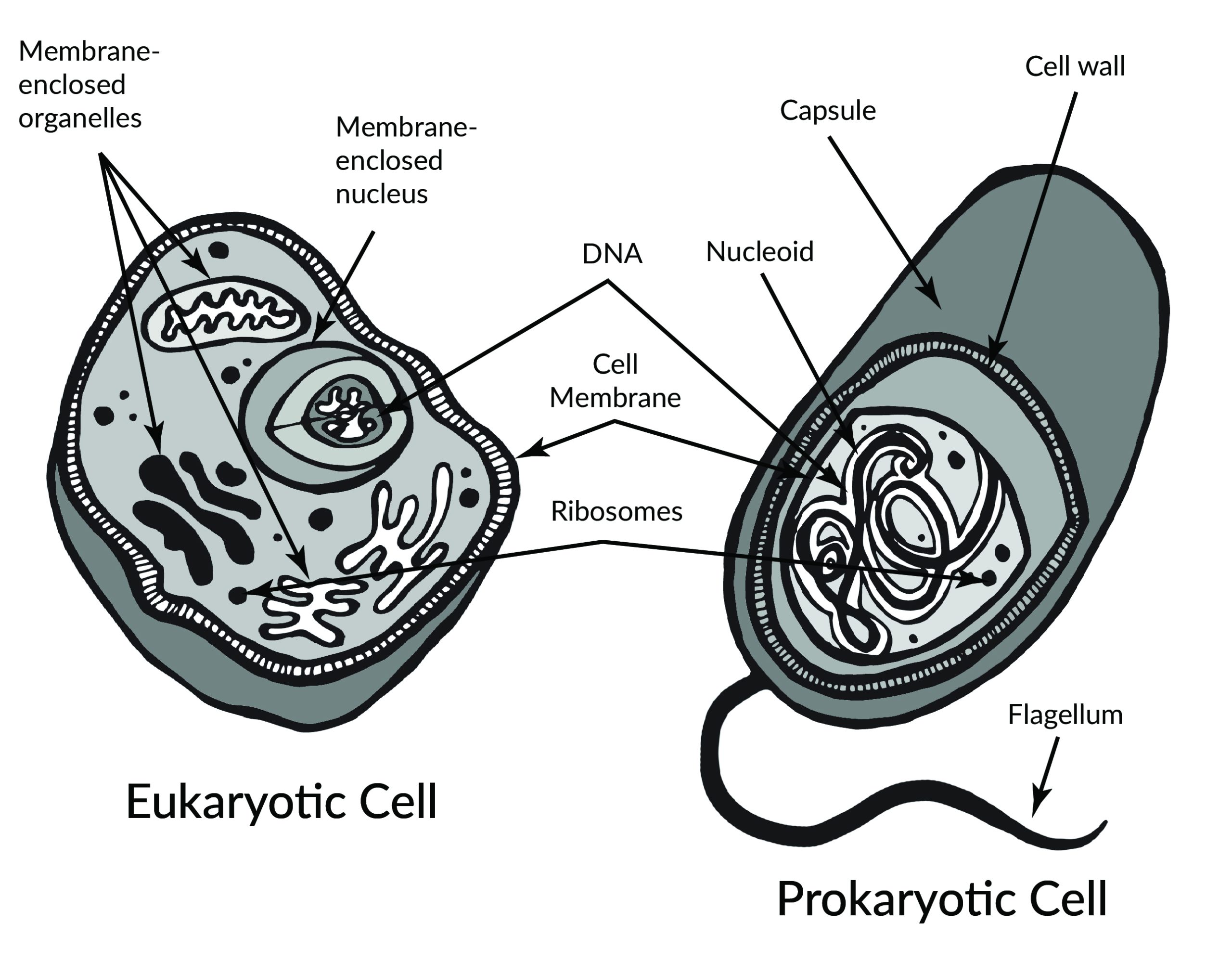
Prokaryotes include bacteria and archaea, and they are composed of a single cell. Additionally, their DNA and organelles are not surrounded by individual membranes. Thus, no compartments separate their DNA from the rest of the cell (see Figure 3.2). It is well known that some bacteria can cause illness in humans. For instance, Escherichia coli (E. coli) and Salmonella contamination can result in food poisoning symptoms. Pneumonia and strep throat are caused by Streptococcal bacteria. Neisseria gonorrhoeae is a sexually transmitted bacterial disease. Although bacteria are commonly associated with illness, not all bacteria are harmful. For example, researchers are studying the relationship between the microbiome and human health. The bacteria that are part of the healthy human microbiome perform beneficial roles, such as digesting food, boosting the immune system, and even making vitamins (e.g., B12 and K).
Eukaryotes can be single-celled or multi-celled in their body composition. In contrast to prokaryotes, eukaryotes possess membranes that surround their DNA and organelles. An example of a single-celled eukaryote is the microscopic algae found in ponds (phytoplankton), which can produce oxygen from the sun. Yeasts are also single-celled, and fungi can be single- or multicellular. Plants and animals are all multicellular.
Although plant and animal cells have a surprising number of similarities, there are some key differences (Figure 3.3). For example, plant cells possess a thick outer cell membrane made of a fibrous carbohydrate called cellulose. Animal and plant cells also have different tissues. For most plants, the outermost layer of cells forms a waxy cuticle that helps to protect the cells and to prevent water loss. Humans have skin, which is the outermost cell layer that is predominantly composed of a tough protein called keratin. Overall, humans have a diversity of tissue types (e.g., cartilage, brain, and heart).
Animal Cell Organelles
An animal cell is surrounded by a double membrane called the phospholipid bilayer (Figure 3.4). A closer look reveals that this protective barrier is made of lipids and proteins that provide structure and function for cellular activities, such as regulating the passage of molecules and ions (e.g., H2O and sodium) into and out of the cell. Cytoplasm is the jelly-like matrix inside of the cell membrane. Part of the cytoplasm comprises organelles, which perform different specialized tasks for the cell (Figure 3.5). An example of an organelle is the nucleus, where the cell’s DNA is located.
Another organelle is the mitochondrion. Mitochondria are often referred to as “powerhouse centers” because they produce energy for the cell in the form of adenosine triphosphate (ATP). Depending on the species and tissue type, multicellular eukaryotes can have hundreds to thousands of mitochondria in each of their cells. Scientists have determined that mitochondria were once symbiotic prokaryotic organisms (i.e., helpful bacteria) that transformed into cellular organelles over time. This evolutionary explanation helps explain why mitochondria also have their own DNA, called mitochondrial DNA (mtDNA). All organelles have important physiological functions and disease can occur when organelles do not perform their role optimally. Figure 3.6 lists other organelles found in the cell and their specialized cellular roles.
|
Cell structure |
Description |
|
Centrioles |
Assist with the organization of mitotic spindles, which extend and contract for the purpose of cellular movement during mitosis and meiosis. |
|
Cytoplasm |
Gelatinous fluid located inside of cell membrane that contains organelles. |
|
Endoplasmic reticulum (ER) |
Continuous membrane with the nucleus that helps transport, synthesize, modify, and fold proteins. Rough ER has embedded ribosomes, whereas smooth ER lacks ribosomes. |
|
Golgi body |
Layers of flattened sacs that receive and transmit messages from the ER to secrete and transport proteins within the cell. |
|
Lysosome |
Located in the cytoplasm; contains enzymes to degrade cellular components. |
|
Microtubule |
Involved with cellular movement including intracellular transport and cell division. |
|
Mitochondrion |
Responsible for cellular respiration, where energy is produced by converting nutrients into ATP. |
|
Nucleolus |
Resides inside of the nucleus and is the site of ribosomal RNA (rRNA) transcription, processing, and assembly. |
|
Nucleopore |
Pores in the nuclear envelope that are selectively permeable. |
|
Nucleus |
Contains the cell’s DNA and is surrounded by the nuclear envelope. |
|
Ribosome |
Located in the cytoplasm and also the membrane of the rough endoplasmic reticulum. Messenger RNA (mRNA) binds to ribosomes and proteins are synthesized. |
Introduction to Genetics
Genetics is the study of heredity. Biological parents pass down their genetic traits to their offspring. Although children resemble their parents, genetic traits often vary in appearance or molecular function. For example, two parents with normal color vision can sometimes produce a son with red-green colorblindness. Molecular geneticists study the biological mechanisms responsible for creating variation between individuals, such as DNA mutations (see Chapter 4), cell division, and genetic regulation.
Molecular anthropologists use genetic data to test anthropological questions. Some of these anthropologists utilize ancient DNA (aDNA), which is DNA that is extracted from anything once living, including human, animal, and plant remains. Over time, DNA becomes degraded (i.e., less intact), but specialized laboratory techniques can make copies of short degraded aDNA segments, which can then be reassembled to provide more complete DNA information.
DNA Structure
The discovery, in 1953, of the molecular structure of deoxyribonucleic acid (DNA) was one of the greatest scientific achievements of all time. Using X-ray crystallography, Rosalind Franklin (Figure 3.7) provided an image that clearly showed the double helix shape of DNA. Due to controversy, Franklin’s colleagues received more recognition for the DNA discovery. In 1962, Watson, Crick, and Wilkins won the Nobel Prize, while Franklin, who had died in 1958, was not honoured. Today, her vital contributions and scientific skill are widely recognized.
The double helix shape of DNA can be described as a twisted ladder (Figure 3.8). More specifically, DNA is a double-stranded molecule with its two strands oriented in opposite directions (i.e., antiparallel). Each strand is composed of nucleotides with a sugar phosphate backbone. There are four different types of DNA nucleotides: adenine (A), thymine (T), cytosine (C), and guanine (G). The two DNA strands are held together by nucleotide base pairs, which have chemical bonding rules. The complementary base-pairing rules are as follows: A and T bond with each other, while C and G form a bond. The chemical bonds between A-T and C-G are formed by “weak” hydrogen atom interactions, which means the two strands can be easily separated. A DNA sequence is the order of nucleotide bases (A, T, G, C) along only one DNA strand. If one DNA strand has the sequence CATGCT, then the other strand will have a complementary sequence GTACGA. This is an example of a short DNA sequence. In reality, there are approximately three billion DNA base pairs in human cells.
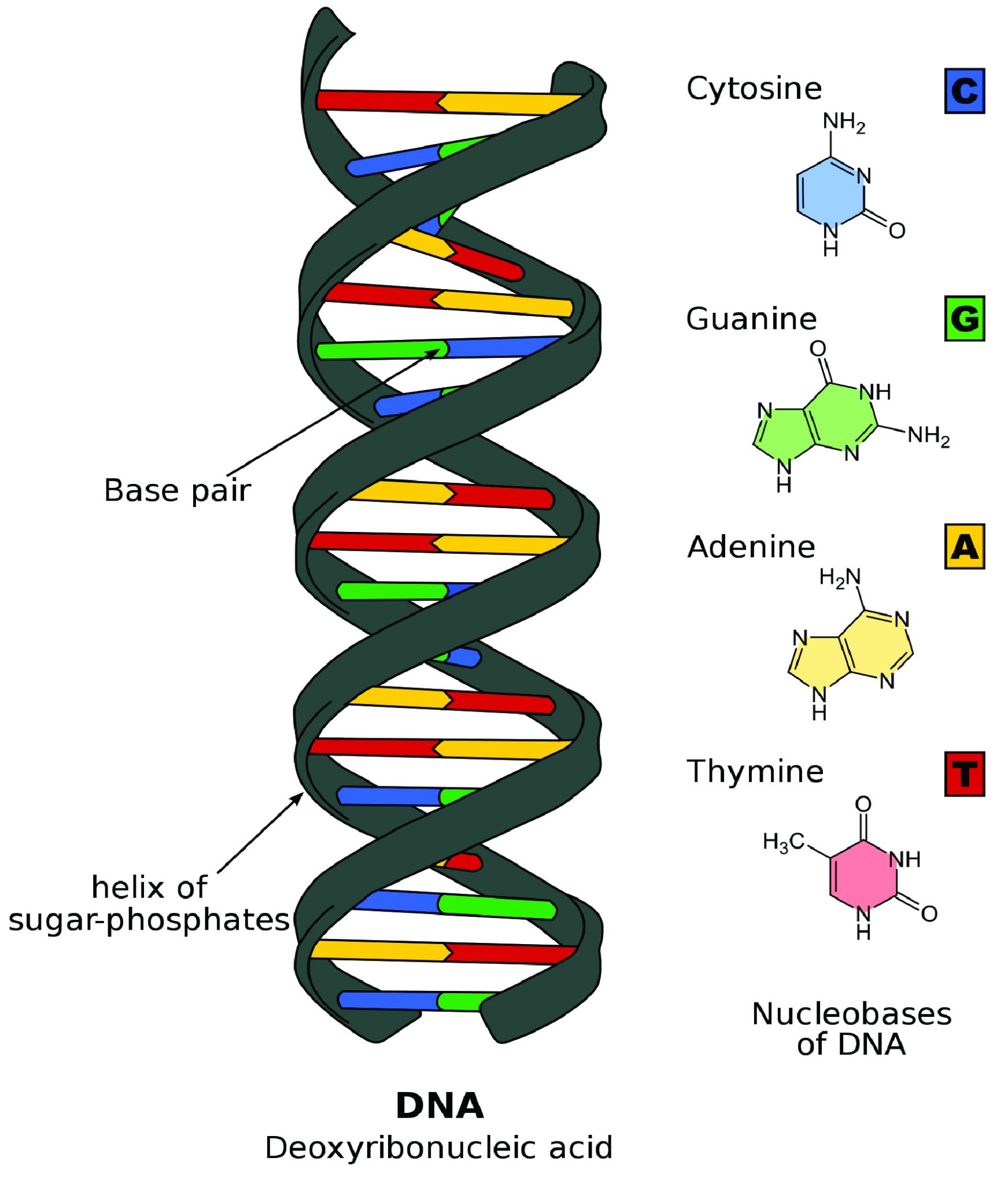
DNA Is Highly Organized within the Nucleus
If you removed the DNA from a single human cell and stretched it out completely, it would measure approximately two meters (about 6.5 feet). Therefore, DNA molecules must be compactly organized in the nucleus. To achieve this, the double helix configuration of DNA undergoes coiling. An analogy would be twisting a string until coils are formed and then continuing to twist so that secondary coils are formed, and so on. To assist with coiling, DNA is first wrapped around proteins called histones. This creates a complex called chromatin, which resembles “beads on a string” (Figure 3.9). Next, chromatin is further coiled into a chromosome. Another important feature of DNA is that chromosomes can be altered from tightly coiled (chromatin) to loosely coiled (euchromatin). Most of the time, chromosomes in the nucleus remain in a euchromatin state so that DNA sequences are accessible for regulatory processes to occur.
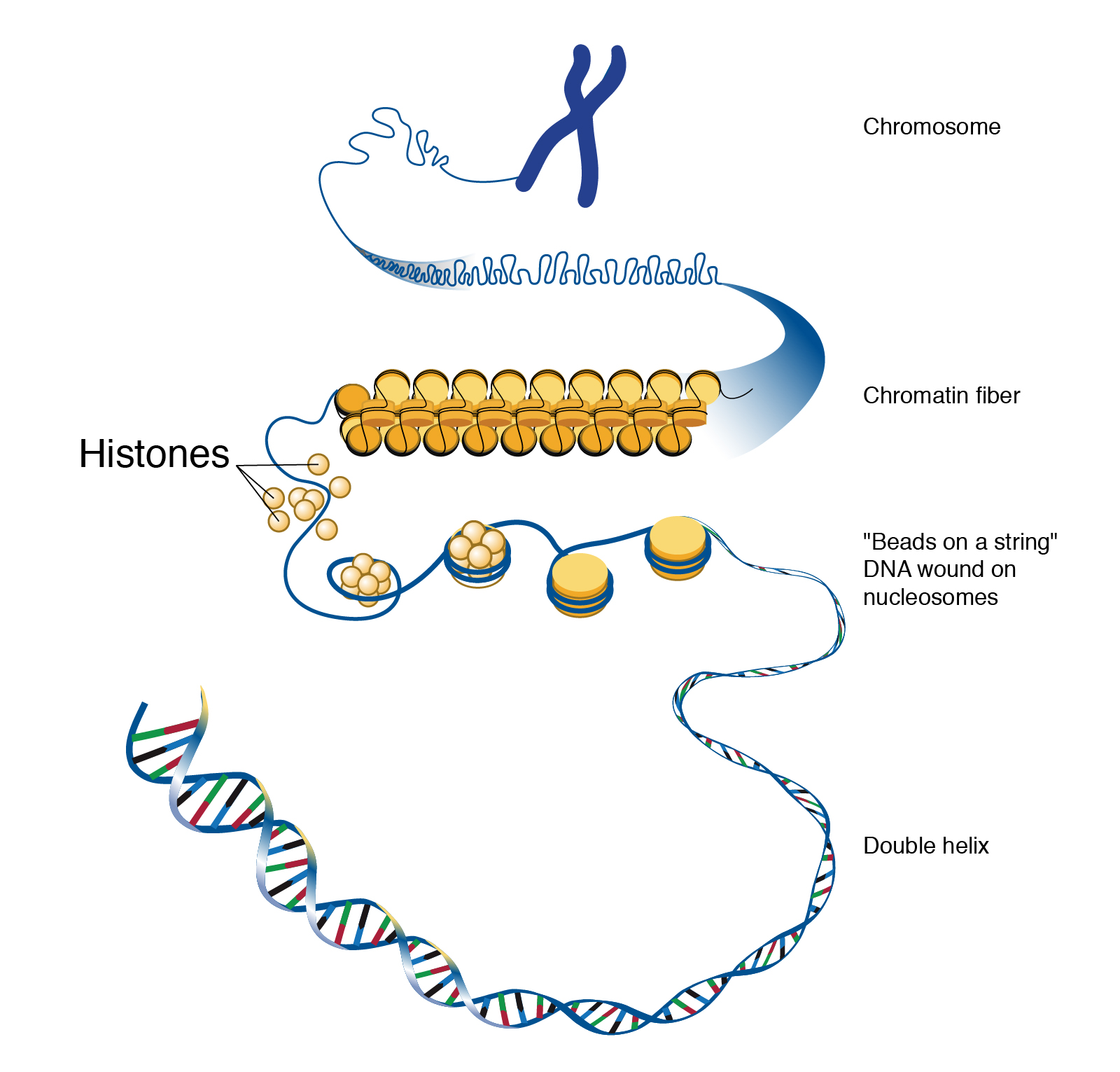
Human body cells typically have 23 pairs of chromosomes, for a total of 46 chromosomes in each cell’s nucleus. An interesting fact is that the number of chromosomes an organism possesses varies by species, and this figure is not dependent upon the size or complexity of the organism. For instance, chimpanzees have a total of 48 chromosomes, while hermit crabs have 254. Chromosomes also have a distinct physical structure, including centromeres (the “center”) and telomeres (the ends) (Figure 3.10). Because of the centromeric region, chromosomes are described as having two different “arms,” where one arm is long and the other is shorter. Centromeres play an important role during cell division, which will be discussed in the next section. Telomeres are located at the ends of chromosomes; they help protect the chromosomes from degradation after every round of cell division.
Special Topic: First Nation Immunity and European Diseases—A Study of Ancient DNA

Beginning in the early fifteenth century, First Nations progressively suffered from high mortality rates as the result of colonization from foreign powers. European-borne diseases such as measles, tuberculosis, influenza, and smallpox are largely responsible for the population collapse of Indigenous peoples in the Americas. Many Europeans who immigrated to the Americas had lived in large sedentary populations, which also included coexisting with domestic animals and pests. Although a few prehistoric Indigenous populations can be characterized as large agricultural societies (especially in Mesoamerica), their overall culture, community lifestyle, and subsistence practices were markedly different from that of Europeans. Therefore, because they did not share the same urban living environments as Europeans, it is believed that Indigenous peoples were susceptible to many European diseases.
In 2016, a Nature article published by John Lindo and colleagues was the first to investigate whether pre-contact Indigenous peoples possessed a genetic susceptibility to European diseases. Their study included Tsimshians, a First Nation community from British Columbia (Figure 3.11a-b). DNA from both present-day and ancient individuals (who lived between 500 and 6,000 years ago) was analyzed. The research team discovered that a change occurred in the HLA-DQA1 gene, which is a member of the major histocompatibility complex (MHC) immune system molecules. MHC molecules are responsible for detecting and triggering an immune response against pathogens. Lindo and colleagues (2016) concluded that HLA-DQA1 gene helped Indigenous peoples adapt to their local environmental ecology. However, when European-borne epidemics occurred in the Northwest during the 1800s, a certain HLA-DQA1 DNA sequence variant (allele) associated with ancient Tsimshian immunity was no longer adaptive. As the result of past selective pressures from European diseases, present-day Tsimshians have different HLA-DQA1 allele frequencies. The precise role that HLA-DQA1 plays in immune adaptation requires further investigation. But overall, this study serves as an example of how studying ancient DNA from the remains of deceased individuals can help provide insight into living human populations and historical events.
DNA Replication and Cell Division
For life to continue and flourish, cells must be able to divide. Tissue growth and cellular damage repair are also necessary to maintain an organism throughout its life. All these rely on the dynamic processes of DNA replication and the cell cycle. The mechanisms highlighted in this section are tightly regulated and represent only part of the life cycle of a cell.
DNA Replication
DNA replication is the process by which new DNA is copied from an original DNA template. It is one phase of the highly coordinated cell cycle, and it requires a variety of enzymes with special functions. The creation of a complementary DNA strand from a template strand is described as semi-conservative replication. The result of semi-conservative replication is two separate double-stranded DNA molecules, each of which is composed of an original “parent” template strand and a newly synthesized “daughter” DNA strand.
DNA replication progresses in three steps referred to as initiation, elongation, and termination. During initiation, enzymes are recruited to specific sites along the DNA sequence (Figure 3.12). For example, an initiator enzyme, called helicase, “unwinds” DNA by breaking the hydrogen bonds between the two parent strands. The unraveling of the helix into two separated strands exposes the strands and creates a fork, which is the active site of DNA replication.
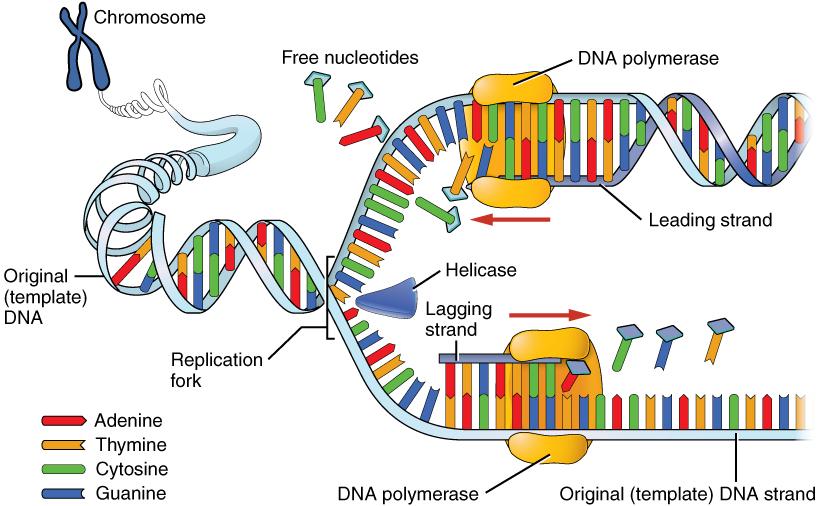
Elongation is the assembly of new DNA daughter strands from the exposed original parent strands. The two parent strands can further be classified as leading strand or lagging strand and are distinguished by the direction of replication. Enzymes called DNA polymerases read parent template strands in a specific direction. Complementary nucleotides are added, and the newly formed daughter strands will grow. On the leading parent strand, a DNA polymerase will create one continuous strand. The lagging parent strand is created in several disconnected sections and other enzymes fill in the missing nucleotide gaps between these sections.
Finally, termination refers to the end of DNA replication activity. It is signaled by a stop sequence in the DNA that is recognized by machinery at the replication fork. The end result of DNA replication is that the number of chromosomes are doubled so that the cell can divide into two.
DNA Mutations
DNA replication should result in the creation of two identical DNA nucleotide sequences. However, although DNA polymerases are quite precise during DNA replication, copying mistakes are estimated to occur every 107 DNA nucleotides. Variation from the original DNA sequence is known as a mutation (Refer to Chapter 4). Briefly, mutations can result in single-nucleotide changes, as well as the insertion or deletion of nucleotides and repeated sequences. Depending on where they occur in the genome, mutations can be deleterious (harmful). For example, mutations may occur in regions that control cell cycle regulation, which can result in cancer (see Special Topic: The Cell Cycle and Immortality of Cancer Cells). Many other types of mutations, however, are not harmful to an organism.
Regardless of their effect, the cell attempts to reduce the frequency of mutations that occur during DNA replication. To accomplish this, there are polymerases with proofreading capacities that can identify and correct mismatched nucleotides. These safeguards reduce the frequency of DNA mutations so that they only occur every 109 nucleotides.
Mitotic Cell Division
There are two types of cells in the body: germ cells (sperm and egg) and somatic cells. The body and its various tissues comprises somatic cells. Organisms that contain two sets of chromosomes in their somatic cells are called diploid organisms. Humans have 46 chromosomes and they are diploid because they inherit one set of chromosomes (n = 23) from each parent. As a result, they have 23 matching pairs of chromosomes, which are known as homologous chromosomes. As seen in Figure 3.13, homologous chromosome pairs vary in size and are generally numbered from largest (chromosome 1) to smallest (chromosome 22) with the exception of the 23rd pair, which is made up of the sex chromosomes (X and Y). Typically, the female sex is XX and the male sex is XY. Individuals inherit an X chromosome from their chromosomal mother and an X or Y from their chromosomal father.
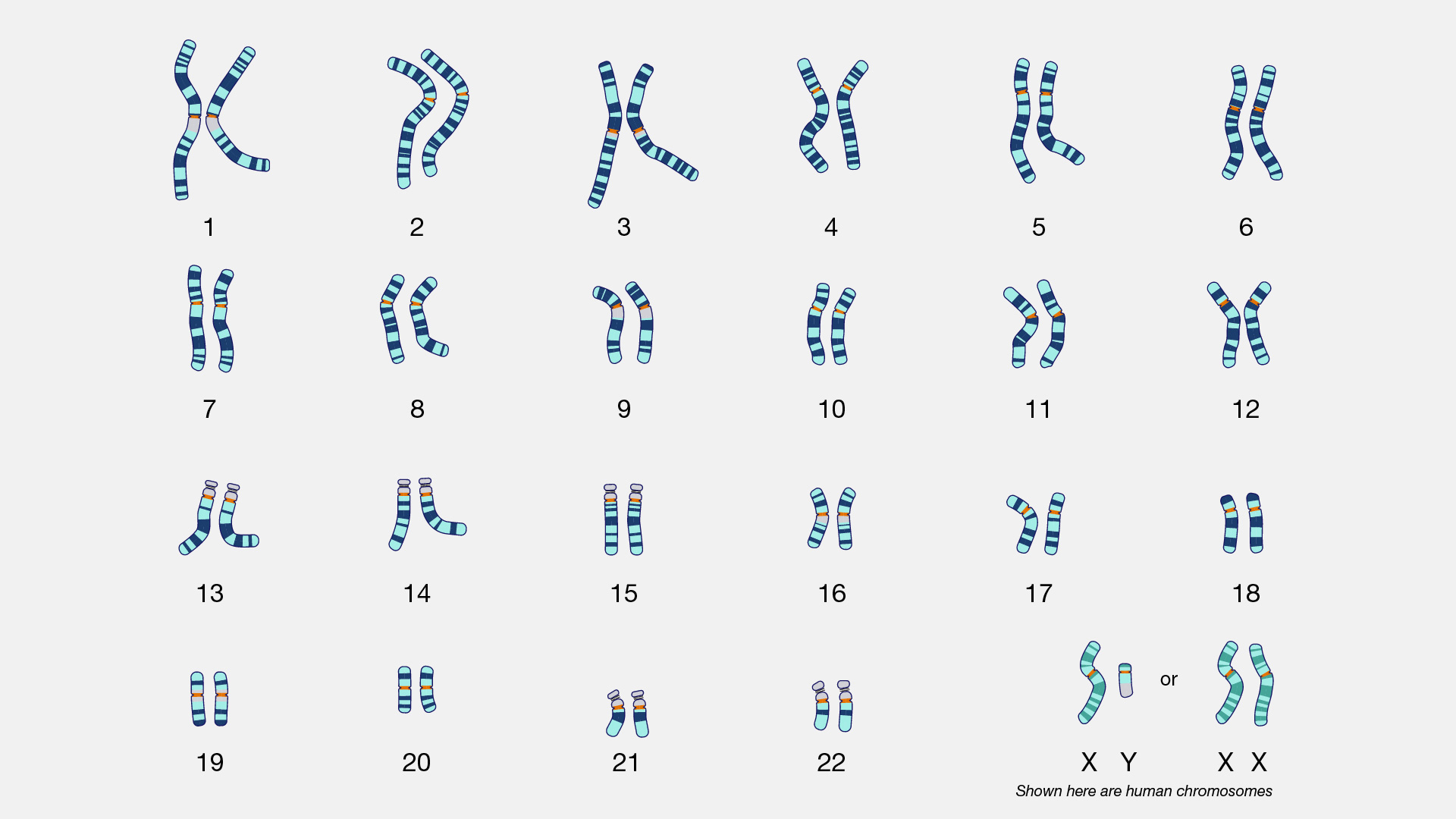
To grow and repair tissues, somatic cells must divide. As discussed previously, for cell division to occur, a cell must first replicate its genetic material. During DNA replication, each chromosome produces double the amount of genetic information. The duplicated arms of chromosomes are known as sister chromatids, and they are attached at the centromeric region. To elaborate, the number of chromosomes stays the same (n = 46); however, the amount of genetic material is doubled in the cell as the result of replication.
Mitosis is the process of somatic cell division that gives rise to two diploid daughter cells (Figure 3.14). Once DNA and other organelles in the cell have finished replication, mitotic spindle fibers physically align each chromosome at the center of the cell. Next, the spindle fibers divide the sister chromatids and move each one to opposite sides of the cell. At this phase, there are 46 chromosomes on each side of a human cell. The cell can now divide into two fully separated daughter cells.
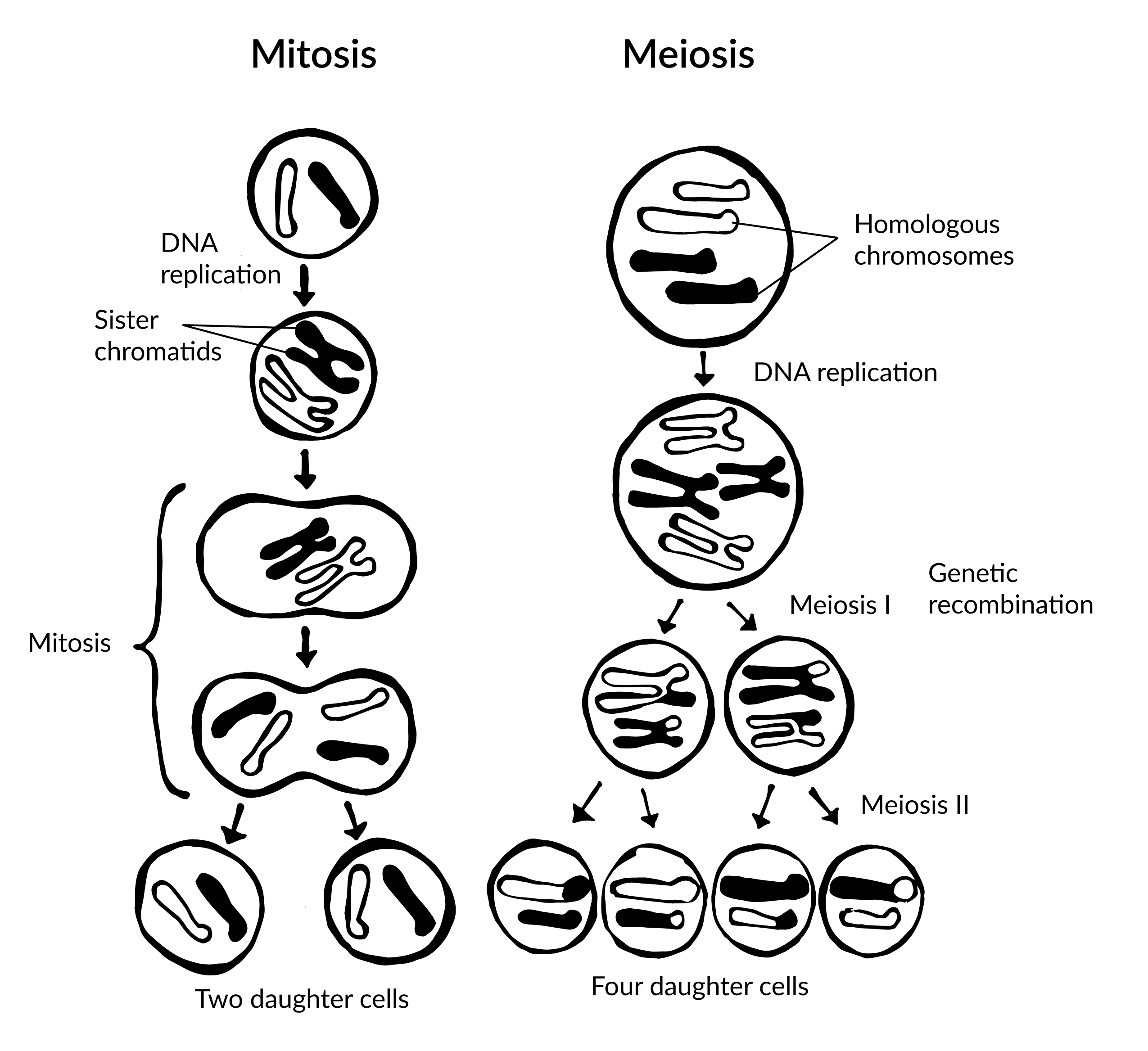
Meiotic Cell Division
Gametogenesis is the production of gametes (sperm and egg cells); it involves two rounds of cell division called meiosis. Similar to mitosis, the parent cell in meiosis is diploid. However, meiosis has a few key differences, including the number of daughter cells produced (four cells, which require two rounds of cell division to produce) and the number of chromosomes each daughter cell has (see Figure 3.14).
During the first round of division (known as meiosis I), each chromosome (n = 46) replicates its DNA so that sister chromatids are formed. Next, with the help of spindle fibers, homologous chromosomes align near the center of the cell and sister chromatids physically swap genetic material. In other words, the sister chromatids of matching chromosomes cross over with each other at matching DNA nucleotide positions. The occurrence of homologous chromosomes crossing over, swapping DNA, and then rejoining segments is called genetic recombination. The “genetic shuffling” that occurs in gametes increases organismal genetic diversity by creating new combinations of genes on chromosomes that are different from the parent cell. Genetic mutations can also arise during recombination. For example, there may be an unequal swapping of genetic material that occurs between the two sister chromatids, which can result in deletions or duplications of DNA nucleotides. Once genetic recombination is complete, homologous chromosomes are separated and two daughter cells are formed.
The daughter cells after the first round of meiosis are haploid, meaning they only have one set of chromosomes (n = 23). During the second round of cell division (known as meiosis II), sister chromatids are separated and two additional haploid daughter cells are formed. Therefore, the four resulting daughter cells have one set of chromosomes (n = 23), and they also have a genetic composition that is not identical to the parent cells nor to each other.
Although both sperm and egg gamete production undergo meiosis, they differ in the final number of viable daughter cells. In the case of spermatogenesis, four mature sperm cells are produced. Although four egg cells are also produced in oogenesis, only one of these egg cells will result in an ovum (mature egg). During fertilization, an egg cell and sperm cell fuse, which creates a diploid cell that develops into an embryo. The ovum also provides the cellular organelles necessary for embryonic cell division. This includes mitochondria, which is why humans, and most other multicellular eukaryotes, have the same mtDNA sequence as their mothers.
Chromosomal Disorders: Aneuploidies
During mitosis or meiosis, entire deletions or duplications of chromosomes can occur due to error. For example, homologous chromosomes may fail to separate properly, so one daughter cell may end up with an extra chromosome while the other daughter cell has one less. Cells with an unexpected (or abnormal) number of chromosomes are known as aneuploid. Adult or embryonic cells can be tested for chromosome number (karyotyping). Aneuploid cells are typically detrimental to a dividing cell or developing embryo, which can lead to a loss of pregnancy. However, the occurrence of individuals being born with three copies of the 21st chromosome is relatively common; this genetic condition is known as Down Syndrome. Moreover, individuals can also be born with aneuploid sex chromosome conditions such as XXY, XXX, and XO (referring to only one X chromosome).
Special Topic: The Cell Cycle and Immortality of Cancer Cells
DNA replication is part of a series of preparatory phases that a cell undergoes prior to cell division, collectively known as interphase (Figure 3.15). During interphase, the cell not only doubles its chromosomes through DNA replication, but it also increases its metabolic capacity to provide energy for growth and division. Transition into each phase of the cell cycle is tightly controlled by proteins that serve as checkpoints. If a cell fails to pass a checkpoint, then DNA replication and/or cell division will not continue. Some of the reasons why a cell may fail at a checkpoint is DNA damage, lack of nutrients to continue the process, or insufficient size. In turn, a cell may undergo apoptosis, which is a mechanism for cell death.
Unchecked cellular growth is a distinguishing hallmark of cancer. In other words, as cancer cells grow and proliferate, they acquire the capacity to avoid death and replicate indefinitely. This uncontrolled and continuous cell division is also known as “immortality.” As previously mentioned, most cells lose the ability to divide due to shortening of telomeres on the ends of chromosomes over time. One way in which cancer cells retain replicative immortality is that the length of their telomeres is continuously protected. Chemotherapy, often used to treat cancer, targets the cell cycle (especially cell division) to halt the propagation of genetically abnormal cells. Another therapeutic approach that continues to be investigated is targeting telomere activity to stop the division of cancer cells.
Researchers have exploited the immortality of cancer cells for molecular research. The oldest immortal cell line is HeLa cells (Figure 3.16), which were harvested from Henrietta Lacks, an African American woman diagnosed with cervical cancer in 1955. At that time, extracted cells frequently died during experiments, but surprisingly HeLa cells continued to replicate. Propagation of Lacks’s cell line has significantly contributed to medical research, including contributing to ongoing cancer research and helping to test the polio vaccine in the 1950s. However, Lacks had not given her consent for her tumor biopsy to be used in cell culture research. Moreover, her family was unaware of the extraction and remarkable application of her cells for two decades. The history of HeLa cell origin was first revealed in 1976. The controversy voiced by the Lacks family was included in an extensive account of HeLa cells published in Rebecca Skloot’s 2010 book, The Immortal Life of Henrietta Lacks. A film based on the book was also released in 2017 (Wolfe 2017).
Protein Synthesis
At the beginning of the chapter, we defined proteins as strings of amino acids that fold into complex 3-D shapes. There are 20 standard amino acids that can be strung together in different combinations in humans, and the result is that proteins can perform an impressive amount of different functions. For instance, muscle fibers are proteins that help facilitate movement. A special class of proteins (immunoglobulins) help protect the organism by detecting disease-causing pathogens in the body. Protein hormones, such as insulin, help regulate physiological activity. Blood hemoglobin is a protein that transports oxygen throughout the body. Enzymes are also proteins, and they are catalysts for biochemical reactions that occur in the cell (e.g., metabolism). Larger-scale protein structures can be visibly seen as physical features of an organism (e.g., hair and nails).
Transcription and Translation
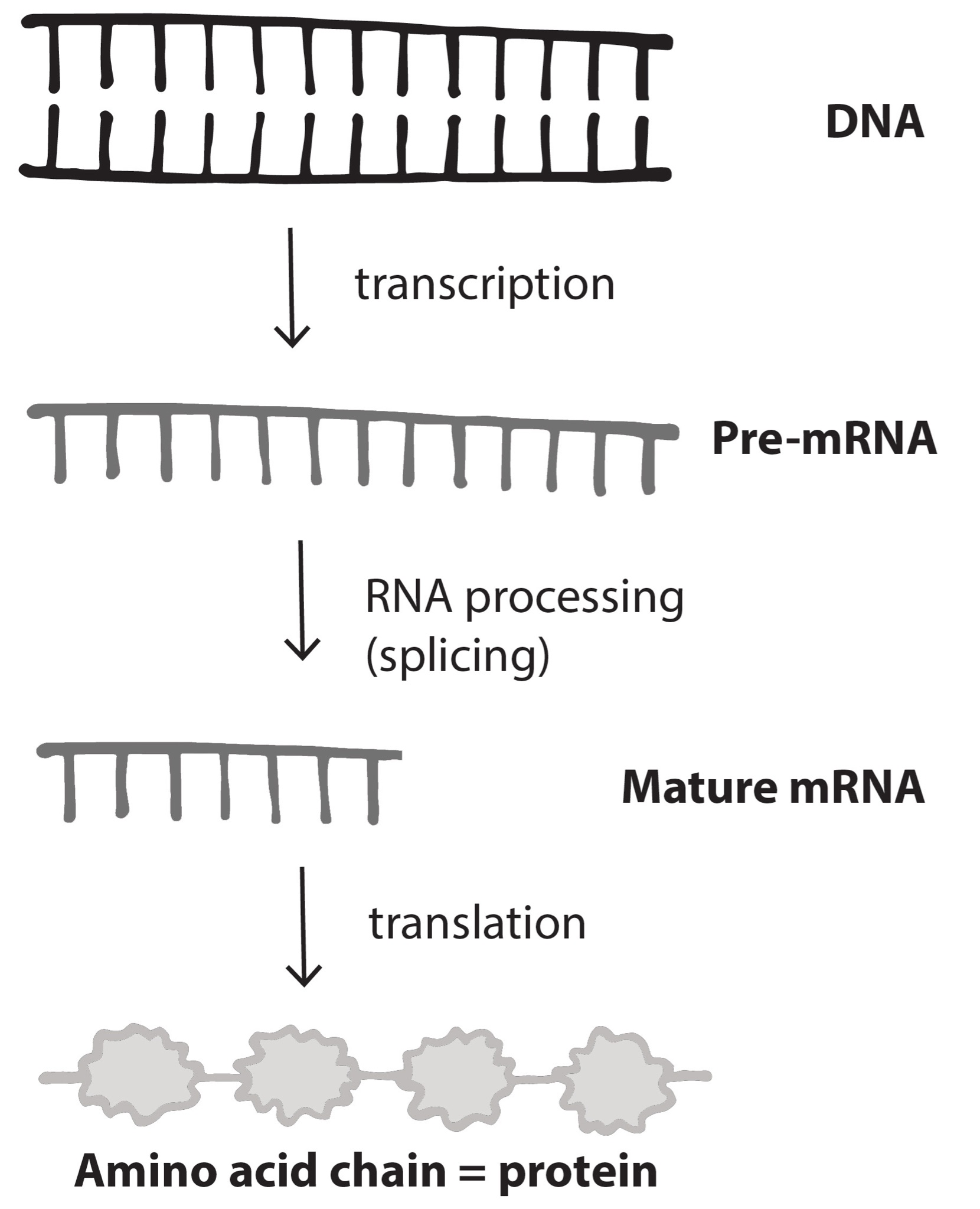
Nucleotides in our DNA provide the coding instructions on how to make proteins. Making proteins, also known as protein synthesis, can be broken down into two main steps referred to as transcription and translation. The purpose of transcription, the first step, is to make an ribonucleic acid (RNA) copy of our genetic code. Although there are many different types of RNA molecules that have a variety of functions within the cell, we will mainly focus on messenger RNA (mRNA). Transcription concludes with the processing (splicing) of the mRNA. The second step, translation, uses mRNA as the instructions for chaining together amino acids into a new protein molecule (Figure 3.17).
Unlike double-stranded DNA, RNA molecules are single-stranded nucleotide sequences (Figure 3.18). Additionally, while DNA contains the nucleotide thymine (T), RNA does not—instead its fourth nucleotide is uracil (U). Uracil is complementary to (or can pair with) adenine (A), while cytosine (C) and guanine (G) continue to be complementary to each other.
For transcription to proceed, a gene must first be turned “on” by the cell. A gene is a segment of DNA that codes for RNA, and genes can vary in length from a few hundred to as many as two million base pairs in length. The double-stranded DNA is then separated, and one side of the DNA is used as a coding template that is read by RNA polymerase. Next, complementary free-floating RNA nucleotides are linked together (Figure 3.19) to form a single-stranded mRNA. For example, if a DNA template is TACGGATGC, then the newly constructed mRNA sequence will be AUGCCUACG.
Genes contain segments called introns and exons. Exons are considered “coding” while introns are considered “noncoding”—meaning the information they contain will not be needed to construct proteins. When a gene is first transcribed into pre-mRNA, introns and exons are both included (Figure 3.20). However, once transcription is finished, introns are removed in a process called splicing. During splicing, a protein/RNA complex attaches itself to the pre-mRNA. Next, introns are removed and the remaining exons are connected, thus creating a shorter mature mRNA that serves as a template for building proteins.
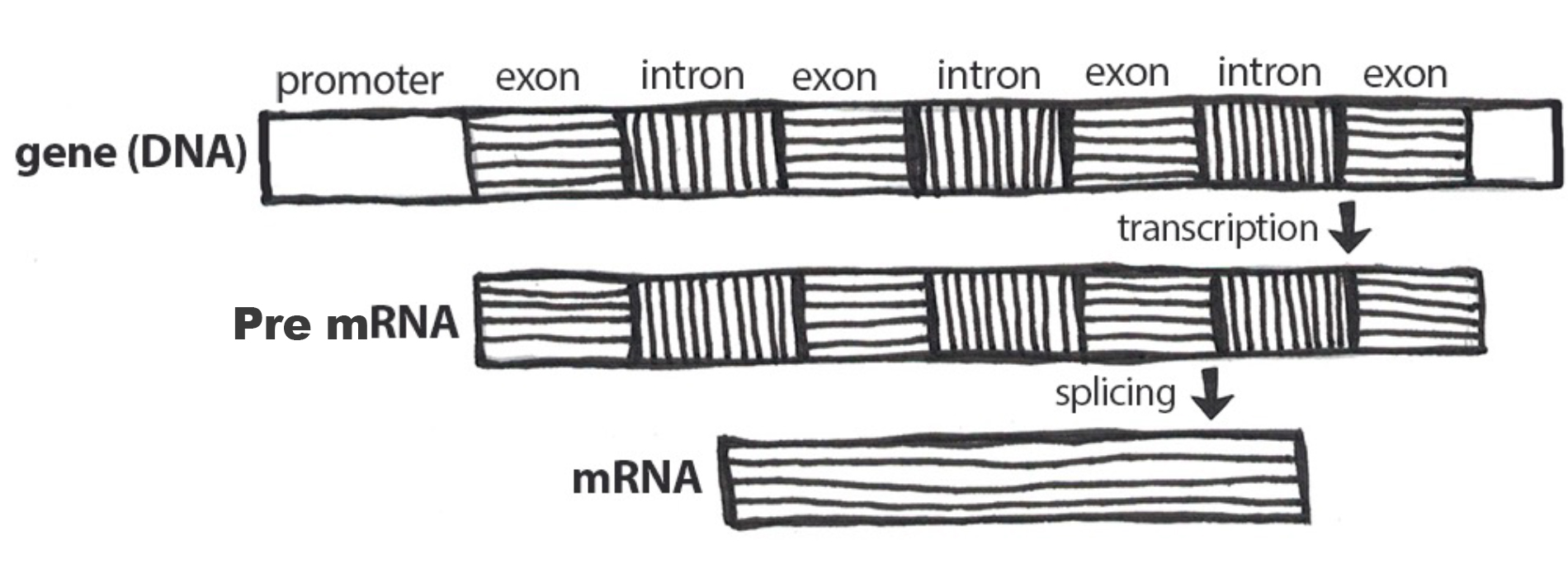
As described above, the result of transcription is a single-stranded mRNA copy of a gene. Translation is the process by which amino acids are chained together to form a new protein. During translation, the mature mRNA is transported outside of the nucleus, where it is bound to a ribosome (Figure 3.21). The nucleotides in the mRNA are read in triplets, which are called codons. Each mRNA codon corresponds to an amino acid, which is carried to the ribosome by a transfer RNA (tRNA). Thus, tRNAs is the link between the mRNA molecule and the growing amino acid chain.
Continuing with our mRNA sequence example from above, the mRNA sequence AUG-CCU-ACG codes for three amino acids. Using a codon table (Figure 3.22), AUG is a codon for methionine (Met), CCU is proline (Pro), and ACG is threonine (Thr). Therefore, the protein sequence is Met-Pro-Thr. Methionine is the most common “start codon” (AUG) for the initiation of protein translation in eukaryotes. As the ribosome moves along the mRNA, the growing amino acid chain exits the ribosome and folds into a protein. When the ribosome reaches a “stop” codon (UAA, UAG, or UGA), the ribosome stops adding any new amino acids, detaches from the mRNA, and the protein is released. Depending upon the amino acid sequence, a linear protein may undergo additional “folding.” The final three-dimensional protein shape is integral to completing a specific structural or functional task.
Dig Deeper: Protein Synthesis
To see protein synthesis in animation, please check out the From DNA to Protein video on YourGenome.org.
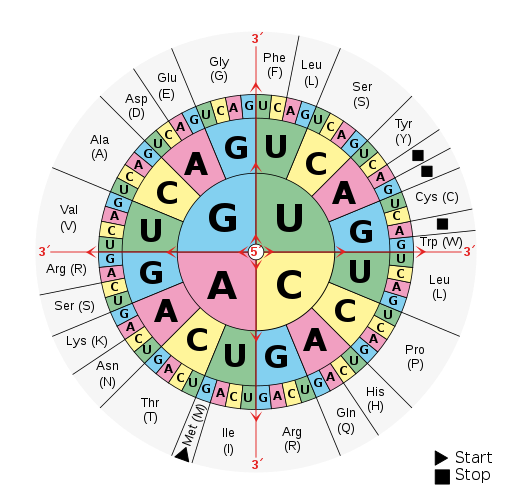
Mendelian Genetics

Gregor Johann Mendel (1822–1884) is often described as the “Father of Genetics.” Mendel was a monk who conducted pea plant breeding experiments in a monastery located in the present-day Czech Republic (Figure 3.23). After several years of experiments, Mendel presented his work to a local scientific community in 1865 and published his findings the following year. Although his meticulous effort was notable, the importance of his work was not recognized for another 35 years. One reason for this delay in recognition is that his findings did not agree with the predominant scientific viewpoints on inheritance at the time. For example, it was believed that parental physical traits “blended” together and offspring inherited an intermediate form of that trait. In contrast, Mendel showed that certain pea plant physical traits (e.g., flower color) were passed down separately to the next generation in a statistically predictable manner. Mendel also observed that some parental traits disappeared in offspring but then reappeared in later generations. He explained this occurrence by introducing the concept of “dominant” and “recessive” traits. Mendel established a few fundamental laws of inheritance, and this section reviews some of these concepts. Moreover, the study of traits and diseases that are controlled by a single gene is commonly referred to as Mendelian genetics.
The physical appearance of a trait is called an organism’s phenotype. Figure 3.24 shows pea plant (Pisum sativum) phenotypes that were studied by Mendel, and in each of these cases the physical traits are controlled by a single gene. In the case of Mendelian genetics, a phenotype is determined by an organism’s genotype. A genotype consists of two gene copies, wherein one copy was inherited from each parent. Gene copies are also known as alleles (Figure 3.25), which means they are found in the same gene location on homologous chromosomes. Alleles have a nonidentical DNA sequence, which means their phenotypic effect can be different. In other words, although alleles code for the same trait, different phenotypes can be produced depending on which two alleles (i.e., genotypes) an organism possesses. For example, Mendel’s pea plants all have flowers, but their flower color can be purple or white. Flower color is therefore dependent upon which two color alleles are present in a genotype.
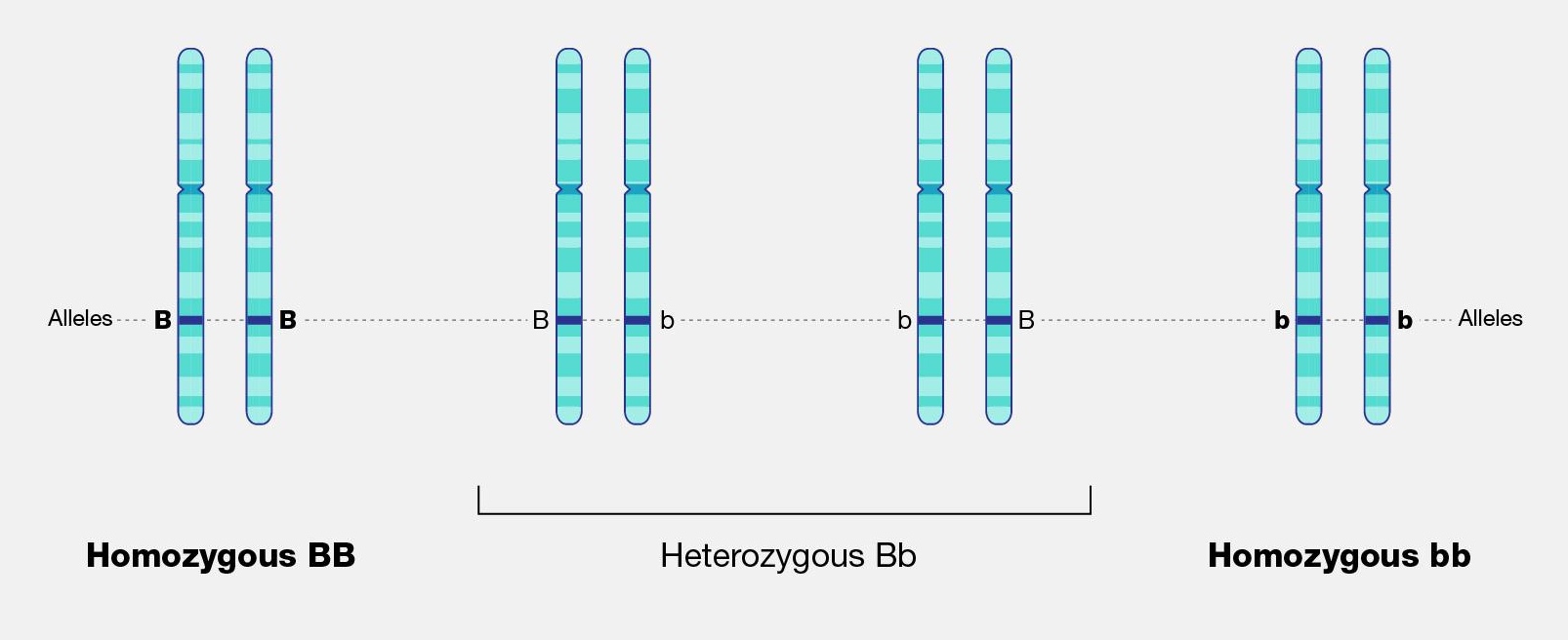
A Punnett square is a diagram that can help visualize Mendelian inheritance patterns. For instance, when parents of known genotypes mate, a Punnett square can help predict the ratio of Mendelian genotypes and phenotypes that their offspring would possess. When discussing genotype, biologists use upper and lower case letters to denote the different allele copies. Figure 3.26 is a Punnett square that includes two heterozygous parents for flower color (Bb). A heterozygous genotype means there are two different alleles for the same gene. Therefore, a pea plant that is heterozygous for flower color has one purple allele and one white allele. When an organism is homozygous for a specific trait, it means their genotype consists of two copies of the same allele. Using the Punnett square example, the two heterozygous pea plant parents can produce offspring with two different homozygous genotypes (BB or bb) or offspring that are heterozygous (Bb).
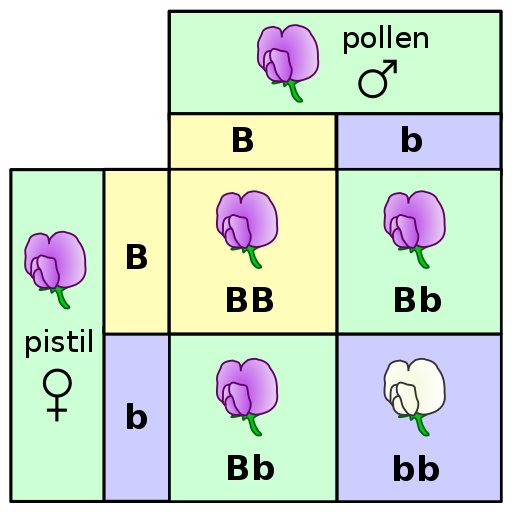
A pea plant with purple flowers could be heterozygous (Bb) or homozygous (BB). This is because the purple color allele (B) is dominant to the white color allele (b), and therefore it only needs one copy of that allele to phenotypically express purple flowers. Because the white flower allele is recessive, a pea plant must be homozygous for the recessive allele in order to have a white color phenotype (bb). As seen by the Punnett square example (Figure 3.26), three of four offspring will have purple flowers and the other one will have white flowers.
The Law of Segregation was introduced by Mendel to explain why we can predict the ratio of genotypes and phenotypes in offspring. As discussed previously, a parent will have two alleles for a certain gene (with each copy on a different homologous chromosome). The Law of Segregation states that the two copies will be segregated from each other and will each be distributed to their own gamete. We now know that the process where that occurs is meiosis.
Offspring are the products of two gametes combining, which means the offspring inherits one allele from each gamete for most genes. When multiple offspring are produced (like with pea plant breeding), the predicted phenotype ratios are more clearly observed. The pea plants Mendel studied provide a simplistic model to understand single-gene genetics. While many traits anthropologists are interested in have a more complicated inheritance (e.g., are informed by many genes), there are a few known Mendelian traits in humans. Additionally, some human diseases also follow a Mendelian pattern of inheritance (Figure 3.27). Because humans do not have as many offspring as other organisms, we may not recognize Mendelian patterns as easily. However, understanding these principles and being able to calculate the probability that an offspring will have a Mendelian phenotype is still important.
| Mendelian disorder | Gene |
| Alpha Thalassemia | HBA1 |
| Cystic Fibrosis | CFTR |
| Fragile X Syndrome | FMR1 |
| Glucose-6-Phosphate Dehydrogenase Deficiency | G6PD |
| Hemophilia A | F8 |
| Huntington disease | HTT |
| Mitochondrial DNA Depletion Syndrome | TYMP |
| Oculocutaneous Albinism: Type 1 | TYR |
| Polycystic Kidney Disease | PKHD1 |
| Sickle-cell anemia | HBB |
| Spinal Muscular Atrophy: SMN1 Linked | SMN1 |
| Tay-Sachs Disease | HEXA |
| Wilson Disease | ATP7B |
Example of Mendelian Inheritance: The ABO Blood Group System
In 1901, Karl Landsteiner at the University of Vienna published his discovery of ABO blood groups. While conducting blood immunology experiments in which he combined the blood of individuals who possess different blood cell types, he observed an agglutination (clotting) reaction. The presence of agglutination implies there is an incompatible immunological reaction; no agglutination will occur in individuals with the same blood type. This work was clearly important because it resulted in a higher survival rate of patients who received blood transfusions. Blood transfusions from someone with a different type of blood causes agglutinations, and the resulting coagulated blood can not easily pass through blood vessels, resulting in death. Landsteiner received the Nobel Prize (1930) for his discovery and explaination of the ABO blood group system.
Blood cell surface antigens are proteins that coat the surface of red blood cells, and antibodies are specifically “against” or “anti” to the antigens from other blood types. Thus, antibodies are responsible for causing agglutination between incompatible blood types. Understanding the interaction of antigens and antibodies helps to determine ABO compatibility amongst blood donors and recipients. To better comprehend blood phenotypes and ABO compatibility, blood cell antigens and plasma antibodies are presented in Figure 3.28. Individuals that are blood type A have A antigens on the red blood cell surface, and anti-B antibodies, which will bind to B antigens should they come in contact. Alternatively, individuals with blood type B have B antigens and anti-A antibodies. Individuals with blood type AB have both A and B antigens but do not produce antibodies for the ABO system. This does not mean type AB does not have any antibodies present, just that specifically anti-A and anti-B antibodies are not produced. Individuals who are blood type O have nonspecific antigens and produce both anti-A and anti-B antibodies.
Figure 3.29 shows a table of the ABO allele system, which has a Mendelian pattern of inheritance. Both the A and B alleles function as dominant alleles, so the A allele always codes for the A antigen, and the B allele codes for the B antigen. The O allele differs from A and B, because it codes for a nonfunctional antigen protein, which means there is no antigen present on the cell surface of O blood cells. To have blood type O, two copies of the O allele must be inherited, one from each parent, thus the O allele is considered recessive. Therefore, someone who is a heterozygous AO genotype is phenotypically blood type A, and a genotype of BO is blood type B. The ABO blood system also provides an example of codominance, which is when both alleles are observed in the phenotype. This is true for blood type AB: when an individual inherits both the A and B alleles, then both A and B antigens will be present on the cell surface.
Also found on the surface of red blood cells is the rhesus group antigen, known as “Rh factor.” In reality, there are several antigens on red blood cells independent from the ABO blood system, however, the Rh factor is the second most important antigen to consider when determining blood donor and recipient compatibility. Rh antigens must also be considered when a pregnant mother and her baby have incompatible Rh factors. In such cases, a doctor can administer necessary treatment steps to prevent pregnancy complications and hemolytic disease, which is when the mother’s antibodies break down the newborn’s red blood cells.
An individual can possess the Rh antigen (be Rh positive) or lack the Rh antigen (be Rh negative). The Rh factor is controlled by a single gene and is inherited independently of the ABO alleles. Therefore, all blood types can either be positive (O+, A+, B+, AB+) or negative (O-, A-, B-, AB-).
Individuals with O+ red blood cells can donate blood to A+, B+, AB+, and O+ blood type recipients. Because O- individuals do not have AB or Rh antigens, they are compatible with all blood cell types and are referred to as “universal donors.” Individuals that are AB+ are considered to be “universal recipients” because they do not possess antibodies against other blood types.
Mendelian Patterns of Inheritance and Pedigrees
A pedigree can be used to investigate a family’s medical history by determining if a health issue is inheritable and will possibly require medical intervention. A pedigree can also help determine if it is a Mendelian recessive or dominant genetic condition. Figure 3.30 is a pedigree example of a family with Huntington’s disease, which has a Mendelian dominant pattern of inheritance. In a standard pedigree, males are represented by a square and females are represented by a circle. Biological family members are connected to a horizontal line, with biological parents above and offspring below. When an individual is affected with a certain condition, the square or circle is filled in as a solid color. With a dominant condition, at least one of the parents will have the disease and an offspring will have a 50% chance of inheriting the affected chromosome. Therefore, dominant genetic conditions tend to be present in every generation. In the case of Huntington’s, some individuals may not be diagnosed until later in adulthood, so parents may unknowingly pass this dominantly inherited disease to their children.
Because the probability of inheriting a disease-causing recessive allele is more rare, recessive medical conditions can skip generations. Figure 3.31 is an example of a family that carries a recessive cystic fibrosis mutation. A parent that is heterozygous for the cystic fibrosis allele has a 50% chance of passing down their affected chromosome to the next generation. If a child has a recessive disease, then it means both of their parents are carriers (heterozygous) for that condition. In most cases, carriers for recessive conditions show no serious medical symptoms. Individuals whose family have a known medical history for certain conditions sometimes seek family planning services (see the Genetic Testing section).
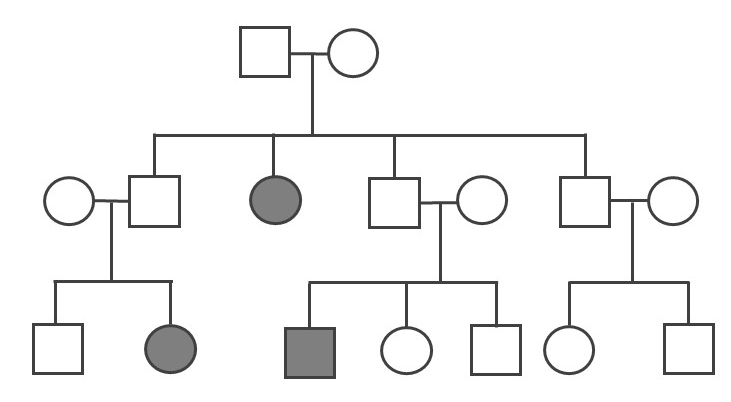
Pedigrees can also help distinguish if a health issue has either an autosomal or X-linked pattern of inheritance. As previously discussed, there are 23 pairs of chromosomes and 22 of these pairs are known as autosomes. The provided pedigree examples (Figure 3.30–31) are autosomally linked genetic diseases. This means the genes that cause the disease are on one of the chromosomes numbered 1 to 22. The conditions caused by genes located on the X chromosome are referred to as X-linked diseases.
Figure 3.32 depicts a family in which the mother is a carrier for the X-linked recessive disease Duchenne Muscular Dystrophy (DMD). The mother is a carrier for DMD, so daughters and sons will have a 50% chance of inheriting the pathogenic DMD allele. Because females have two X chromosomes, females who inherit only one copy will not have the disease (although in rare cases, female carriers may show some symptoms of the disease). On the other hand, males who inherit a copy of an X-linked pathogenic DMD allele will typically be affected with the condition. Thus, males are more susceptible to X-linked conditions because they only have one X chromosome. Therefore, when evaluating a pedigree, if a higher proportion of males are affected with the disease, this could suggest the disease is X-linked recessive.
Compared to the X chromosome, the Y chromosome is smaller with only a few genes. Y-linked traits are therefore rare and can only be passed from a chromosomal father to a biological XY child.
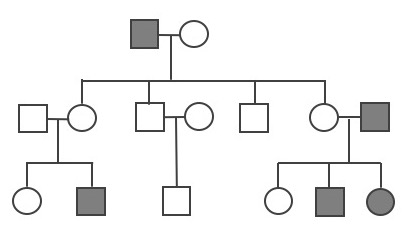
Other Patterns of Inheritance
Complexity Surrounding Mendelian Inheritance
Pea plant trait genetics are relatively simple compared to what we know about genetic inheritance today. The vast majority of genetically controlled traits are not strictly dominant or recessive, so the relationship among alleles and predicting phenotype is often more complicated. For example, traits that exhibit incomplete dominance occur when a heterozygote exhibits a phenotype that is an intermediate phenotype of both alleles. In snapdragon flowers, the red flower color (R) is dominant and white is recessive (r). Therefore, the homozygous dominant RR is red and homozygous recessive rr is white. However, because the R allele is not completely dominant, the heterozygote Rr is a blend of red and white, which results in a pink flower (Figure 3.33).

An example of incomplete dominance in humans is the enzyme β-hexosaminidase A (Hex A), which is encoded by the gene HEXA. Patients with two dysfunctional HEXA alleles are unable to metabolize a specific lipid-sugar molecule (GM2 ganglioside); because of this, the molecule builds up and causes damage to nerve cells in the brain and spinal cord. This condition is known as Tay-Sachs disease, and it usually appears in infants who are three to six months old. Most children with Tay-Sachs do not live past early childhood. Individuals who are heterozygous for the functional type HEXA allele and one dysfunctional allele have reduced Hex A activity. However, the amount of enzyme activity is still sufficient, so carriers do not exhibit any neurological phenotypes and appear healthy.
Some genes and alleles can also have higher penetrance than others. Penetrance can be defined as the proportion of individuals who have a certain allele and also express an expected phenotype. If a genotype always produces an expected phenotype, then those alleles are said to be fully penetrant. However, in the case of incomplete (or reduced) penetrance, an expected phenotype may not occur even if an individual possesses the alleles that are known to control a trait or cause a disease.
A well-studied example of genetic penetrance is the cancer-related genes BRCA1 and BRCA2. Mutations in these genes can affect crucial processes such as DNA repair, which can lead to breast and ovarian cancers. Although BRCA1 and BRCA2 mutations have an autosomal dominant pattern of inheritance, it does not mean an individual will develop cancer if they inherit a pathogenic allele. Several lifestyle and environmental factors can also influence the risk for developing cancer. Regardless, if a family has a history of certain types of cancers, then it is often recommended that genetic testing be performed for individuals who are at risk. Moreover, publically available genetic testing companies are now offering health reports that include BRCA1 and BRCA2 allele testing (see the Genetic Testing section).
Polygenic Traits
While Mendelian traits tend to be influenced by a single gene, the vast majority of human phenotypes are polygenic traits. The term polygenic means “many genes.” Therefore, a polygenic trait is influenced by many genes that work together to produce the phenotype. Human phenotypes such as hair color, eye color, height, and weight are examples of polygenic traits. Hair color, for example, is largely determined by the type and quantity of a pigment called melanin, which is produced by a specialized cell type within the skin called melanocytes. The quantity and ratio of melanin pigments determine black, brown, blond, and red hair colors. MC1R is a well-studied gene that encodes a protein expressed on the surface of melanocytes that is involved in the production of eumelanin pigment. Typically, people with two functional copies of MC1R have brown hair. People with reduced functioning MC1R allele copies tend to produce pheomelanin, which results in blond or red hair. However, MC1R alleles have variable penetrance, and studies are continually identifying new genes (e.g., TYR, TYRP1, SLC24A5, and KITLG) that also influence hair color. Individuals with two nonfunctioning copies of the gene TYR have a condition called oculocutaneous albinism—their melanocytes are unable to produce melanin so these individuals have white hair, light eyes, and pale skin.
In comparison to Mendelian diseases, complex diseases (e.g., Type II diabetes, coronary heart disease, Alzheimer's, and schizophrenia) are more prevalent in humans. Complex diseases are polygenic, but their development is also influenced by physical, environmental, sociocultural, and individual lifestyle factors. Families can be more predisposed to certain diseases; however, complex diseases often do not have a clear pattern of inheritance.
Although research of complex traits and diseases continue, geneticists may not know all of the genes involved with a given complex disease. Additionally, how much genetic versus nongenetic determinants contribute to a disease phenotype can be difficult to decipher. Therefore, predicting individual medical risk and risk across different human populations is often a significant challenge. For instance, cardiovascular diseases (CVDs) continue to be one of the leading causes of death around the world. Development of CVDs has been linked to nutrient exposure during fetal development, high fat and sedentary lifestyles, drug usage, adverse socioeconomic conditions, and various genes. Human environments are diverse, and public health research including the field of Human Biology can help identify risk factors and behaviors associated with chronic diseases. Large-scale clinical genetic studies with powerful bioinformatic approaches can also help elucidate some of these complex relationships.
Genomics and Epigenetics
A genome is all of the genetic material of an organism. In the case of humans, this includes 46 chromosomes and mtDNA. The human genome contains approximately three billion base pairs of DNA and has regions that are both noncoding and coding. Scientists now estimate that the human genome contains 20,000–25,000 protein-coding genes, with each chromosome containing a few hundred to a few thousand genes. As our knowledge of heredity increases, researchers have begun to realize the importance of epigenetics, or changes in gene expression that do not result in a change of the underlying DNA sequence. Epigenetics research is also crucial for unraveling gene regulation, which involves complex interactions between DNA, RNA, proteins, and the environment.
Genomics
The vast majority of the human genome is noncoding, meaning there are no instructions to make a protein or RNA product in these regions. Historically, noncoding DNA was referred to as “junk DNA” because these vast segments of the genome were thought to be irrelevant and nonfunctional. However, continual improvement of DNA sequencing technology along with worldwide scientific collaborations and consortia have contributed to our increased understanding of how the genome functions. Through these technological advances and collaborations, we have since discovered that many of these noncoding DNA regions are involved in dynamic genetic regulatory processes.
Genomics is a diverse field of molecular biology that focuses on genomic evolution, structure, and function; gene mapping; and genotyping (determining the alleles present). Evolutionary genomics determined that humans share about 98.8% percent of their DNA with chimpanzees. Given the phenotypic differences between humans and chimpanzees, having a DNA sequence difference of 1.2% seems surprising. However, a lot of genomics research is also focused on understanding how noncoding genomic regions influence how individual genes are turned “on” and “off” (i.e., regulated). Therefore, although DNA sequences are identical, regulatory differences in noncoding genetic regions (e.g., promoters) are believed to be largely responsible for the physical differences between humans and chimpanzees.
Further understanding of genomic regulatory elements can lead to new therapies and personalized treatments for a broad range of diseases. For example, targeting the regulatory region of a pathogenic gene to “turn off” its expression can prevent its otherwise harmful effects. Such molecular targeting approaches can be personalized based on an individual’s genetic makeup. Genome-wide association studies (GWAS), which seek to determine genes that are linked to complex traits and diseases, typically require significant computational efforts. This is because millions of DNA sequences must be analyzed and GWAS sometimes include thousands of participants. During the beginning of the genomics field, most of the large-scale genomics studies only included North American, European, and East Asian participants and patients. Researchers are now focusing on increasing ethnic diversity in genomic studies and databases. In turn, accuracy of individual disease risk across all human populations will be improved and more rare disease–causing alleles will be identified.
Epigenetics
All cells within your body have the same copy of DNA. For example, a brain neuron has the same DNA blueprint as does a skin cell on your arm. Although these cells have the same genetic information, they are considered specialized. The reason all cells within the body have the same DNA but different morphologies and functions is that different subsets of genes are turned “on” and “off” within the different cell types. A more precise explanation is that there is differential expression of genes among different cell types. In the case of neuronal cells, a unique subset of genes are active that allow them to grow axons to send and receive messages. This subset of genes will be inactive in non-neuronal cell types such as skin cells. Epigenetics is a branch of genetics that studies how these genes are regulated through mechanisms that do not change the underlying DNA sequence.
The prefix epi- means “on, above, or near,” and epigenetic mechanisms such as DNA methylation and histone modifications occur on, above, or near DNA. The addition of a methyl group (— CH₃) to DNA is known as DNA methylation (Figure 3.34). DNA methylation and other modifications made to the histones around which DNA are wrapped are thought to make chromatin more compact. This DNA is inaccessible to transcription factors and RNA polymerases, thus preventing genes from being turned on (i.e., transcribed). Other histone modifications have the opposite effect by loosening chromatin, which makes genes accessible to transcription factors.
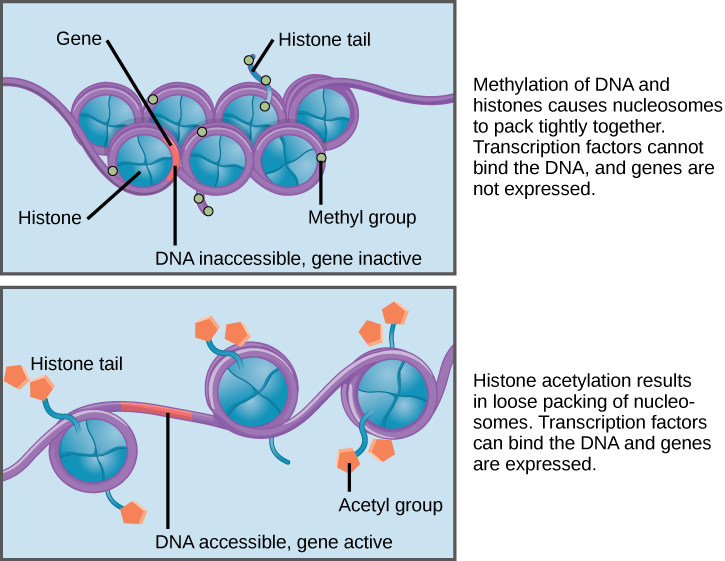
It is important to note that environmental factors can alter DNA methylation and histone modifications and also that these changes can be passed from generation to generation. For example, someone’s epigenetic profile can be altered during a stressful time (e.g., natural disasters, famine, etc.), and those regulatory changes can be inherited by the next generation. Moreover, our epigenetic expression profile changes as we age. For example, certain places in our genome become “hyper” or “hypo” methylated over time. Identical twins also have epigenetic profiles that become more different as they age. Researchers are only beginning to understand the significance of these genome-wide epigenetic changes. Scientists have also discovered that changes in epigenetic modifications can alter gene expression in ways that contribute to diseases. It is also important to note that, unlike DNA mutations (which permanently change the nucleotide sequence), epigenetic changes can be easily reversed. A lot of research now focuses on how drugs can alter or modulate changes in DNA methylation and histone modifications to treat diseases such as cancer.
Environmental Disruptors and Their Impact on Human Reproductive Systems
The National Institute of Environmental Health Sciences (NIEHS) defines endocrine-disrupting chemicals (EDCs) as synthetic or natural compounds that interfere with the body’s hormonal systems. Found in pesticides, plastics, industrial chemicals, and pollutants, EDCs can mimic, block, or alter the natural action of hormones (NIEHS, 2024). Their effects on reproductive health are profound, particularly during critical developmental windows while also affecting subsequent generations through epigenetic changes.
NIEHS declared EDC’s:
| Atrazine | one of the most commonly applied herbicides in the world, often used to control weeds in corn, sorghum, and sugarcane crops. |
| Bisphenol A (BPA) | used to make polycarbonate plastics and epoxy resins. It is used in manufacturing, food packaging, toys, and other applications. BPA resins may be found in the lining of some canned foods and beverages. |
| Dioxins | a byproduct of certain manufacturing processes, such as herbicide production and paper bleaching. They can be released into the air from waste burning and wildfires. |
| Perchlorate | a colorless salt manufactured and used as an industrial chemical to make rockets, explosives, and fireworks, which can be found in some groundwater. |
| Polyfluoroalkyl Substances (PFAS) | a large group of chemicals used widely in industrial applications, such as firefighting foam, nonstick pans, paper, and textile coatings. |
| Phthalates | a large group of compounds used as liquid plasticizers. They are found in hundreds of products including some food packaging, cosmetics, fragrances, children’s toys, and medical device tubing. Cosmetics that may contain phthalates include nail polish, hair spray, aftershave lotion, cleanser, and shampoo. |
| Phytoestorgens | naturally occurring substances with hormone-like activity found in some plants; they may have a similar effect to estrogen produced by the body. Soy foods, for example, contain phytoestrogens. |
| Polybrominated diphenyl ethers (PBDE) | used to make flame retardants for products such as furniture foam and carpet. |
| Polychlorinated biphenyls (PCBs) | used to make electrical equipment, such as transformers, and are in hydraulic fluids, heat transfer fluids, lubricants, and plasticizers. PCBs were mass-produced globally until they were banned in 1979. |
| Triclosan | an ingredient that was previously added to some antimicrobial and personal care products, like liquid body wash and soaps. |
The Male Reproductive System: Vulnerabilities, Epigenetics, and Disruptions
The male reproductive system is highly sensitive to hormonal interference, especially during prenatal and early postnatal development. Over the past 50 years, epidemiological data gathered by the NIEHS has revealed alarming changes: increased cases of prostate and testicular cancers, male-descended testes, and anatomical malformations of male genitalia (Sweeney et al., 2015). These changes are accompanied by a global decline in sperm quality, underscoring the widespread vulnerability of male reproductive health to environmental factors. The testes, as the site of sperm production and testosterone synthesis, are particularly susceptible to EDC interference. Proper testicular development depends on tightly regulated hormonal signalling, which EDCs can disrupt by mimicking or blocking hormones like testosterone and estrogen, leading to improper testicular formation and increased risk of testicular cancer. Prostate development is also a target for EDC interference. African American men, for example, exhibit twice the risk of developing prostate cancer than Caucasian men. This disparity has been attributed to hereditary, lifestyle, and environmental factors, often causing elevated maternal estrogen levels during gestation. This prenatal exposure to EDCs can mimic estrogen and predispose developing prostate tissues to cancerous changes in adulthood (2015).
The Female Reproductive System: Epigenetics and Fertility Challenges
Female fertility relies on a delicate hormonal balance to regulate processes such as ovulation, implantation, and pregnancy. EDCs can disrupt this balance by mimicking, antagonizing, or altering the action of hormones. Their interference contributes to a wide range of reproductive disorders, including early puberty, premature ovarian failure, anovulation, and infertility. Epigenetics plays a central role in female reproductive health. DNA methylation, histone modifications, and ncRNA generation are crucial for regulating ovarian and uterine function; However, EDCs can affect these regulatory mechanisms. An example of this is primordial germ cells (PGCs) in female embryos, which need to undergo extensive epigenetic reprogramming during development (Biswas et al., 2021). This process erases genomic imprinting and reactivates the inactive X chromosome, creating a "blank slate" for the next generation; however, EDCs can disrupt this critical period of epigenetic resetting, leading to long-term consequences for reproductive health.
The ovarian follicle–the functional unit of female reproduction–is particularly vulnerable to these chemicals. Being exposed to EDCs can deplete the pool of these follicles, leading to temporary or permanent infertility (2021). Additionally, EDCs interfere with estrogen receptor function, a crucial regulator of female reproductive processes. These chemicals bind to these receptors, altering the recruitment of enzymes involved in histone modification and chromatin remodelling; this disrupts gene expression patterns critical for ovarian and uterine health. One striking example is diethylstilbestrol (DES), a synthetic estrogen once prescribed to pregnant women (2021). DES exposure has been linked to ovarian cancer in subsequent generations, highlighting the transgenerational effects of EDCs on the female reproductive system. In severe cases, EDCs induce multigenerational reproductive disorders, as observed in studies linking DES to ovarian cancer in the grandchildren of exposed individuals.
Epigenetic Therapy
Heritable Changes and Some Related Drugs
As has been said, epigenetics involves heritable changes in gene expression, without involving DNA alteration. These changes, being heritable and often involving abnormal DNA methylation patterns within the four DNA methyltransferases (DNMTs) or histone modifications in chromatin, can lead to disease development. DNMTs (DNMT1, DNMT2, DNMT3A, and DNMT3B) have functions specific to themselves and are at the core of the DNA methylation process. Regarding the histone modifications mentioned, histones have been recognized to mutate under various mechanisms, such as acetylation, methylation and phosphorylation. The acetylation of histones involves histone acetyltransferases (HATs), which are associated with the activation of gene transcription. This process is reversed by the deacetylation of histones, which is associated with the silencing of gene transcription under histone deacetylases (HDACs). (Peedicayil, 2006)
Epigenetic therapy, with the use of specialized drug developments, aims to correct epigenetic defects, which are reversible under pharmacological intervention, by targeting enzymes such as HATs, HDACs and DNMTs, as well as histone methyltransferases. For instance, certain drugs are being developed as DNMT inhibitors, stopping the methylation of DNA associated with inappropriate transcriptional silencing of genes, and potentially increasing haemoglobin F to help patients affected by sickle cell anemia. These DNMT inhibitor drugs have been classified under three categories based on their structures: nucleoside analogue DNMT inhibitors, non-nucleoside analogue DNMT inhibitors, and antisense oligonucleotides (2006). Nucleoside analogue DNMT inhibitors are analogues of cytosine, the nucleotide affected by methylation from DNMTs, and are incorporated into replicating DNA, replacing cytosine, thus being S-phase-specific drugs. Non-nucleoside analogue DNMT inhibitors are researched to reduce the myelotoxic effects of drugs directly incorporated into the DNA, and are brought into the patient differently. Antisense oligonucleotides are drugs made up of sequences of nucleotides complementary to mRNAs, made to block translation, by acting on the DNMT1 for instance. Additionally, drugs such as HDAC inhibitors help maintain the acetylation of histones, leading to apoptosis, growth arrest or differentiation of tumour cells, giving this drug an anticancer effect, suppressing tumour growth. (2006)
Implications with Cancers
Research published in The Indian Journal for Medical Research has shown that these drugs show promising results in cancer treatment trials involving solid tumours and hematological malignancies. However, they have limitations, for instance, the fact that DNMT and HDAC inhibitors could activate oncogenes due to limited specificity, leading to further tumor progression; or their high myelotoxicity levels, a side effect thought to be due to their incorporation into DNA, and nucleotide analogue inhibitors (2006). Though that is the case, it is important to know that epigenetic drugs alone or in combination with conventional anticancer drugs, may prove to be a significant advance over the use of conventional anticancer drugs, and may also be a way to prevent diseases. Additionally, combination therapy strategies targeting various epigenetic markers, such as DNMTs for cancer-related genes and non-selective HDAC inhibitors, have been shown to yield promising results, simultaneously inducing the expression of tumor suppressor genes and inhibiting the expression of key oncogenes. As recently explored by researchers in Cell Death Discovery, this specific case of combination therapy would synergistically induce gene expression while maintaining the selectivity required to increase targeting of particular tumor types based on gene expression profiles. (Yu et al., 2024)
To date, the majority of cases in which epigenetic defects have led to disease pathogenesis are cancers (Peedicayil, 2006), cancer cells often developing due to uncontrolled cell growth and resistance to cell death mechanisms, made possible with abnormal DNA methylation patterns as well as histone modifications (Yu et al., 2024). Epigenetic alterations have therefore been identified within the core of tumor progression mechanisms in cancer cells, including tumorigenesis, promotion, progression, and recurrence, suggesting epigenetic heterogeneity at the cellular level (2024). Certain drugs have been developed, showing specifically good results for cancer treatments, by inhibiting enzymes such as KMTs and KDMs. These can be added to the growing list of drugs fitting into epigenetic therapy, including DNMT and HDAC inhibitors, as well as combination therapy treatments, for cancer and other diseases.
Purpose of Study and Future Developments
Studying the link between epigenetics and diseases is crucial for multiple reasons, one of which is enabling scientists and researchers to better understand disease mechanisms, detect abnormal epigenetic changes, and, in turn, develop more effective treatments or possibly even prevent diseases from developing in the first place. As previously mentioned, epigenetic therapy has been shown to bring promising results in drug trials surrounding cancer treatments. Still, the range of diseases to be treated with this new pharmacology approach is vast, molecules other than DNMTs and HDACs being related to epigenetic mechanisms within gene expression, such as BET proteins and KDMs, potentially being a source of new medications or treatments (Yu et al., 2024; Peedicayil, 2006). Additionally, by understanding someone's epigenetic profile, a form of personalized “precision medicine” (Yu et al., 2024, p. 8) is developed, offering less toxic and more effective treatments with fewer undesired side effects. Researchers expanding this field of knowledge would be able to understand, in more concrete terms, how external factors are linked to epigenetic changes and, consequently, disease risk, potentially halting disease progression and developing new prevention mechanisms. Personalized medicine combines both genetic and epigenetic data, including gene expression profiles, DNA methylation patterns, histone modification profiles, and identified biomarkers, to create precise disease management and prediction.
It is crucial to keep in mind that diseases like cancer are linked to major causes of morbidity and mortality worldwide, which could be reduced with therapeutic medicine such as epigenetic therapy, aiming to detect cancer biomarkers to improve risk assessment, diagnosis, and targeted treatment interventions, limiting the burden of chronic and life-threatening diseases. With the advancement of epigenetic therapies, new sequencing techniques, as well as AI (2024), have opened avenues to establish precision diagnostics and therapeutics for patients.
With this said, epigenetics is a relatively new area of scientific research. This field has exploded in the last few decades, especially with the advancement of technologies that allow researchers to examine DNA methylation patterns, histone modifications, and non-coding RNA molecules across the genome. While the potential of epigenetics in explaining complex diseases, including those linked to environmental factors such as endocrine-disrupting chemicals (EDCs), is immense, we’ve identified two key challenges. One major limitation is the complexity and variability of epigenetic marks. These modifications can differ significantly across cell types, tissues, and even individuals, making it difficult to generalize findings.
Additionally, epigenetic changes are dynamic and can fluctuate over time, which complicates the task of linking them to specific environmental exposures or health outcomes. Another challenge lies in the transgenerational aspect of epigenetics. While it's clear that epigenetic changes can be passed from one generation to the next, the mechanisms behind this inheritance are not fully understood. It's also difficult to pinpoint exactly when and how these modifications occur in development, especially since environmental exposures may affect individuals at different stages of their life, with varying effects depending on the timing and dose.
Special Topic: Epigenetics and X Chromosome Inactivation

Mary Lyon was a British geneticist who presented a hypothesis for X chromosome inactivation (called the Lyon hypothesis) based on her work and other studies of the day. Females inherit two X chromosomes, one from each parent. Males have one functional X chromosome; however, this does not mean females have more active genes than males. During the genetic embryonic development of many female mammals, one of the X chromosomes is inactivated at random, so females have one functional X chromosome. The process of X chromosome inactivation in females occurs through epigenetic mechanisms, such as DNA methylation and histone modifications. Recent studies have analyzed the role of a long noncoding RNA called X-inactive specific transcript (XIST), which is largely responsible for the random silencing of one of the X chromosomes. The presence of two X chromosomes is the signal for XIST RNA to be expressed so that one X chromosome can be inactivated. However, some cells may have an active paternal X chromosome while other cells may have an active maternal X chromosome. This phenomenon is easily seen in calico and tortoiseshell cats (Figure 3.35). In cats, the gene that controls coat color is found on the X chromosome. During early embryo development, random inactivation of X chromosomes gives rise to populations of cells that express black or orange, which results in the unique coat patterning. Therefore, calico cats are typically always female.
Genetic Testing
To assist with public health efforts, newborn screening for genetic diseases has been available in the United States for over 50 years. One of the first available genetic tests was to confirm a phenylketonuria (PKU) diagnosis in infants, which is easily treatable with a dietary change. Currently, each state decides what genes are included on newborn screening panels and some states even have programs to help with infant medical follow-ups. There are now hundreds of laboratories that provide testing for a few thousand different genes that can inform medical decisions for infants and adults. Moreover, genetic testing has been made available publicly to anyone without the assistance of medical professionals.
Clinical Testing
Clinical genetics tests assist patients with making medically informed decisions about family planning and health. Applications of this technology include assistance with in vitro fertilization (IVF) procedures, embryo genetic screening, and personalized medicine such as matching patients to cancer therapies. To ensure accuracy of patient genetic screening, it is important that all clinical laboratories are regulated. The Clinical Laboratory Improvement Amendments (CLIA) are United States federal standards that all human laboratory testing clinics must follow. A major benefit provided by some clinical genetic testing companies is access to genetic counselors, who have specialized education and training in medical genetics and counseling. For individuals with a family history of genetic disease, a physician may recommend genetic carrier screening to see if there is a risk for passing on a disease to a child. Genetic counselors provide expertise with interpretation of genetic testing results, as well as help guide and support patients when making impactful medical decisions.
Review Questions
- What is the purpose of DNA replication? Explain in a few sentences what happens during DNA replication. When do DNA mutations happen? And how does this create phenotypic variation (i.e., different phenotypes of the same physical trait)?
- Using your own words, what are homologous chromosomes and sister chromatids? What are the key differences between mitosis and meiosis?
- Determine if the pedigree diagram below (Figure 3.41) represents an autosomal dominant, autosomal recessive, or X-linked recessive pattern of inheritance. You should write the genotype (i.e., AA, Aa, or aa) above each square to help you (note: there may sometimes be two possible answers for a square’s genotype). Please also explain why you concluded a particular pattern of inheritance.
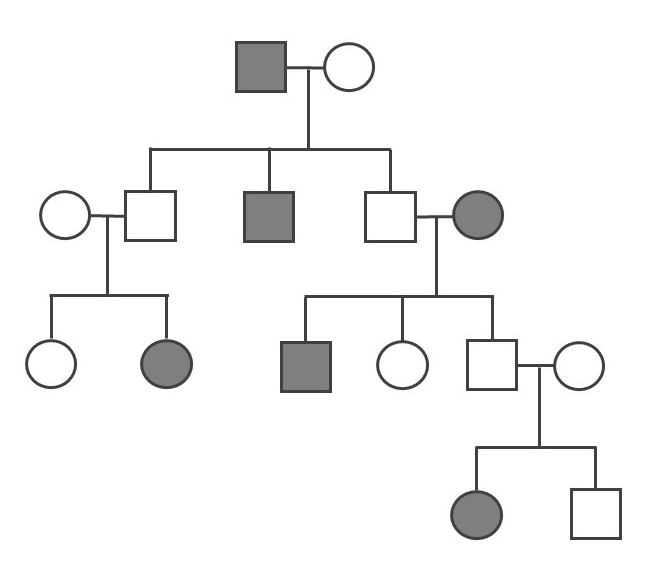
- Use base pairing rules to transcribe the following DNA template sequence into mRNA: GTAAAGGTGCTGGCCATC. Next, use the protein codon table (see Figure 3.21) to translate the sequence. In regard to transcription, explain what the significance is of the first and last codon/protein in the sequence.
- In your opinion, what do you think the benefits are of direct-to-consumer (DTC) genetic testing? What are the drawbacks and/or greater ethical concerns? Do you think benefits outweigh concerns?
- Imagine that you submit your DNA sample to a genetic testing company and among the various diseases for which they test, there is an allele that is associated with late-onset Alzheimer’s disease. You have the option to view your Alzheimer’s result or to not view your result. What do you do and why?
Key Terms
Adenosine triphosphate (ATP): A high-energy compound produced by mitochondria that powers cellular processes.
Allele: A nonidentical DNA sequence found in the same gene location on a homologous chromosome, or gene copy, that codes for the same trait but produces a different phenotype.
Amino acids: Organic molecules that are the building blocks of protein. Each of the 20 different amino acids have their own unique chemical property. Amino acids are chained together to form proteins.
Ancient DNA (aDNA): DNA that is extracted from organic remains and that often dates from hundreds to thousands of years ago. Also, aDNA is typically degraded (i.e., damaged) due to exposure to the elements such as heat, acidity, and humidity.
Aneuploid: A cell with an unexpected amount of chromosomes. The loss or gain of chromosomes can occur during mitotic or meiotic division.
Antibodies: Immune-related proteins that can detect and bind to foreign substances in the blood such as pathogens.
Apoptosis: A series of molecular steps that is activated leading to cell death. Apoptosis can be activated when a cell fails checkpoints during the cell cycle; however, cancer cells have the ability to avoid apoptosis.
Autosomal: Refers to a pattern of inheritance in which an allele is located on an autosome (non sex chromosome).
Base pairs: Chemical bonding between nucleotides. In DNA, adenine (A) pairs with thymine (T) and cytosine (C) pairs with guanine (G); in RNA, adenine (A) always pairs with uracil (U).
Carbohydrate: Molecules composed of carbon and hydrogen atoms that can be broken down to supply energy.
Carrier: An individual who has a heterozygous genotype that is typically associated with a disease.
Cell cycle: A cycle the cell undergoes with checkpoints between phases to ensure that DNA replication and cell division occur properly.
Cell surface antigen: A protein that is found on a red blood cell’s surface.
Centromere: A structural feature that is defined as the “center” of a chromosome and that creates two different arm lengths. This term also refers to the region of attachment for microtubules during mitosis and meiosis.
Chromatin: DNA wrapped around histone complexes. During cell division, chromatin becomes a condensed chromosome.
Chromosome: DNA molecule that is wrapped around protein complexes, including histones.
Codominance: The effects of both alleles in a genotype can be seen in the phenotype.
Codons: A sequence that comprises three DNA nucleotides that together code for a protein.
Complex diseases: A category of diseases that are polygenic and are also influenced by environment and lifestyle factors.
Cytoplasm: The “jelly-like” matrix inside of the cell that contains many organelles and other cellular molecules.
Deleterious: A mutation that increases an organism’s susceptibility to disease.
Deoxyribonucleic acid (DNA): A molecule that carries the hereditary information passed down from parents to offspring. DNA can be described as a “double helix”’ shape. It includes two chains of nucleotides held together by hydrogen bonds with a sugar phosphate backbone.
Diploid: Refers to an organism or cell with two sets of chromosomes.
DNA methylation: Methyl groups bind DNA, which modifies the transcriptional activity of a gene by turning it “on” or “off.”
DNA polymerase: Enzyme that adds nucleotides to existing nucleic acid strands during DNA replication. These enzymes can be distinguished by their processivity (e.g., DNA replication).
DNA replication: Cellular process in which DNA is copied and doubled.
DNA sequence: The order of nucleotide bases. A DNA sequence can be short, long, or representative of entire chromosomes or organismal genomes.
Dominant: Refers to an allele for which one copy is sufficient to be visible in the phenotype.
Elongation: The assembly of new DNA from template strands with the help of DNA polymerases.
Enzymes: Proteins responsible for catalyzing (accelerating) various biochemical reactions in cells.
Epigenetic profile: The methylation pattern throughout a genome—that is, which genes (and other genomic sites) are methylated and unmethylated.
Epigenetics: Changes in gene expression that do not result in a change of the underlying DNA sequence. These changes typically involve DNA methylation and histone modifications. These changes are reversible and can also be inherited by the next generation.
Euchromatin: Loosely coiled chromosomes found within the nucleus that are accessible for regulatory processing of DNA.
Eukaryote: Single-celled or multicelled organism characterized by a distinct nucleus, with each organelle surrounded by its own membrane.
Exon: Protein-coding segment of a gene.
Gametes: Haploid cells referred to as an egg and sperm that will fuse together during sexual reproduction to form a diploid organism.
Gene: Segment of DNA that contains protein-coding information and various regulatory (e.g., promoter) and noncoding (e.g., introns) regions.
Genetic recombination: A cellular process that occurs during meiosis I in which homologous chromosomes pair up and sister chromatids on different chromosomes physically swap genetic information.
Genome: All the genetic information of an organism.
Genotype: The combination of two alleles that code for or are associated with the same gene.
Genotyping: A molecular procedure that is performed to test for the presence of certain alleles or to discover new ones.
Germ cells: Specialized cells that form gametes (egg and sperm cells).
Haploid: Cell or organism with one set of chromosomes (n = 23).
Helicase: A protein that breaks the hydrogen bonds that hold double-stranded DNA together.
Heterozygous: Genotype that consists of two different alleles.
Histones: Proteins that DNA wraps around to assist with DNA organization within the nucleus.
Homologous chromosomes: A matching pair of chromosomes wherein one chromosome is maternally inherited and the other is paternally inherited.
Homozygous: Genotype that consists of two identical alleles.
Incomplete dominance: Heterozygous genotype that produces a phenotype that is a blend of both alleles.
Initiation: The recruitment of proteins to separate DNA strands and begin DNA replication.
Interphase: Preparatory period of the cell cycle when increased metabolic demand allows for DNA replication and doubling of the cell prior to cell division.
Introns: Segment of DNA that does not code for proteins.
Karyotyping: The microscopic procedure wherein the number of chromosomes in a cell is determined.
Lagging strand: DNA template strand that is opposite to the leading strand during DNA replication. This strand is created in several disconnected sections and other enzymes fill in the missing nucleotide gaps between these sections.
Leading strand: DNA template strand in which replication proceeds continuously.
Lipids: Fatty acid molecules that serve various purposes in the cell, including energy storage, cell signaling, and structure.
Meiosis: The process that gametes undergo to divide. The end of meiosis results in four haploid daughter cells.
Mendelian genetics: A classification given to phenotypic traits that are controlled by a single gene.
Messenger RNA (mRNA): RNA molecule that is transcribed from DNA. Its tri-nucleotide codons are “read” by a ribosome to build a protein.
Microarray technology: A genotyping procedure that utilizes a microarray chip, which is a collection of thousands of short nucleotide sequences attached to a solid surface that can probe genomic DNA.
Microbiome: The collective genomes of the community of microorganisms that humans have living inside of their bodies.
Mitochondrial DNA (mtDNA): Circular DNA segment found in mitochondria that is inherited maternally.
Mitochondrion: Specialized cellular organelle that is the site for energy production. It also has its own genome (mtDNA).
Mitosis: The process that somatic cells undergo to divide. The end of mitosis results in two diploid daughter cells.
Molecular anthropologists: Individuals who use molecular techniques (primarily genetics) to compare ancient and modern populations and to study living populations of humans and nonhuman primates.
Molecular geneticists: Biologists that study the structure and function of genes.
Mutation: A nucleotide sequence variation from the template DNA strand that can occur during replication. Mutations can also happen during recombination.
Next-generation sequencing: A genotyping technology that involves producing millions of nucleotide sequences (from a single DNA sample) that are then read with a sequencing machine. It can be used for analyzing entire genomes or specific regions and requires extensive program-based applications.
Nuclear envelope: A double-layered membrane that encircles the nucleus.
Nucleic acid: A complex structure (like DNA or RNA) that carries genetic information about a living organism.
Nucleotide: The basic structural component of nucleic acids, which includes DNA (A, T, C, and G) and RNA (A, U, C, and G).
Nucleus: Double-membrane cellular organelle that helps protect DNA and also regulates nuclear activities.
Organelle: A structure within a cell that performs specialized tasks that are essential for the cell. There are different types of organelles, each with its own function.
Pathogenic: A genetic mutation (i.e., allele) that has a harmful phenotypic disease-causing effect.
Pedigree: A diagram of family relationships that indicates which members may have or carry certain genetic and/or phenotypic traits.
Penetrance: The proportion of how often the possession of an allele results in an expected phenotype. Some alleles are more penetrant than others.
Phenotype: The physical appearance of a given trait.
Phospholipid bilayer: Two layers of lipids that form a barrier due to the properties of a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail.
Polygenic trait: A phenotype that is controlled by two or more genes.
Polymerase chain reaction (PCR): A molecular biology procedure that can make copies of genomic DNA segments. A small amount of DNA is used as a starting template and is then used to make millions of copies.
Prokaryote: A single-celled organism characterized by the lack of a nucleus and membrane-enclosed organelles.
Promoter: The region of a gene that initiates transcription. Transcription factors can bind and DNA methylation may occur at a promoter site, which can modify the transcriptional activities of a gene.
Protein: Chain of amino acids that folds into a three-dimensional structure that allows a cell to function in a variety of ways.
Protein synthesis: A multi-step process by which amino acids are strung together by RNA machinery read from a DNA template.
Recessive: Refers to an allele whose effect is not normally seen unless two copies are present in an individual’s genotype.
Ribonucleic acid (RNA): Single-stranded nucleic acid molecule.There are different RNAs found within cells and they perform a variety of functions, such as cell signaling and involvement in protein synthesis.
Ribosomal RNA (rRNA): A ribosome-bound molecule that is used to correctly assemble amino acids into proteins.
Ribosome: An organelle in the cell found in the cytoplasm or endoplasmic reticulum. It is responsible for reading mRNA and protein assemblage.
RNA polymerase: An enzyme that catalyzes the process of making RNA from a DNA template.
Sanger-sequencing: A process that involves the usage of fluorescently labeled nucleotides to visualize DNA (PCR fragments) at the nucleotide level.
Semi-conservative replication: DNA replication in which new DNA is replicated from an existing DNA template strand.
Sequencing: A molecular laboratory procedure that produces the order of nucleotide bases (i.e., sequences).
Sister chromatids: During DNA replication, sister chromatids are produced on the chromosome. In cell division, sister chromatids are pulled apart so that two cells can be formed. In meiosis, sister chromatids are also the sites of genetic recombination.
Somatic cells: Diploid cells that comprise body tissues and undergo mitosis for maintenance and repair of tissues.
Splicing: The process by which mature mRNAs are produced. Introns are removed (spliced) and exons are joined together.
Sugar phosphate backbone: A biochemical structural component of DNA. The “backbone” consists of deoxyribose sugars and phosphate molecules.
Telomere: A compound structure located at the ends of chromosomes to help protect the chromosomes from degradation after every round of cell division.
Termination: The halt of DNA replication activity that occurs when a DNA sequence “stop” codon is encountered.
Tissue: A cluster of cells that are morphologically similar and perform the same task.
Transcription: The process by which DNA nucleotides (within a gene) are copied, which results in a messenger RNA molecule.
Transcription factors: Proteins that bind to regulatory regions of genes (e.g., promoter) and increase or decrease the amount of transcriptional activity of a gene, including turning them “on” or “off.”
Transfer RNA (tRNA): RNA molecule involved in translation. Transfer RNA transports amino acids from the cell’s cytoplasm to a ribosome.
Translation: The process by which messenger RNA codons are read and amino acids are “chained together” to form proteins.
X-linked: Refers to a pattern of inheritance where the allele is located on the X or Y chromosome.
For Further Exploration
National Human Genome Research Institute
NOVA. 2018. Gene Sequencing Speeds Diagnosis of Deadly Newborn Diseases. NOVA, March 7, 2018. Accessed January 31, 2023. https://www.pbs.org/wgbh/nova/next/body/newborn-gene-sequencing/.
Zimmer, Carl. N.d. “Carl Zimmer’s Game of Genomes.” STATnews. Accessed January 31, 2023. https://www.statnews.com/feature/game-of-genomes/season-one/.
Illumina. 2016. “Illumina Sequencing by Synthesis.” YouTube.com, October 5, 2016. Accessed January 31, 2023. https://www.youtube.com/watch?v=fCd6B5HRaZ8.
References
Aartsma-Rus, Annemieke, Ieke B. Ginjaar, and Kate Bushby. 2016. “The Importance of Genetic Diagnosis for Duchenne Muscular Dystrophy.” Journal of Medical Genetics 53 (3): 145–151.
Acuna-Hidalgo, Rocio, Joris A. Veltman, and Alexander Hoischen. 2016. “New Insights into the Generation and Role of De Novo Mutations in Health and Disease.” Genome Biology 17 (241): 1–19.
Albert, Benjamin, Susanna Tomassetti, Yvonne Gloor, Daniel Dilg, Stefano Mattarocci, Slawomir Kubik, Lukas Hafner, and David Shore. 2019. "Sfp1 Regulates Transcriptional Networks Driving Cell Growth and Division through Multiple Promoter-Binding Modes." Genes & Development 33 (5–6): 288–293.
Almathen, Faisal, Haitham Elbir, Hussain Bahbahani, Joram Mwacharo, and Olivier Hanotte. 2018. “Polymorphisms in Mc1r and Asip Genes Are Associated with Coat Color Variation in the Arabian Camel.” Journal of Heredity 109 (6): 700–706.
Ballester, Leomar Y., Rajyalakshmi Luthra, Rashmi Kanagal-Shamanna, and Rajesh R. Singh. 2016. “Advances in Clinical Next-Generation Sequencing: Target Enrichment and Sequencing Technologies.” Expert Review of Molecular Diagnostics 16 (3): 357–372.
Baranovskiy, Andrey G., Vincent N. Duong, Nigar D. Babayeva, Yinbo Zhang, Youri I. Pavlov, Karen S. Anderson, and Tahir H. Tahirov. 2018. “Activity and Fidelity of Human DNA Polymerase Alpha Depend on Primer Structure.” Journal of Biological Chemistry 293 (18): 6824–6843.
Biswas, S., Ghosh, S., Das, S., & Maitra, S. (2021). Female Reproduction: At the Crossroads of Endocrine Disruptors and Epigenetics. Proceedings of the Zoological Society, 74(4), 532–545. https://doi.org/10.1007/s12595-021-00403-4
Brezina, Paulina R., Raymond Anchan, and William G. Kearns. 2016. “Preimplantation Genetic Testing for Aneuploidy: What Technology Should You Use and What Are the Differences?” Journal of Assisted Reproduction and Genetics 33 (7): 823–832.
Bultman, Scott J. 2017. “Interplay Between Diet, Gut Microbiota, Epigenetic Events, and Colorectal Cancer." Molecular Nutrition & Food Research 61 (1):1–12.
Cutting, Garry R. 2015. “Cystic Fibrosis Genetics: From Molecular Understanding to Clinical Application.” Nature Reviews Genetics 16 (1): 45–56.
D'Alessandro, Giuseppina., and Fabrizio d'Adda di Fagagna. 2017. “Transcription and DNA Damage: Holding Hands or Crossing Swords?” Journal of Molecular Biology 429 (21): 3215–3229.
De Craene, Johan-Owen, Dimitri L. Bertazzi, Séverine Bar, and Sylvie Friant. 2017. “Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways.” International Journal of Molecular Sciences 18 (3): 1–20.
Deng, Lian, and Shuhua Xu. 2018. “Adaptation of Human Skin Color in Various Populations.” Hereditas 155 (1): 1–12.
Dever, Thomas E., Terri G. Kinzy, and Graham D. Pavitt. 2016. “Mechanism and Regulation of Protein Synthesis in Saccharomyces Cerevisiae.” Genetics 203 (1): 65–107.
Eme, Laura, Anja Spang, Jonathan Lombard, Courtney W. Stairs, and Thijs J. G. Ettema. 2017. “Archaea and the Origin of Eukaryotes.” Nature Reviews Microbiology 15 (12): 711–723.
Gomez-Carballa, Alberto, Jacobo Pardo-Seco, Stefania Brandini, Alessandro Achilli, Ugo A. Perego, Michael D. Coble, Toni M. Diegoli, et al. 2018. “The Peopling of South America and the Trans-Andean Gene Flow of the First Settlers.” Genome Research 28 (6): 767–779.
Gvozdenov, Zlata, Janhavi Kolhe, and Brian C. Freeman. 2019. “The Nuclear and DNA-Associated Molecular Chaperone Network.” Cold Spring Harbor Perspectives in Biology 11 (10): a034009.
Harkins, Kelly M., and Anne C. Stone. 2015. “Ancient Pathogen Genomics: Insights into Timing and Adaptation.” Journal of Human Evolution 79: 137–149.
Jackson, Maria, Leah Marks, Gerhard H. W. May, and Joanna B. Wilson. 2018. “The Genetic Basis of Disease.” Essays in Biochemistry 62 (5): 643–723.
Lenormand, Thomas, Jan Engelstadter, Susan E. Johnston, Erik Wijnker, and Christopher R. Haag. 2016. “Evolutionary Mysteries in Meiosis.” Philosophical Transactions of the Royal Society B 371: 1–14.
Levy, Shawn E., and Richard M. Myers. 2016. “Advancements in Next-Generation Sequencing.” Annual Review of Genomics and Human Genetics 17: 95–115.
Lindo, John, Emilia Huerta-Sánchez, Shigeki Nakagome, Morten Rasmussen, Barbara Petzelt, Joycelynn Mitchell, Jerome S. Cybulski, et al. 2016. "A Time Transect of Exomes from a Native American Population Before and After European Contact." Nature Communications 7: 1–11. https://doi.org/10.1038/ncomms13175.
Lu, Mengfei, Cathryn M. Lewis, and Matthew Traylor. 2017. “Pharmacogenetic Testing through the Direct-to-Consumer Genetic Testing Company 23andme.” BMC Medical Genomics 10 (47): 1–8.
Ly, Lundi, Donovan Chan, Mahmoud Aarabi, Mylene Landry, Nathalie A. Behan, Amanda J. MacFarlane, and Jacquetta Trasler. 2017. “Intergenerational Impact of Paternal Lifetime Exposures to Both Folic Acid Deficiency and Supplementation on Reproductive Outcomes and Imprinted Gene Methylation.” Molecular Human Reproduction 23 (7): 461–477.
Ma, Wenxiu, Giancarlo Bonora, Joel B. Berletch, Xinxian Deng, William S. Noble, and Christine M. Disteche. 2018. “X-Chromosome Inactivation and Escape from X Inactivation in Mouse.” Methods in Molecular Biology 1861: 205–219.
Machiela, Mitchell J., Weiyin Zhou, Eric Karlins, Joshua N. Sampson, Neal D. Freedman, Qi Yang, Belynda Hicks, et al. 2016. “Female Chromosome X Mosaicism Is Age-Related and Preferentially Affects the Inactivated X Chromosome.” Nature Communications 7: 1–9. https://doi.org/10.1038/ncomms11843.
Mahdavi, Morteza, Mohammadreza Nassiri, Mohammad M. Kooshyar, Masoume Vakili-Azghandi, Amir Avan, Ryan Sandry, Suja Pillai, Alfred K. Lam, and Vinod Gopalan. 2019. “Hereditary Breast Cancer; Genetic Penetrance and Current Status with BRCA.” Journal of Cellular Physiology 234 (5): 5741–5750.
McDade, Thomas W., Calen P. Ryan, Meaghan J. Jones, Morgan K. Hoke, Judith Borja, Gregory E. Miller, Christopher W. Kuzawa, and Michael S. Kobor. 2019. “Genome-Wide Analysis of DNA Methylation in Relation to Socioeconomic Status During Development and Early Adulthood.” American Journal of Physical Anthropology 169 (1): 3–11.
Migeon, Barbara R. 2017. “Choosing the Active X: The Human Version of X Inactivation.” Trends in Genetics 33 (12): 899–909.
Myerowitz, Rachel. 1997. “Tay-Sachs Disease-Causing Mutations and Neutral Polymorphisms in the Hex A Gene.” Human Mutation 9 (3): 195–208.
National Institute of Environmental Health Sciences. (2024, July 22). Endocrine Disruptors. National Institute of Environmental Health Sciences; United States Government. https://www.niehs.nih.gov/health/topics/agents/endocrine
Onufriev, Alexey V., and Helmut Schiessel. 2019. “The Nucleosome: From Structure to Function through Physics.” Current Opinion in Structural Biology 56: 119–130.
Peedicayil J. (2006). Epigenetic therapy--a new development in pharmacology. The Indian journal of medical research, 123(1), 17–24.
Quillen, Ellen E., Heather L. Norton, Esteban J. Parra, Frida Lona-Durazo, Khai C. Ang, Florin M. Illiescu, Laurel N. Pearson, et al. 2019. “Shades of Complexity: New Perspectives on the Evolution and Genetic Architecture of Human Skin.” American Journal of Physical Anthropology 168 (67): 4–26.
Raspelli, Erica, and Roberta Fraschini. 2019. “Spindle Pole Power in Health and Disease.” Current Genetics 65 (4): 851–855.
Ravinet, M., R. Faria, R. K. Butlin, J. Galindo, N. Bierne, M. Rafajlovic, M. A. F. Noor, B. Mehlig, and A. M. Westram. 2017. “Interpreting the Genomic Landscape of Speciation: A Road Map for Finding Barriers to Gene Flow.” Journal of Evolutionary Biology 30 (8): 1450–1477.
Regev, Aviv, Sarah A. Teichmann, Eric S. Lander, Ido Amit, Christophe Benoist, Ewan Birney, Bernd Bodenmiller, et al. 2017. “The Human Cell Atlas.” Elife 6e27041: 1–30. https://doi.org/10.7554.eLife.27041.
Roberts, Andrea L., Nicole Gladish, Evan Gatev, Meaghan J. Jones, Ying Chen, Julia L. MacIsaac, Shelley S. Tworoger, et al. 2018. “Exposure to Childhood Abuse Is Associated with Human Sperm DNA Methylation.” Translational Psychiatry 8 (194): 1–11.
Roger, Andrew J., Sergio A. Muñoz-Gómez, and Ryoma Kamikawa. 2017. “The Origin and Diversification of Mitochondria.” Current Biology 27 (21): R1177–R1192. https://www.sciencedirect.com/science/article/pii/S096098221731179X?via%3Dihub#!
Ségurel, Laure, and Céline Bon. 2017. “On the Evolution of Lactase Persistence in Humans.” Annual Review of Genomics and Human Genetics 18: 297–319.
Sheth, Bhavisha P., and Vrinda S. Thaker. 2017. “DNA Barcoding and Traditional Taxonomy: An Integrated Approach for Biodiversity Conservation.” Genome 60 (7): 618–628.
Skloot, Rebecca. 2010. The Immortal Life of Henrietta Lacks. New York: Crown Publishing Group.
Snedeker, Jonathan, Matthew Wooten, and Xin Chen. 2017. “The Inherent Asymmetry of DNA Replication.” Annual Review of Cell and Developmental Biology 33: 291–318.
Sullivan-Pyke, Chantae, and Anuja Dokras. 2018. “Preimplantation Genetic Screening and Preimplantation Genetic Diagnosis.” Obstetrics and Gynecology Clinics of North America 45 (1): 113–125.
Sweeney, M. F., Hasan, N., Soto, A. M., & Sonnenschein, C. (2015). Environmental endocrine disruptors: Effects on the human male reproductive system. Reviews in Endocrine and Metabolic Disorders, 16(4), 341–357. https://doi.org/10.1007/s11154-016-9337-4
Szostak, Jack W. 2017. “The Narrow Road to the Deep Past: In Search of the Chemistry of the Origin of Life.” Angewandte Chemie International Edition 56 (37): 11037–11043.
Tessema, Mathewos, Ulrich Lehmann, and Hans Kreipe. 2004. “Cell Cycle and No End.” Virchows Archiv European Journal of Pathology 444 (4): 313–323.
Tishkoff, Sarah A., Floyd A. Reed, Alessia Ranciaro, Benjamin F. Voight, Courtney C. Babbitt, Jesse S. Silverman, Kweli Powell, et al. 2007. “Convergent Adaptation of Human Lactase Persistence in Africa and Europe.” Nature Genetics 39 (1): 31–40.
Visootsak, Jeannie, and John M. Graham, Jr. 2006. “Klinefelter Syndrome and Other Sex Chromosomal Aneuploidies.” Orphanet Journal of Rare Diseases 1:42. https://doi.org/10.1186/1750-1172-1-42.
Wolfe, George C., dir. 2017. The Immortal Life of Henrietta Lacks. HBO Films, April 22, 2017. TV Movie.
Yamamoto, Fumi-ichiro, Henrik Clausen, Thayer White, John Marken, and Sen-itiroh Hakomori. 1990. “Molecular Genetic Basis of the Histo-Blood Group ABO System.” Nature 345 (6272): 229–233.
Yu, X., Zhao, H., Wang, R., Chen, Y., Ouyang, X., Li, W., Sun, Y., & Peng, A. (2024). Cancer epigenetics: from laboratory studies and clinical trials to precision medicine. Cell Death Discovery, 10(1), 1–12. https://doi.org/10.1038/s41420-024-01803-z
Zlotogora, Joël. 2003. “Penetrance and Expressivity in the Molecular Age.” Genetics in Medicine 5 (5): 347–352.
Zorina-Lichtenwalter, Katerina, Ryan N. Lichtenwalter, Dima V. Zaykin, Marc Parisien, Simon Gravel, Andrey Bortsov, and Luda Diatchenko. 2019. “A Study in Scarlet: MC1R as the Main Predictor of Red Hair and Exemplar of the Flip-Flop Effect.” Human Molecular Genetics 28 (12): 2093-2106.
Zwart, Haeh. 2018. “In the Beginning Was the Genome: Genomics and the Bi-Textuality of Human Existence.” New Bioethics 24 (1): 26–43.
Keith Chan, Ph.D., Grossmont-Cuyamaca Community College District and MiraCosta College
This chapter is a revision from "Chapter 12: Modern Homo sapiens” by Keith Chan. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Identify the skeletal and behavioral traits that represent modern Homo sapiens.
- Critically evaluate different types of evidence for the origin of our species in Africa and our expansion around the world.
- Understand how the human lifestyle changed when people transitioned from foraging to agriculture.
- Hypothesize how human evolutionary trends may continue into the future.
The walls of a pink limestone cave in the hillside of Jebel Irhoud jutted out of the otherwise barren landscape of the Moroccan desert (Figure 12.1). Miners had excavated the cave in the 1960s, revealing some fossils. In 2007, a re-excavation of the site became a momentous occasion for science. A fossil cranium unearthed by a team of researchers was barely visible to the untrained eye. Just the fossil’s robust brows were peering out of the rock. This research team from the Max Planck Institute for Evolutionary Anthropology was the latest to explore the ancient human presence in this part of North Africa after a find by miners in 1960. Excavating near the first discovery, the researchers wanted to learn more about how Homo sapiens lived far from East Africa, where we thought our species originated.

The scientists were surprised when they analyzed the cranium, named Irhoud 10, and other fossils. Statistical comparisons with other human crania concluded that the Irhoud face shapes were typical of recent modern humans while the braincases matched ancient modern humans. Based on the findings of other scientists, the team expected these modern Homo sapiens fossils to be around 200,000 years old. Instead, dating revealed that the cranium had been buried for around 315,000 years.
Together, the modern-looking facial dimensions and the older date reshaped the interpretation of our species: modern Homo sapiens. Some key evolutionary changes from the archaic Homo sapiens (described in Chapter 11) to our species today happened 100,000 years earlier than we had thought and across the vast African continent rather than concentrated in its eastern region.
This revelation in the study of modern Homo sapiens is just one of the latest in this continually advancing area of biological anthropology. Researchers today are still discovering amazing fossils and ingenious ways to collect data and test hypotheses about our past. Through the collective work of many scientists, we are building an overall theory of modern human origins.
Defining Modernity
What defines modern Homo sapiens when compared to archaic Homo sapiens? Modern humans, like you and me, have a set of derived traits that are not seen in archaic humans or any other hominin. As with other transitions in hominin evolution, such as increasing brain size and bipedal ability, modern traits do not appear fully formed or all at once. In other words, the first modern Homo sapiens was not just born one day from archaic parents. The traits common to modern Homo sapiens appeared in a mosaic manner: gradually and out of sync with one another. There are two areas to consider when tracking the complex evolution of modern human traits. One is the physical change in the skeleton. The other is behavior inferred from the size and shape of the cranium and material culture evidence.
Skeletal Traits
The skeleton of modern Homo sapiens is less robust than that of archaic Homo sapiens. In other words, the modern skeleton is gracile, meaning that the structures are thinner and smoother. Differences related to gracility in the cranium are seen in the braincase, the face, and the mandible. There are also broad differences in the rest of the skeleton.
Cranial Traits
Several elements of the braincase differ between modern and archaic Homo sapiens. Overall, the shape is much rounder, or more globular, on a modern skull (Lieberman, McBratney, and Krovitz 2002; Neubauer, Hublin, and Gunz 2018; Pearson 2008; Figure 12.2). You can feel the globularity of your own modern human skull. Feel the height of your forehead with the palm of your hand. Viewed from the side, the tall vertical forehead of a modern Homo sapiens stands out when compared to the sloping archaic version. This is because the frontal lobe of the modern human brain is larger than the one in archaic humans, and the skull has to accommodate the expansion. The vertical forehead reduces a trait that is common to all other hominins: the brow ridge or supraorbital torus. The parietal lobes of the brain and the matching parietal bones on either side of the skull both bulge outward more in modern humans. At the back of the skull, the archaic occipital bun is no longer present. Instead, the occipital region of the modern human cranium has a derived tall and smooth curve, again reflecting the globular brain inside.
The trend of shrinking face size across hominins reaches its extreme with our species as well. The facial bones of a modern Homo sapiens are extremely gracile compared to all other hominins (Lieberman, McBratney, and Krovitz 2002). Continuing a trend in hominin evolution, technological innovations kept reducing the importance of teeth in reproductive success (Lucas 2007). As natural selection favored smaller and smaller teeth, the surrounding bone holding these teeth also shrank.
Related to smaller teeth, the mandible is also gracile in modern humans when compared to archaic humans and other hominins. Interestingly, our mandibles have pulled back so far from the prognathism of earlier hominins that we gained an extra structure at the most anterior point, called the mental eminence. You know this structure as the chin. At the skeletal level, it resembles an upside-down “T” at the centerline of the mandible (Pearson 2008). Looking back at archaic humans, you will see that they all lack a chin. Instead, their mandibles curve straight back without a forward point. What is the chin for and how did it develop? Flora Gröning and colleagues (2011) found evidence of the chin’s importance by simulating physical forces on computer models of different mandible shapes. Their results showed that the chin acts as structural support to withstand strain on the otherwise gracile mandible.
Postcranial Gracility
The rest of the modern human skeleton is also more gracile than its archaic counterpart. The differences are clear when comparing a modern Homo sapiens with a cold-adapted Neanderthal (Sawyer and Maley 2005), but the trends are still present when comparing modern and archaic humans within Africa (Pearson 2000). Overall, a modern Homo sapiens postcranial skeleton has thinner cortical bone, smoother features, and more slender shapes when compared to archaic Homo sapiens (Figure 12.3). Comparing whole skeletons, modern humans have longer limb proportions relative to the length and width of the torso, giving us lankier outlines.
Why is our skeleton so gracile compared to those of other hominins? Natural selection can drive the gracilization of skeletons in several ways (Lieberman 2015). A slender frame is believed to be adapted for the efficient long-distance running ability that started with Homo erectus. Furthermore, it is argued that slenderness is a genetic adaptation for cooling an active body in hotter climates, which aligns with the ample evidence that Africa was the home continent of our species.
Behavioral Modernity
Aside from physical differences in the skeleton, researchers have also uncovered evidence of behavioral changes associated with increased cultural complexity from archaic to modern humans. How did cultural complexity develop? Two investigations into this question are archaeology and the analysis of reconstructed brains.
Archaeology tells us much about the behavioral complexity of past humans by interpreting the significance of material culture. In terms of advanced culture, items created with an artistic flair, or as decoration, speak of abstract thought processes (Figure 12.4). The demonstration of difficult artistic techniques and technological complexity hints at social learning and cooperation as well. According to paleoanthropologist John Shea (2011), one way to track the complexity of past behavior through artifacts is by measuring the variety of tools found together. The more types of tools constructed with different techniques and for different purposes, the more modern the behavior. Researchers are still working on an archaeological way to measure cultural complexity that is useful across time and place.

The interpretation of brain anatomy is another promising approach to studying the evolution of human behavior. When looking at investigations on this topic in modern Homo sapiens brains, researchers found a weak association between brain size and test-measured intelligence (Pietschnig et al. 2015). Additionally, they found no association between intelligence and biological sex. These findings mean that there are more significant factors that affect tested intelligence than just brain size. Since the sheer size of the brain is not useful for weighing intelligence within a species, paleoanthropologists are instead investigating the differences in certain brain structures. The differences in organization between modern Homo sapiens brains and archaic Homo sapiens brains may reflect different cognitive priorities that account for modern human culture. As with the archaeological approach, new discoveries will refine what we know about the human brain and apply that knowledge to studying the distant past.
Taken together, the cognitive abilities in modern humans may have translated into an adept use of tools to enhance survival. Researchers Patrick Roberts and Brian A. Stewart (2018) call this concept the generalist-specialist niche: our species is an expert at living in a wide array of environments, with populations culturally specializing in their own particular surroundings. The next section tracks how far around the world these skeletal and behavioral traits have taken us.
First Africa, Then the World
What enabled modern Homo sapiens to expand its range further in 300,000 years than Homo erectus did in 1.5 million years? The key is the set of derived biological traits from the last section. It is theorized that the gracile frame and neurological anatomy allowed modern humans to survive and even flourish in the vastly different environments they encountered. Based on multiple types of evidence, the source of all of these modern humans was Africa. Instead of originating from just one location, evidence shows that modern Homo sapiens evolution occurred in a complex gene flow network across Africa, a concept called African multiregionalism (Scerri et al. 2018).
This section traces the origin of modern Homo sapiens and the massive expansion of our species across all of the continents (except Antarctica) by 12,000 years ago. While modern Homo sapiens first shared geography with archaic humans, modern humans eventually spread into lands where no human had gone before. Figure 12.5 shows the broad routes that our species took expanding around the world. I encourage you to make your own timeline with the dates in this part to see the overall trends.
Modern Homo sapiens Biology and Culture in Africa
We start with the ample fossil evidence supporting the theory that modern humans originated in Africa during the Middle Pleistocene, having evolved from African archaic Homo sapiens. The earliest dated fossils considered to be modern actually have a mosaic of archaic and modern traits, showing the complex changes from one type to the other. Experts have various names for these transitional fossils, such as Early Modern Homo sapiens or Early Anatomically Modern Humans. However they are labeled, the presence of some modern traits means that they illustrate the origin of the modern type. Three particularly informative sites with fossils of the earliest modern Homo sapiens are Jebel Irhoud, Omo, and Herto.
Recall from the start of the chapter that the most recent finds at Jebel Irhoud are now the oldest dated fossils that exhibit some facial traits of modern Homo sapiens. Besides Irhoud 10, the cranium that was dated to 315,000 years ago (Hublin et al. 2017; Richter et al. 2017), there were other fossils found in the same deposit that we now know are from the same time period. In total there are at least five individuals, representing life stages from childhood to adulthood. These fossils form an image of high variation in skeletal traits. For example, the skull named Irhoud 1 has a primitive brow ridge, while Irhoud 2 and Irhoud 10 do not (Figure 12.6). The braincases are lower than what is seen in the modern humans of today but higher than in archaic Homo sapiens. The teeth also have a mix of archaic and modern traits that defy clear categorization into either group.
Research separated by nearly four decades uncovered fossils and artifacts from the Kibish Formation in the Lower Omo Valley in Ethiopia. These Omo Kibish hominins were represented by braincases and fragmented postcranial bones of three individuals found kilometers apart, dating back to around 233,000 years ago (Day 1969; McDougall, Brown, and Fleagle 2005; Vidal et al. 2022). One interesting finding was the variation in braincase size between the two more-complete specimens: while the individual named Omo I had a more globular dome, Omo II had an archaic-style long and low cranium.
Also in Ethiopia, a team led by Tim White (2003) excavated numerous fossils at Herto. There were fossilized crania of two adults and a child, along with fragments of more individuals. The dates ranged between 160,000 and 154,000 years ago. The skeletal traits and stone-tool assemblage were both intermediate between the archaic and modern types. Features reminiscent of modern humans included a tall braincase and thinner zygomatic (cheek) bones than those of archaic humans (Figure 12.7). Still, some archaic traits persisted in the Herto fossils, such as the supraorbital tori. Statistical analysis by other research teams concluded that at least some cranial measurements fit just within the modern human range (McCarthy and Lucas 2014), favoring categorization with our own species.

The timeline of material culture suggests a long period of relying on similar tools before a noticeable diversification of artifacts types. Researchers label the time of stable technology shared with archaic types the Middle Stone Age, while the subsequent time of diversification in material culture is called the Later Stone Age.
In the Middle Stone Age, the sites of Jebel Irhoud, Omo, and Herto all bore tools of the same flaked style as archaic assemblages, even though they were separated by almost 150,000 years. The consistency in technology may be evidence that behavioral modernity was not so developed. No clear signs of art dating back this far have been found either. Other hypotheses not related to behavioral modernity could explain these observations. The tool set may have been suitable for thriving in Africa without further innovation. Maybe works of art from that time were made with media that deteriorated or perhaps such art was removed by later humans.
Evidence of what Homo sapiens did in Africa from the end of the Middle Stone Age to the Later Stone Age is concentrated in South African cave sites that reveal the complexity of human behavior at the time. For example, Blombos Cave, located along the present shore of the Cape of Africa facing the Indian Ocean, is notable for having a wide variety of artifacts. The material culture shows that toolmaking and artistry were more complex than previously thought for the Middle Stone Age. In a layer dated to 100,000 years ago, researchers found two intact ochre-processing kits made of abalone shells and grinding stones (Henshilwood et al. 2011). Marine snail shell beads from 75,000 years ago were also excavated (Figure 12.8; d’Errico et al. 2005). Together, the evidence shows that the Middle Stone Age occupation at Blombos Cave incorporated resources from a variety of local environments into their culture, from caves (ochre), open land (animal bones and fat), and the sea (abalone and snail shells). This complexity shows a deep knowledge of the region’s resources and their use—not just for survival but also for symbolic purposes.
On the eastern coast of South Africa, Border Cave shows new African cultural developments at the start of the Later Stone Age. Paola Villa and colleagues (2012) identified several changes in technology around 43,000 years ago. Stone-tool production transitioned from a slower process to one that was faster and made many microliths, small and precise stone tools. Changes in decorations were also found across the Later Stone Age transition. Beads were made from a new resource: fragments of ostrich eggs shaped into circular forms resembling present-day breakfast cereal O’s (d’Errico et al. 2012). These beads show a higher level of altering one’s own surroundings and a move from the natural to the abstract in terms of design.
Expansion into the Middle East and Asia
While modern Homo sapiens lived across Africa, some members eventually left the continent. These pioneers could have used two connections to the Middle East or West Asia. From North Africa, they could have crossed the Sinai Peninsula and moved north to the Levant, or eastern Mediterranean. Finds in that region show an early modern human presence. Other finds support the Southern Dispersal model, with a crossing from East Africa to the southern Arabian Peninsula through the Straits of Bab-el-Mandeb. It is tempting to think of one momentous event in which people stepped off Africa and into the Middle East, never to look back. In reality, there were likely multiple waves of movement producing gene flow back and forth across these regions as the overall range pushed east. The expanding modern human population could have thrived by using resources along the southern coast of the Arabian Peninsula to South Asia, with side routes moving north along rivers. The maximum range of the species then grew across Asia.
Modern Homo sapiens in the Middle East
Geographically, the Middle East is the ideal place for the African modern Homo sapiens population to inhabit upon expanding out of their home continent. In the Eastern Mediterranean coast of the Levant, there is a wealth of skeletal and material culture linked to modern Homo sapiens. Recent discoveries from Saudi Arabia further add to our view of human life just beyond Africa.
The Caves of Mount Carmel in present-day Israel have preserved skeletal remains and artifacts of modern Homo sapiens, the first-known group living outside Africa. The skeletal presence at Misliya Cave is represented by just part of the left upper jaw of one individual, but it is notable for being dated to a very early time, between 194,000 and 177,000 years ago (Hershkovitz et al. 2018). Later, from 120,000 to 90,000 years ago, fossils of multiple individuals across life stages were found in the caves of Es-Skhul and Qafzeh (Shea and Bar-Yosef 2005). The skeletons had many modern Homo sapiens traits, such as globular crania and more gracile postcranial bones when compared to Neanderthals. Still, there were some archaic traits. For example, the adult male Skhul V also possessed what researchers Daniel Lieberman, Osbjorn Pearson, and Kenneth Mowbray (2000) called marked or clear occipital bunning. Also, compared to later modern humans, the Mount Carmel people were more robust. Skhul V had a particularly impressive brow ridge that was short in height but sharply jutted forward above the eyes (Figure 12.9). The high level of preservation is due to the intentional burial of some of these people. Besides skeletal material, there are signs of artistic or symbolic behavior. For example, the adult male Skhul V had a boar’s jaw on his chest. Similarly, Qafzeh 11, a juvenile with healed cranial trauma, had an impressive deer antler rack placed over his torso (Figure 12.10; Coqueugniot et al. 2014). Perforated seashells colored with ochre, mineral-based pigment, were also found in Qafzeh (Bar-Yosef Mayer, Vandermeersch, and Bar-Yosef 2009).

One remaining question is, what happened to the modern humans of the Levant after 90,000 years ago? Another site attributed to our species did not appear in the region until 47,000 years ago. Competition with Neanderthals may have accounted for the disappearance of modern human occupation since the Neanderthal presence in the Levant lasted longer than the dates of the early modern Homo sapiens. John Shea and Ofer Bar-Yosef (2005) hypothesized that the Mount Carmel modern humans were an initial expansion from Africa that failed. Perhaps they could not succeed due to competition with the Neanderthals who had been there longer and had both cultural and biological adaptations to that environment.
Modern Homo sapiens of China
A long history of paleoanthropology in China has found ample evidence of modern human presence. Four notable sites are the caves at Fuyan, Liujiang, Tianyuan, and Zhoukoudian. In the distant past, these caves would have been at least seasonal shelters that unintentionally preserved evidence of human presence for modern researchers to discover.
At Fuyan Cave in Southern China, paleoanthropologists found 47 adult teeth associated with cave formations dated to between 120,000 and 80,000 years ago (Liu et al. 2015). It is currently the oldest-known modern human site in China, though other researchers question the validity of the date range (Michel et al. 2016). The teeth have the small size and gracile features of modern Homo sapiens dentition.
The fossil Liujiang (or Liukiang) hominin (67,000 years ago) has derived traits that classified it as a modern Homo sapiens, though primitive archaic traits were also present. In the skull, which was found nearly complete, the Liujiang hominin had a taller forehead than archaic Homo sapiens but also had an enlarged occipital region (Figure 12.11; Brown 1999; Wu et al. 2008). Other parts of the skeleton also had a mix of modern and archaic traits: for example, the femur fragments suggested a slender length but with thick bone walls (Woo 1959).
Another Chinese site to describe here is the one that has been studied the longest. In the Zhoukoudian Cave system (Figure 12.12), where Homo erectus and archaic Homo sapiens have also been found, there were three crania of modern Homo sapiens. These crania, which date to between 34,000 and 10,000 years ago, were all more globular than those of archaic humans but still lower and longer than those of later modern humans (Brown 1999; Harvati 2009). When compared to one another, the crania showed significant differences from one another. Comparison of cranial measurements to other populations past and present found no connection with modern East Asians, again showing that human variation was very different from what we see today.

Crossing to Australia
Expansion of the first modern human Asians, still following the coast, eventually entered an area that researchers call Sunda before continuing on to modern Australia. Sunda was a landmass made up of the modern-day Malay Peninsula, Sumatra, Java, and Borneo. Lowered sea levels connected these places with land bridges, making them easier to traverse. Proceeding past Sunda meant navigating Wallacea, the archipelago that includes the Indonesian islands east of Borneo. In the distant past, there were many megafauna, large animals that migrating humans would have used for food and materials (such as utilizing animals’ hides and bones). Further southeast was another landmass called Sahul, which included New Guinea and Australia as one contiguous continent. Based on fossil evidence, this land had never seen hominins or any other primates before modern Homo sapiens arrived. Sites along this path offer clues about how our species handled the new environment to live successfully as foragers.

The skeletal remains at Lake Mungo, land traditionally owned by Mutthi Mutthi, Ngiampaa, and Paakantji peoples, are the oldest known in the continent. The now-dry lake was one of a series located along the southern coast of Australia in New South Wales, far from where the first people entered from the north (Barbetti and Allen 1972; Bowler et al. 1970). Two individuals dating to around 40,000 years ago show signs of artistic and symbolic behavior, including intentional burial. The bones of Lake Mungo 1 (LM1), an adult female, were crushed repeatedly, colored with red ochre, and cremated (Bowler et al. 1970). Lake Mungo 3 (LM3), a tall, older male with a gracile cranium but robust postcranial bones, had his fingers interlocked over his pelvic region (Brown 2000).
Kow Swamp, within traditional Yorta Yorta land also in southern Australia, contained human crania that looked distinctly different from the ones at Lake Mungo (Durband 2014; Thorne and Macumber 1972). The crania, dated between 9,000 and 20,000 years ago, had extremely robust brow ridges and thick bone walls, but these were paired with globular features on the braincase (Figure 12.13).
While no fossil humans have been found at the Madjedbebe rock shelter in the North Territory of Australia, more than 10,000 artifacts found there show both behavioral modernity and variability (Clarkson et al. 2017). They include a diverse array of stone tools and different shades of ochre for rock art, including mica-based reflective pigment (similar to glitter). These impressive artifacts are as far back as 56,000 years old, providing the date for the earliest-known presence of humans in Australia.
From the Levant to Europe
The first modern human expansion into Europe occurred after other members of our species settled in East Asia and Australia. As the evidence from the Levant suggests, modern human movement to Europe may have been hampered by the presence of Neanderthals. It is suggested that another obstacle was the colder climate, which was incompatible with the biology of modern Homo sapiens from Africa, as they were adapted to high temperatures and ultraviolet radiation. Still, by 40,000 years ago, modern Homo sapiens had a detectable presence. This time was also the start of the Later Stone Age or Upper Paleolithic, when there was an expansion in cultural complexity. There is a wealth of evidence from this region due to a Western bias in research, the proximity of these findings to Western scientific institutions, and the desire of Western scientists to explore their own past.
In Romania, the site of Peștera cu Oase (Cave of Bones) had the oldest-known remains of modern Homo sapiens in Europe, dated to around 40,000 years ago (Trinkaus et al. 2003a). Among the bones and teeth of many animals were the fragmented cranium of one person and the mandible of another (the two bones did not fit each other). Both bones have modern human traits similar to the fossils from the Middle East, but they also had Neanderthal traits. Oase 1, the mandible, had a mental eminence but also extremely large molars (Trinkaus et al. 2003b). This mandible has yielded DNA that surprisingly is equally similar to DNA from present-day Europeans and Asians (Fu et al. 2015). This means that Oase 1 was not the direct ancestor of modern Europeans. The Oase 2 cranium has the derived traits of reduced brow ridges along with archaic wide zygomatic cheekbones and an occipital bun (Figure 12.14; Rougier et al. 2007).
Dating to around 26,000 years ago, Předmostí near Přerov in the Czech Republic was a site where people buried over 30 individuals along with many artifacts. Eighteen individuals were found in one mass burial area, a few covered by the scapulae of woolly mammoths (Germonpré, Lázničková-Galetová, and Sablin 2012). The Předmostí crania were more globular than those of archaic humans but tended to be longer and lower than in later modern humans (Figure 12.15; Velemínská et al. 2008). The height of the face was in line with modern residents of Central Europe. There was also skeletal evidence of dog domestication, such as the presence of dog skulls with shorter snouts than in wild wolves (Germonpré, Lázničková-Galetová, and Sablin et al. 2012). In total, Předmostí could have been a settlement dependent on mammoths for subsistence and the artificial selection of early domesticated dogs.
The sequence of modern Homo sapiens technological change in the Later Stone Age has been thoroughly dated and labeled by researchers working in Europe. Among them, the Gravettian tradition of 33,000 years to 21,000 years ago is associated with most of the known curvy female figurines, often assumed to be “Venus” figures. Hunting technology also advanced in this time with the first known boomerang, atlatl (spear thrower), and archery. The Magdalenian tradition spread from 17,000 to 12,000 years ago. This culture further expanded on fine bone tool work, including barbed spearheads and fishhooks (Figure 12.16).
Among the many European sites dating to the Later Stone Age, the famous cave art sites deserve mention. Chauvet-Pont-d'Arc Cave in southern France dates to separate Aurignacian occupations 31,000 years ago and 26,000 years ago. Over a hundred art pieces representing 13 animal species are preserved, from commonly depicted deer and horses to rarer rhinos and owls. Another French cave with art is Lascaux, which is several thousand years younger at 17,000 years ago in the Magdalenian period. At this site, there are over 6,000 painted figures on the walls and ceiling (Figure 12.17). Scaffolding and lighting must have been used to make the paintings on the walls and ceiling deep in the cave. Overall, visiting Lascaux as a contemporary must have been an awesome experience: trekking deeper in the cave lit only by torches giving glimpses of animals all around as mysterious sounds echoed through the galleries.

Peopling of the Americas
By 25,000 years ago, our species was the only member of Homo left on Earth. Gone were the Neanderthals, Denisovans, Homo naledi, and Homo floresiensis. The range of modern Homo sapiens kept expanding eastward into—using the name given to this area by Europeans much later—the Western Hemisphere. This section will address what we know about the peopling of the Americas, from the first entry to these continents to the rapid spread of Indigenous Americans across its varied environments.
While evidence points to an ancient land bridge called Beringia that allowed people to cross from what is now northeastern Siberia into modern-day Alaska, what people did to cross this land bridge is still being investigated. For most of the 20th century, the accepted theory was the Ice-Free Corridor model. It stated that northeast Asians (East Asians and Siberians) first expanded across Beringia inland through a passage between glaciers that opened into the western Great Plains of the United States, just east of the Rocky Mountains, around 13,000 years ago (Swisher et al. 2013). While life up north in the cold environment would have been harsh, migrating birds and an emerging forest might have provided sustenance as generations expanded through this land (Potter et al. 2018).
However, in recent decades, researchers have accumulated evidence against the Ice-Free Corridor model. Archaeologist K. R. Fladmark (1979) brought the alternate Coastal Route model into the archaeological spotlight; researcher Jon M. Erlandson has been at the forefront of compiling support for this theory (Erlandson et al. 2015). The new focus is the southern edge of the land bridge instead of its center: About 16,000 years ago, members of our species expanded along the coastline from northeast Asia, east through Beringia, and south down the Pacific Coast of North America while the inland was still sealed off by ice. The coast would have been free of ice at least part of the year, and many resources would have been found there, such as fish (e.g., salmon), mammals (e.g., whales, seals, and otters), and plants (e.g., seaweed).
South through the Americas
When the first modern Homo sapiens reached the Western Hemisphere, the spread through the Americas was rapid. Multiple migration waves crossed from North to South America (Posth et al. 2018). Our species took advantage of the lack of hominin competition and the bountiful resources both along the coasts and inland. The Americas had their own wide array of megafauna, which included woolly mammoths (Figure 12.18), mastodons, camels, horses, ground sloths, giant tortoises, and—a favorite of researchers—a two-meter-tall beaver. The reason we cannot see these amazing animals today may be that resources gained from these fauna were crucial to the survival for people over 12,000 years ago (Araujo et al. 2017). Several sites are notable for what they add to our understanding of the distant past in the Americas, including interactions with megafauna and other elements of the environment.

A 2019 discovery may allow researchers to improve theories about the peopling of the Americas. In White Sands National Park, New Mexico, 60 human footprints have been astonishingly dated to around 22,000 years ago (Bennett et al. 2021). This date and location do not match either the Ice-Free Corridor or Coastal Route models. Researchers are now working to verify the find and adjust previous models to account for the new evidence. This groundbreaking find is sparking new theories; it is another example of the fast pace of research performed on our past.
Monte Verde is a landmark site that shows that the human population had expanded down the whole vertical stretch of the Americas to Chile by 14,600 years ago. The site has been excavated by archaeologist Tom D. Dillehay and his team (2015). The remains of nine distinct edible species of seaweed at the site shows familiarity with coastal resources and relates to the Coastal Route model by showing a connection between the inland people and the sea.

Named after the town in New Mexico, the Clovis stone-tool style is the first example of a widespread culture across much of North America, between 13,400 and 12,700 years ago (Miller, Holliday, and Bright 2013). Clovis points were fluted with two small projections, one on each end of the base, facing away from the head (Figure 12.19). The stone points found at this site match those found as far as the Canadian border and northern Mexico, and from the west coast to the east coast of the United States. Fourteen Clovis sites also contained the remains of mammoths or mastodons, suggesting that hunting megafauna with these points was an important part of life for the Clovis people. After the spread of the Clovis style, it diversified into several regional styles, keeping some of the Clovis form but also developing their own unique touches.
The Big Picture: The Assimilation Hypothesis
How do researchers make sense of all of these modern Homo sapiens discoveries that cover over 300,000 years of time and stretch across every continent except Antarctica? How was modern Homo sapiens related to archaic Homo sapiens?
The Assimilation hypothesis proposes that modern Homo sapiens evolved in Africa first and expanded out but also interbred with the archaic Homo sapiens they encountered outside Africa (Figure 12.20). This hypothesis is powerful since it explains why Africa has the oldest modern human fossils, why early modern humans found in Europe and Asia bear a resemblance to the regional archaics, and why traces of archaic DNA can be found in our genomes today (Dannemann and Racimo 2018; Reich et al. 2010; Reich et al. 2011; Slatkin and Racimo 2016; Smith et al. 2017; Wall and Yoshihara Caldeira Brandt 2016).
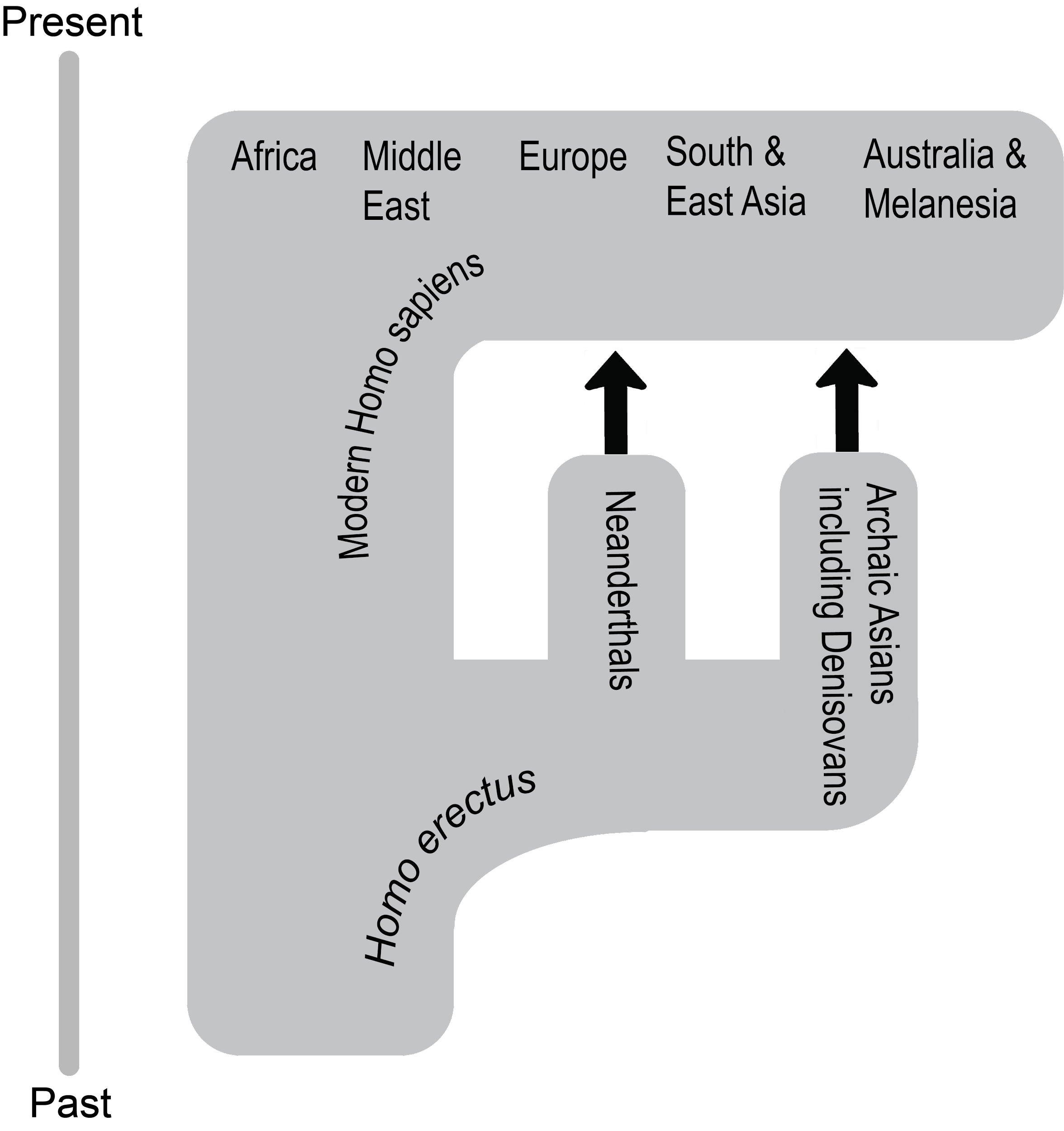
While researchers have produced a model that satisfies the data, there are still a lot of questions for paleoanthropologists to answer regarding our origins. What were the patterns of migration in each part of the world? Why did the archaic humans go extinct? In what ways did archaic and modern humans interact? The definitive explanation of how our species started and what our ancestors did is still out there to be found. You are now in a great place to welcome the next discovery about our distant past—maybe you’ll even contribute to our understanding as well.
The Chain Reaction of Agriculture
While it may be hard to imagine today, for most of our species’ existence we were nomadic: moving through the landscape without a singular home. Instead of a refrigerator or pantry stocked with food, we procured nutrition and other resources as needed based on what was available in the environment. This section gives an overview of how the foraging lifestyle enabled the expansion of our species and how the invention of a new way of life caused a chain reaction of cultural change.
The Foraging Tradition
There are a variety of possible subsistence strategies, or methods of finding sustenance and resources. To understand our species is to understand the subsistence strategy of foraging, or the search for resources in the environment. While most (but not all) humans today live in cultures that practice agriculture (whereby we greatly shape the environment to mass produce what we need), we have spent far more time as nomadic foragers than as settled agriculturalists. As such, it has been suggested that our traits have evolved to be primarily geared toward foraging. For instance, our efficient bipedalism allows persistence-hunting across long distances as well as movement from resource to resource.
How does human foraging, also known as hunting and gathering, work? Anthropologists have used all four fields to answer this question (see Ember n.d.). Typically, people formed bands, or kin-based groups of around 50 people or less (rarely over 100). A band’s organization would be egalitarian, with a flexible hierarchy based on an individual’s age, level of experience, and relationship with others. Everyone would have a general knowledge of the skills assigned to their gender roles, rather than specializing in different occupations. A band would be able to move from place to place in the environment, using knowledge of the area to forage (Figure 12.21). In varied environments—from savannas to tropical forests, deserts, coasts, and the Arctic circle—people found sustenance needed for survival.

Humans made extensive use of the foraging subsistence strategy, but this lifestyle did have limitations. The ease of foraging depended on the richness of the environment. Due to the lack of storage, resources had to be dependably found when needed. While a bountiful environment would require just a few hours of foraging a day and could lead to a focus on one location, the level and duration of labor increased greatly in poor or unreliable environments. Labor was also needed to process the acquired resources, which contributed to the foragers’ daily schedule (Crittenden and Schnorr 2017).
The adaptations to foraging found in modern Homo sapiens may explain why our species became so successful both within Africa and in the rapid expansion around the world. Overcoming the limitations, each generation at the edge of our species’s range would have found it beneficial to expand a little further, keeping contact with other bands but moving into unexplored territory where resources were more plentiful. The cumulative effect would have been the spread of modern Homo sapiens across continents and hemispheres.
Why Agriculture?
After hundreds of thousands of years of foraging, some groups of people around 12,000 years ago started to practice agriculture. This transition, called the Neolithic Revolution, occurred at the start of the Holocene epoch. While the reasons for this global change are still being investigated, two likely co-occurring causes are a growing human population and natural global climate change.
Overcrowding could have affected the success of foraging in the environment, leading to the development of a more productive subsistence strategy (Cohen 1977). Foraging works best with low population densities since each band needs a lot of space to support itself. If too many people occupy the same environment, they deplete the area faster. The high population could exceed the carrying capacity, or number of people a location can reliably support. Reaching carrying capacity on a global level due to growing population and limited areas of expansion would have been an increasingly pressing issue after the expansion through the major continents by 14,600 years ago.
A changing global climate immediately preceded the transition to agriculture, so researchers have also explored a connection between the two events. Since the Last Glacial Maximum of 23,000 years ago, the Earth slowly warmed. Then, from 13,000 to 11,700 years ago, the temperature in most of the Northern Hemisphere dropped suddenly in a phenomenon called the Younger Dryas. Glaciers returned in Europe, Asia, and North America. In Mesopotamia, which includes the Levant, the climate changed from warm and humid to cool and dry. The change would have occurred over decades, disrupting the usual nomadic patterns and subsistence of foragers around the world. The disruption to foragers due to the temperature shift could have been a factor in spurring a transition to agriculture. Researchers Gregory K. Dow and colleagues (2009) believe that foraging bands would have clustered in the new resource-rich places where people started to direct their labor to farming the limited area. After the Younger Dryas ended, people expanded out of the clusters with their agricultural knowledge (Figure 12.22).
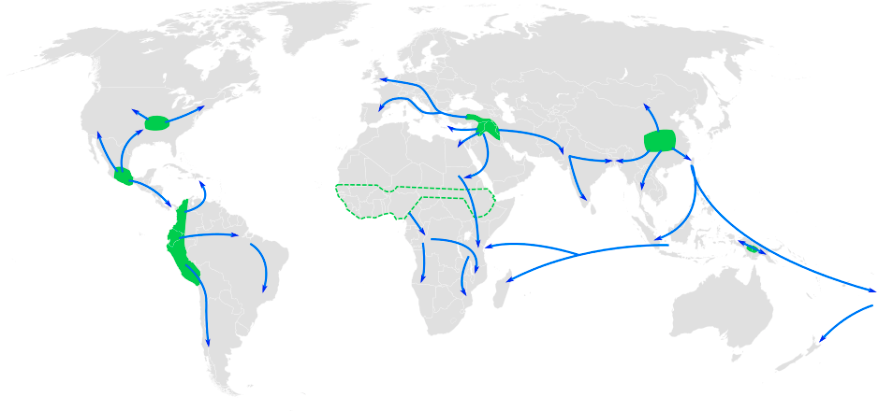
The double threat of the limitation of human continental expansion and the sudden global climate change may have placed bands in peril as more populations outpaced their environment’s carrying capacity. Not only had a growing population led to increased competition with other bands, but environments worldwide had shifted to create more uncertainty. As such, it has been proposed that as people in different areas around the world faced this unpredictable situation, they became the independent inventors of agriculture.
Agriculture around the World
Due to global changes to the human experience starting from 12,000 years ago, it has been suggested that cultures with no knowledge of each other turned toward intensely farming their local resources (see Figure 12.22). It is proposed that the first farmers engaged in artificial selection of their domesticates to enhance useful traits over generations. The switch to agriculture took time and effort with no guarantee of success and constant challenges (e.g. fires, droughts, diseases, and pests). The regions with the most widespread impact in the face of these obstacles became the primary centers of agriculture (Figure 12.23; Fuller 2010):
- Mesopotamia: The Fertile Crescent from the Tigris and Euphrates rivers through the Levant was where bands started to domesticate plants and animals around 12,000 years ago. The connection between the development of agriculture and the Younger Dryas was especially strong here. Farmed crops included wheat, barley, peas, and lentils. This was also where cattle, pigs, sheep, and goats were domesticated.
- South and East Asia: Multiple regions across this land had varieties of rice, millet, and soybeans by 10,000 years ago. Pigs were farmed with no connection to Mesopotamia. Chickens were also originally from this region, bred for fighting first and food second.
- New Guinea: Agriculture started here 10,000 years ago. Bananas, sugarcane, and taro were native to this island. Sweet potatoes were brought back from voyages to South America around the year C.E. 1000. No known animal farming occurred here.
- Mesoamerica: Agriculture from Central Mexico to northern South America also occurred from 10,000 years ago; it was also only plant based. Maize was a crop bred from teosinte grass, which has become one of the global staples. Beans, squash, and avocados were also grown in this region.
- The Andes: Starting around 8,000 years ago, local domesticated plants started with squash but later included potatoes, tomatoes, beans, and quinoa. Maize was brought down from Mesoamerica. The main farm animals were llamas, alpacas, and guinea pigs.
- Sub-Saharan Africa: This region went through a change 5,000 years ago called the Bantu expansion. The Bantu agriculturalists were established in West Central Africa and then expanded south and east. Native varieties of rice, yams, millet, and sorghum were grown across this area. Cattle were also domesticated here.
- Eastern North America: This region was the last major independent agriculture center, from 4,000 years ago. Squash and sunflower are the produce from this region that are most known today, though sumpweed and pitseed goosefoot were also farmed. Hunting was still the main source of animal products.

By 5,000 years ago, our species was well within the Neolithic Revolution. Agriculturalists spread to neighboring parts of the world with their domesticates, further expanding the use of this subsistence strategy. From this point, the human species changed from being primarily foragers to primarily agriculturalists with skilled control of their environments. The planet changed from mostly unaffected by human presence to being greatly transformed by humans. The revolution took millennia, but it was a true revolution as our species’ lifestyle was dramatically reshaped.
Cultural Effects of Agriculture
The worldwide adoption of agriculture altered the course of human culture and history forever. The core change in human culture due to agriculture is the move toward not moving: rather than live a nomadic lifestyle, farmers had to remain in one area to tend to their crops and livestock. The term for living bound to a certain location is sedentarism. This led to new aspects of life that were uncommon among foragers: the construction of permanent shelters and agricultural infrastructure, such as fields and irrigation, plus the development of storage technology, such as pottery, to preserve extra resources in case of future instability.

The high productivity of successful agriculture sparked further changes (Smith 2009). It is argued that since successful agriculture produced a much greater amount of food and other resources per unit of land compared to foraging, the population growth rate skyrocketed. The surplus of a bountiful harvest also provided insurance for harder times, reducing the risk of famine. Changes happened to society as well. With a few farming households producing enough food to feed many others, other people could focus on other tasks. So began specialization into different occupations such as craftspeople, traders, religious figures, and artists, spurring innovation in these areas as people could now devote time and effort toward specific skills. These interdependent people would settle an area together for convenience. The growth of these settlements led to urbanization, the founding of cities that became the foci of human interaction (Figure 12.24).
The formation of cities led to new issues that sparked the growth of further specializations, called institutions. These are cultural constructs that exist beyond the individual and have wide control over a population. Leadership of these cities became hierarchical with different levels of rank and control. The stratification of society increased social inequality between those with more or less power over others. Under leadership, people built impressive monumental architecture, such as pyramids and palaces, that embodied the wealth and power of these early cities. Alliances could unite cities, forming the earliest states. In several regions of the world, state organization expanded into empires, wide-ranging political entities that covered a variety of cultures.
(Inlcude Special Topic about the Haudesaunee/Iroquois confederacy)
Urbanization brought new challenges as well. The concentration of sedentary peoples was ideal for infectious diseases to thrive since they could jump from person to person and even from livestock to person (Armelagos, Brown, and Turner 2005). While successful agriculture provided a large surplus of food to thwart famine, the food produced offered less diverse food sources than foragers’ diets (Cohen and Armelagos 1984; Cohen and Crane-Kramer 2007). This shift in nutrition caused other diseases to flourish among those who adopted farming, such as dental cavities and malocclusion (the misalignment of teeth caused by soft, agricultural diets). The need to extract “wisdom teeth” or third molars seen in agricultural cultures today stems from this misalignment between the environment our ancestors adapted to and our lifestyles today.
As the new disease trends show, the adoption of agriculture and the ensuing cultural changes were not entirely positive. It is also important to note that this is not an absolutely linear progression of human culture from simple to complex. In many cases, empires have collapsed and, in some cases, cities dispersed to low-density bands that rejected institutions. However, a global trend has emerged since the adoption of agriculture, wherein population and social inequality have increased, leading to the massive and influential nation-states of today.
The rise of states in Europe has a direct impact on many of this book’s topics. Science started as a European cultural practice by the upper class that became a standardized way to study the world. Education became an institution to provide a standardized path toward producing and gaining knowledge. The scientific study of human diversity, embroiled in the race concept that still haunts us today, was connected to the European slave trade and colonialism.
Also starting in Europe, the Industrial Revolution of the 19th century turned cities into centers of mass manufacturing and spurred the rapid development of inventions (Figure 12.25). In the technologically interconnected world of today, human society has reached a new level of complexity with globalization. In this system, goods are mass-produced and consumed in different parts of the world, weakening the reliance on local farms and factories. The imbalanced relationship between consumers and producers of goods further increases economic inequality.

As states based on agriculture and industry keep exerting influence on humanity today, there are people, like the Hadzabe of Tanzania, who continue to live a lifestyle centered on foraging. Due to the overwhelming force that agricultural societies exert, foragers today have been marginalized to live in the least habitable parts of the world—the areas that are not conducive to farming, such as tropical rainforests, deserts, and the Arctic (Headland et al. 1989). Foragers can no longer live in the abundant environments that humans would have enjoyed before the Neolithic Revolution. Interactions with agriculturalists are typically imbalanced, with trade and other exchanges heavily favoring the larger group. One of anthropology’s important roles today is to intelligently and humanely manage equitable interactions between people of different backgrounds and levels of influence.
Special Topic: Indigenous Land Management
Insight into the lives of past modern humans has evolved as researchers revise previous theories and establish new connections with Indigenous knowledge holders.
The outdated view of foraging held that people lived off of the land without leaving an impact on the environment. Accompanying this idea was anthropologist Marshall Sahlins’s (1968) proposal that foragers were the “original affluent society” since they were meeting basic needs and achieving satisfaction with less work hours than agriculturalists and city-dwellers. This view countered an earlier idea that foragers were always on the brink of starvation. Sahlins’s theory took hold in the public eye as an attractive counterpoint to our busy contemporary lives in which we strive to meet our endless wants.
A fruitful type of study involving researchers collaborating with Indigenous experts has found that foragers did not just live off the land with minimal effort nor were they barely surviving in unchanging environments. Instead, they shaped the landscape to their needs using labor and strategies that were more subtle than what European colonizers and subsequent researchers were used to seeing. Research from two regions shows the latest developments in understanding Indigenous land management.
In British Columbia, Canada, the bridging of scientific and Indigenous perspectives has shown that the forests of the region are not untouched wilderness but, rather, have been crafted by Indigenous peoples thousands of years ago. Forest gardens adjacent to archaeological sites show higher plant diversity than unmanaged places even after 150 years (Armstrong et al. 2021). On the coast, 3,500-year-old archaeological sites are evidence of constructed clam gardens, according to Indigenous experts (Lepofsky et al. 2015). Another project, in consultation with Elders of the T’exelc (William Lakes First Nation) in British Columbia, introduced researchers to explanations of how forests were managed before the practice was disrupted by European colonialism (Copes-Gerbitz et al. 2021). Careful management of controlled fires reduced the density of the forest to favor plants such as raspberries and allow easier movement through the landscape.
Similarly, the study of landscapes in Australia, in consultation with Aboriginal Australians today, shows that areas previously considered wilderness by scientists were actually the result of controlling fauna and fires. The presence of grasslands with adjacent forests were purposely constructed to attract kangaroos for hunting (Gammage 2008). People also managed other animal and insect life, from emus to caterpillars. In Tasmania, a shift from productive grassland to wildfire-prone rainforest occurred after Aboriginal Australian land management was replaced by British colonial rule (Fletcher, Hall, and Alexander 2021). The site of Budj Bim of the Gunditjmara people has archaeological features of aquaculture, or the farming of fish, that date back 6,600 years (McNiven et al. 2012; McNiven et al. 2015). These examples show that Indigenous knowledge of how to manipulate the environment may be invaluable at the state level, such as by creating an Aboriginal ranger program to guide modern land management.
The Future of Humanity
A common question stemming from understanding human evolution is: What will the genetic and biological traits of our species be hundreds of thousands of years in the future? When faced with this question, people tend to think of directional selection. Maybe our braincases will be even larger, resembling the large-headed and small-bodied aliens of science fiction (Figure 12.26). Or, our hands could be specialized for interacting with our touch-based technology with less risk of repetitive injury. These ideas do not stand up to scrutiny. Since natural selection is based on adaptations that increase reproductive success, any directional change must be due to a higher rate of producing successful offspring compared to other alleles. Larger brains and more agile fingers would be convenient to possess, but they do not translate into an increase in the underlying allele frequencies.
Scientists are hesitant to professionally speculate on the unknowable, and we will never know what is in store for our species one thousand or one million years from now, but there are two trends in human evolution that may carry on into the future: increased genetic variation and a reduction in regional differences.
Rather than a directional change, genetic variation in our species could expand. Our technology can protect us from extreme environments and pathogens, even if our biological traits are not tuned to handle these stressors. The rapid pace of technological advancement means that biological adaptations will become less and less relevant to reproductive success, so nonbeneficial genetic traits will be more likely to remain in the gene pool. Biological anthropologist Jay T. Stock (2008) views environmental stress as needing to defeat two layers of protection before affecting our genetics. The first layer is our cultural adaptations. Our technology and knowledge can reduce pressure on one’s genotype to be “just right” to pass to the next generation. The second defense is our flexible physiology, such as our acclimatory responses. Only stressors not handled by these powerful responses would then cause natural selection on our alleles. These shields are already substantial, and cultural adaptations will only keep increasing in strength.
The increasing ability to travel far from one’s home region means that there will be a mixing of genetic variation on a global level in the future of our species. In recent centuries, gene flow of people around the world has increased, creating admixture in populations that had been separated for tens of thousands of years. For skin color, this means that populations all around the world could exhibit the whole range of skin colors, rather than the current pattern of decreasing melanin pigment farther from the equator. The same trend of intermixing would apply to all other traits, such as blood types. While our genetics will become more varied, the variation will be more intermixed instead of regionally isolated.
Our distant descendants will not likely be dextrous ultraintellectuals; more likely, they will be a highly variable and mobile species supported by novel cultural adaptations that make up for any inherited biological limitations. Technology may even enable the editing of DNA directly, changing these trends. With the uncertainty of our future, these are just the best-educated guesses for now. Our future is open and will be shaped little by little by the environment, our actions, and the actions of our descendants.
Summary
Modern Homo sapiens is the species that took the hominin lifestyle the furthest to become the only living member of that lineage. The largest factor that allowed us to persist while other hominins went extinct was likely our advanced ability to culturally adapt to a wide variety of environments. Our species, with its skeletal and behavioral traits, was well-suited to be generalist-specialists who successfully foraged across most of the world’s environments. The biological basis of this adaptation was our reorganized brain that facilitated innovation in cultural adaptations and intelligence for leveraging our social ties and finding ways to acquire resources from the environment. As the brain’s ability increased, it shaped the skull by reducing the evolutionary pressure to have large teeth and robust cranial bones to produce the modern Homo sapiens face.
Our ability to be generalist-specialists is seen in the geographical range that modern Homo sapiens covered in 300,000 years. In Africa, our species formed from multiregional gene flow that loosely connected archaic humans across the continent. People then expanded out to the rest of the continental Eurasia and even further to the Americas.
For most of our species’s existence, foraging was the general subsistence strategy within which people specialized to culturally adapt to their local environment. With omnivorousness and mobility, people found ways to extract and process resources, shaping the environment in return. When resource uncertainty hit the species, people around the world focused on agriculture to have a firmer control of sustenance. The new strategy shifted human history toward exponential growth and innovation, leading to our high dependence on cultural adaptations today.
While a cohesive image of our species has formed in recent years, there is still much to learn about our past. The work of many driven researchers shows that there are amazing new discoveries made all the time that refine our knowledge of human evolution. Technological innovations such as DNA analysis enable scientists to approach lingering questions from new angles. The answers we get allow us to ask even more insightful questions that will lead us to the next revelation. Like the pink limestone strata at Jebel Irhoud, previous effort has taken us so far and you are now ready to see what the next layer of discovery holds.
Hominin Species Summary
|
Hominin |
Modern Homo sapiens |
|
Dates |
315,000 years ago to present |
|
Region(s) |
Starting in Africa, then expanding around the world |
|
Famous discoveries |
Cro-Magnon individuals, discovered 1868 in Dordogne, France. Otzi the Ice Man, discovered 1991 in the Alps between Austria and Italy. Kennewick man, discovered 1996 in Washington state. |
|
Brain size |
1400 cc average |
|
Dentition |
Extremely small with short cusps. |
|
Cranial features |
An extremely globular brain case and gracile features throughout the cranium. The mandibular symphysis forms a chin at the anterior-most point. |
|
Postcranial features |
Gracile skeleton adapted for efficient bipedal locomotion at the expense of the muscular strength of most other large primates. |
|
Culture |
Extremely extensive and varied culture with many spoken and written languages. Art is ubiquitous. Technology is broad in complexity and impact on the environment. |
|
Other |
The only living hominin. Chimpanzees and bonobos are the closest living relatives. |
Review Questions
- What are the skeletal and behavioral traits that define modern Homo sapiens? What are the evolutionary explanations for its presence?
- What are some creative ways that researchers have learned about the past by studying fossils and artifacts?
- How do the discoveries mentioned in “First Africa, Then the World” fit the Assimilation model?
- What is foraging? What adaptations do we have for this subsistence strategy? Could you train to be a skilled forager?
- What are aspects of your life that come from dependence on agriculture and its cultural effects? Where did the ingredients of your favorite foods originate from?
Key Terms
African multiregionalism: The idea that modern Homo sapiens evolved as a complex web of small regional populations with sporadic gene flow among them.
Agriculture: The mass production of resources through farming and domestication.
Aquaculture: The farming of fish using techniques such as trapping, channels, and artificial ponds.
Assimilation hypothesis: Current theory of modern human origins stating that the species evolved first in Africa and interbred with archaic humans of Europe and Asia.
Atlatl: A handheld spear thrower that increased the force of thrown projectiles.
Band: A small group of people living together as foragers.
Beringia: Ancient landmass that connected Siberia and Alaska. The ancestors of Indigenous Americans would have crossed this area to reach the Americas.
Carrying capacity: The amount of organisms that an environment can reliably support.
Coastal Route model: Theory that the first Paleoindians crossed to the Americas by following the southern coast of Beringia.
Early Modern Homo sapiens, Early Anatomically Modern Human: Terms used to refer to transitional fossils between archaic and modern Homo sapiens that have a mosaic of traits. Humans like ourselves, who mostly lack archaic traits, are referred to as Late Modern Homo sapiens and simply Anatomically Modern Humans.
Egalitarian: Human organization without strict ranks. Foraging societies tend to be more egalitarian than those based on other subsistence strategies.
Foraging: Lifestyle consisting of frequent movement through the landscape and acquiring resources with minimal storage capacity.
Generalist-specialist niche: The ability to survive in a variety of environments by developing local expertise. Evolution toward this niche may have been what allowed modern Homo sapiens to expand past the geographical range of other human species.
Globalization: A recent increase in the interconnectedness and interdependence of people that is facilitated with long-distance networks.
Globular: Having a rounded appearance. Increased globularity of the braincase is a trait of modern Homo sapiens.
Gracile: Having a smooth and slender quality; the opposite of robust.
Holocene: The epoch of the Cenozoic Era starting around 12,000 years ago and lasting arguably through the present.
Ice-Free Corridor model: Theory that the first Native Americans crossed to the Americas through a passage between glaciers.
Institutions: Long-lasting and influential cultural constructs. Examples include government, organized religion, academia, and the economy.
Last Glacial Maximum: The time 23,000 years ago when the most recent ice age was the most intense.
Later Stone Age: Time period following the Middle Stone Age with a diversification in tool types, starting around 50,000 years ago.
Levant: The eastern coast of the Mediterranean. The site of early modern human expansion from Africa and later one of the centers of agriculture.
Megafauna: Large ancient animals that may have been hunted to extinction by people around the world.
Mental eminence: The chin on the mandible of modern H. sapiens. One of the defining traits of our species.
Microlith: Small stone tool found in the Later Stone Age; also called a bladelet.
Middle Stone Age: Time period known for Mousterian lithics that connects African archaic to modern Homo sapiens.
Monumental architecture: Large and labor-intensive constructions that signify the power of the elite in a sedentary society. A common type is the pyramid, a raised crafted structure topped with a point or platform.
Mosaic: Composed from a mix or composite of traits.
Neolithic Revolution: Time of rapid change to human cultures due to the invention of agriculture, starting around 12,000 years ago.
Ochre: Iron-based mineral pigment that can be a variety of yellows, reds, and browns. Used by modern human cultures worldwide since at least 80,000 years ago.
Sahul: Ancient landmass connecting New Guinea and Australia.
Sedentarism: Lifestyle based on having a stable home area; the opposite of nomadism.
Southern Dispersal model: Theory that modern H. sapiens expanded from East Africa by crossing the Red Sea and following the coast east across Asia.
Subsistence strategy: The method an organism uses to find nourishment and other resources.
Sunda: Ancient Asian landmass that incorporated modern Southeast Asia.
Supraorbital torus: The bony brow ridge across the top of the eye orbits on many hominin crania.
Upper Paleolithic: Time period considered synonymous with the Later Stone Age.
Urbanization: The increase of population density as people settled together in cities.
Wallacea: Archipelago southeast of Sunda with different biodiversity than Asia.
Younger Dryas: The rapid change in global climate—notably a cooling of the Northern Hemisphere—13,000 years ago.
For Further Exploration
Websites
First-person virtual tour of Lascaux cave with annotated cave art: Ministère de la Culture and Musée d’Archéologie Nationale. “Visit the cave” Lascaux website.
Online anthropology magazine articles related to paleoanthropology and human evolution: SAPIENS. “Evolution.” SAPIENS website.
Various presentations of information about hominin evolution: Smithsonian Institution. “What does it mean to be human?” Smithsonian National Museum of Natural History website.
Magazine-style articles on archaeology and paleoanthropology: ThoughtCo. “Archaeology.” ThoughtCo. Website.
Database of comparisons across hominins and primates: University of California, San Diego. “MOCA Domains.” Center for Academic Research & Training in Anthropogeny website.
Books
Engaging book that covers human-made changes to the environment with industrialization and globalization: Kolbert, Elizabeth. 2014. The Sixth Extinction: An Unnatural History. New York: Bloomsbury.
Overview of what human life was like among the environmental shifts of the Ice Age: Woodward, Jamie. 2014. The Ice Age: A Very Short Introduction. Oxford: OUP Press.
Articles
Recent review paper about the current state of paleoanthropology research: Stringer, C. 2016. “The Origin and Evolution of Homo sapiens.” Philosophical Transactions of the Royal Society B 371 (1698).
Overview of the history of American paleoanthropology and the many debates that have occurred over the years: Trinkaus, E. 2018. “One Hundred Years of Paleoanthropology: An American Perspective.” American Journal of Physical Anthropology 165 (4): 638–651.
Amazing magazine article that synthesizes hominin evolution and why it is important to study this subject: Wheelwright, Jeff. 2015. “Days of Dysevolution.” Discover 36 (4): 33–39.
Fascinating research on Ötzi, a mummy from 5,000 years ago: Wierer, Ursula, Simona Arrighi, Stefano Bertola, Günther Kaufmann, Benno Baumgarten, Annaluisa Pedrotti, Patrizia Pernter, and Jacques Pelegrin. 2018. “The Iceman’s Lithic Toolkit: Raw Material, Technology, Typology and Use.” PLOS One 13 (6): e0198292. https://doi.org/10.1371/journal.pone.0198292.
Documentaries
PBS NOVA series covering the expansion of modern Homo sapiens and interbreeding with archaic humans: Brown, Nicholas, dir. 2015. First Peoples. Edmonton: Wall to Wall Television. Amazon Prime Video.
PBS NOVA special featuring the footprints found in White Sands National Park: Falk, Bella, dir. 2016. Ice Age Footprints. Boston: Windfall Films. https://www.pbs.org/wgbh/nova/video/ice-age-footprints/.
PBS NOVA special about how modern humans evolved adaptations to different environments. Shows how present-day people live around the world: Thompson, Niobe, dir. 2016. Great Human Odyssey. Edmonton: Clearwater Documentary. https://www.pbs.org/wgbh/nova/evolution/great-human-odyssey.html.
References
Araujo, Bernardo B. A., Luiz Gustavo R. Oliveira-Santos, Matheus S. Lima-Ribeiro, José Alexandre F. Diniz-Filho, and Fernando A. S. Fernandez. 2017. “Bigger Kill Than Chill: The Uneven Roles of Humans and Climate on Late Quaternary Megafaunal Extinctions.” Quaternary International 431: 216–222.
Armelagos, George J., Peter J. Brown, and Bethany Turner. 2005. “Evolutionary, Historical, and Political Economic Perspectives on Health and Disease.” Social Science & Medicine 61 (4): 755–765.
Armstrong, C. G., J. E. D. Miller, A. C. McAlvay, P. M. Ritchie, and D. Lepofsky. 2021. “Historical Indigenous Land-Use Explains Plant Functional Trait Diversity. Ecology and Society 26 (2): 6.
Bar-Yosef Mayer, Daniella E., Bernard Vandermeersch, and Ofer Bar-Yosef. 2009. “Shells and Ochre in Middle Paleolithic Qafzeh Cave, Israel: Indications for Modern Behavior.” Journal of Human Evolution 56 (3): 307–314.
Barbetti, M., and H. Allen. 1972. “Prehistoric Man at Lake Mungo, Australia, by 32,000 Years Bp.” Nature 240 (5375): 46–48.
Bennett, M. R., D. Bustos, J. S. Pigati, K. B. Springer, T. M. Urban, V. T. Holliday, Sally C. Reynolds, et al. (2021). “Evidence of Humans in North America during the Last Glacial Maximum.” Science 373 (6562): 1528–1531.
Bowler, J. M., Rhys Jones, Harry Allen, and A. G. Thorne. 1970. “Pleistocene Human Remains from Australia: A Living Site and Human Cremation from Lake Mungo, Western New South Wales.” World Archaeology 2 (1): 39–60.
Brown, Peter. 1999. “The First Modern East Asians? Another Look at Upper Cave 101, Liujiang and Minatogawa 1.” In Interdisciplinary Perspectives on the Origins of the Japanese, edited by K. Omoto, 105–131. Kyoto: International Research Center for Japanese Studies.
Brown, Peter. 2000. “Australian Pleistocene Variation and the Sex of Lake Mungo 3.” Journal of Human Evolution 38 (5): 743–749.
Clarkson, Chris, Zenobia Jacobs, Ben Marwick, Richard Fullagar, Lynley Wallis, Mike Smith, Richard G. Roberts, et al. 2017. “Human Occupation of Northern Australia by 65,000 Years Ago.” Nature 547 (7663): 306–310.
Cohen, Mark Nathan. 1977. The Food Crisis in Prehistory: Overpopulation and the Origins of Agriculture. New Haven, CT: Yale University Press.
Cohen, Mark Nathan, and George J. Armelagos, eds. 1984. Paleopathology at the Origins of Agriculture. Orlando, FL: Academic Press.
Cohen, Mark Nathan, and Gillian M. M. Crane-Kramer, eds. 2007. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. Gainesville, FL: University Press of Florida.
Copes-Gerbitz, K., S. Hagerman, and L. Daniels. 2021. “Situating Indigenous Knowledge for Resilience in Fire-Dependent Social-Ecological Systems.” Ecology and Society 26(4): 25. https://www.ecologyandsociety.org/vol26/iss4/art25/.
Coqueugniot, Hélène, Olivier Dutour, Baruch Arensburg, Henri Duday, Bernard Vandermeersch, and Anne-Marie Tillier. 2014. “Earliest Cranio-Encephalic Trauma from the Levantine Middle Palaeolithic: 3-D Reappraisal of the Qafzeh 11 Skull, Consequences of Pediatric Brain Damage on Individual Life Condition and Social Care.” PLOS ONE 9 (7): e102822.
Crittenden, Alyssa N., and Stephanie L. Schnorr. 2017. “Current Views on Hunter‐Gatherer Nutrition and the Evolution of the Human Diet.” American Journal of Physical Anthropology 162 (S63): 84–109.
d’Errico, Francesco, Lucinda Backwell, Paola Villa, Ilaria Degano, Jeannette J. Lucejko, Marion K. Bamford, Thomas F. G. Higham, Maria Perla Colombini, and Peter B. Beaumont. 2012. “Early Evidence of San Material Culture Represented by Organic Artifacts from Border Cave, South Africa.” Proceedings of the National Academy of Sciences 109 (33): 13214–13219.
d’Errico, Francesco, Christopher Henshilwood, Marian Vanhaeren, and Karen Van Niekerk. 2005. “Nassarius Kraussianus Shell Beads from Blombos Cave: Evidence for Symbolic Behaviour in the Middle Stone Age.” Journal of Human Evolution 48 (1): 3–24.
Dannemann, Michael, and Fernando Racimo. 2018. “Something Old, Something Borrowed: Admixture and Adaptation in Human Evolution.” Current Opinion in Genetics & Development 53: 1–8.
Day, M. H. 1969. “Omo Human Skeletal Remains.” Nature 222: 1135–1138.
Dillehay, Tom D., Carlos Ocampo, José Saavedra, Andre Oliveira Sawakuchi, Rodrigo M. Vega, Mario Pino, Michael B. Collins, et al. 2015. “New Archaeological Evidence for an Early Human Presence at Monte Verde, Chile.” PLOS ONE 10 (11): e0141923. doi:10.1371/journal.pone.0141923.
Dow, Gregory K., Clyde G. Reed, and Nancy Olewiler. 2009. “Climate Reversals and the Transition to Agriculture.” Journal of Economic Growth 14 (1): 27–53.
Durband, Arthur C. 2014. “Brief Communication: Artificial Cranial Modification in Kow Swamp and Cohuna.” American Journal of Physical Anthropology 155 (1): 173–178.
Ember, Carol R. N.d. “Hunter-Gatherers.” Explaining Human Culture. Human Relations Area Files. Accessed March 4, 2023. https://hraf.yale.edu/ehc/summaries/hunter-gatherers.
Erlandson, Jon M., Todd J. Braje, Kristina M. Gill, and Michael H. Graham. 2015. “Ecology of the Kelp Highway: Did Marine Resources Facilitate Human Dispersal from Northeast Asia to the Americas?” The Journal of Island and Coastal Archaeology 10 (3): 392–411.
Fladmark, K. R. 1979. “Routes: Alternate Migration Corridors for Early Man in North America.” American Antiquity 44 (1): 55–69.
Fletcher, M. S., T. Hall, and A. N. Alexandra. 2021. “The Loss of an Indigenous Constructed Landscape Following British Invasion of Australia: An Insight into the Deep Human Imprint on the Australian Landscape.” Ambio 50(1): 138–149.
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216–219.
Fuller, Dorian Q. 2010. “An Emerging Paradigm Shift in the Origins of Agriculture.” General Anthropology 17 (2): 1, 8–11.
Gammage, B. 2008. “Plain Facts: Tasmania under Aboriginal Management.” Landscape Research 33 (2): 241–254.
Germonpré, Mietje, Martina Lázničková-Galetová, and Mikhail V. Sablin. 2012. “Palaeolithic Dog Skulls at the Gravettian Předmostí Site, the Czech Republic.” Journal of Archaeological Science 39 (1): 184–202.
Gröning, Flora, Jia Liu, Michael J. Fagan, and Paul O’Higgins. 2011. “Why Do Humans Have Chins? Testing the Mechanical Significance of Modern Human Symphyseal Morphology with Finite Element Analysis.” American Journal of Physical Anthropology 144 (4): 593–606.
Harvati, Katerina. 2009. “Into Eurasia: A Geometric Morphometric Reassessment of the Upper Cave (Zhoukoudian) Specimens.” Journal of Human Evolution 57 (6): 751–762.
Headland, Thomas N., Lawrence A. Reid, M. G. Bicchieri, Charles A. Bishop, Robert Blust, Nicholas E. Flanders, Peter M. Gardner, Karl L. Hutterer, Arkadiusz Marciniak, and Robert F. Schroeder. 1989. “Hunter-Gatherers and Their Neighbors from Prehistory to the Present.” Current Anthropology 30 (1): 43–66.
Henshilwood, Christopher S., Francesco d’Errico, Karen L. van Niekerk, Yvan Coquinot, Zenobia Jacobs, Stein-Erik Lauritzen, Michel Menu, and Renata García-Moreno. 2011. “A 100,000-Year-Old Ochre-Processing Workshop at Blombos Cave, South Africa.” Science 334 (6053): 219–222.
Hershkovitz, Israel, Gerhard W. Weber, Rolf Quam, Mathieu Duval, Rainer Grün, Leslie Kinsley, Avner Ayalon, et al. 2018. “The Earliest Modern Humans Outside Africa.” Science 359 (6374): 456–459.
Hublin, Jean-Jacques, Abdelouahed Ben-Ncer, Shara E. Bailey, Sarah E. Freidline, Simon Neubauer, Matthew M. Skinner, Inga Bergmann, et al. 2017. “New Fossils from Jebel Irhoud, Morocco, and the Pan-African Origin of Homo sapiens.” Nature 546 (7657): 289–292.
Lepofsky, D., N. F. Smith, N. Cardinal, J. Harper, M. Morris, M., Gitla (Elroy White), Randy Bouchard, et al. 2015. “Ancient Shellfish Mariculture on the Northwest Coast of North America.” American Antiquity 80 (2): 236–259.
Lieberman, Daniel E. 2015. “Human Locomotion and Heat Loss: An Evolutionary Perspective.” Comprehensive Physiology 5 (1): 99–117.
Lieberman, Daniel E., Brandeis M. McBratney, and Gail Krovitz. 2002. “The Evolution and Development of Cranial Form in Homo sapiens.” Proceedings of the National Academy of Sciences 99 (3): 1134–1139.
Lieberman, Daniel E., Osbjorn M. Pearson, and Kenneth M. Mowbray. 2000. “Basicranial Influence on Overall Cranial Shape.” Journal of Human Evolution 38 (2): 291–315.
Liu, Wu, María Martinón-Torres, Yan-jun Cai, Song Xing, Hao-wen Tong, Shu-wen Pei, Mark Jan Sier, Xiao-hong Wu, R. Lawrence Edwards, and Hai Cheng. 2015. “The Earliest Unequivocally Modern Humans in Southern China.” Nature 526 (7575): 696-699.
Lucas, Peter W. 2007. “The Evolution of the Hominin Diet from a Dental Functional Perspective.” In Evolution of the Human Diet: The Known, the Unknown, and the Unknowable, edited by Peter S. Ungar, 31–38 Oxford, UK: Oxford University Press.
McCarthy, Robert C., and Lynn Lucas. 2014. “A Morphometric Reassessment of Bou-Vp-16/1 from Herto, Ethiopia.” Journal of Human Evolution 74: 114–117.
McDougall, Ian, Francis H. Brown, and John G. Fleagle. 2005. “Stratigraphic Placement and Age of Modern Humans from Kibish, Ethiopia.” Nature 433 (7027): 733–736.
McNiven, I. J., J. Crouch, T. Richards, N. Dolby, and G. Jacobsen. 2012. “Dating Aboriginal Stone-Walled Fishtraps at Lake Condah, Southeast Australia.” Journal of Archaeological Science 39 (2): 268–286.
McNiven, I., J. Crouch, T. Richards, K. Sniderman, N. Dolby, and G. Mirring. 2015. “Phased Redevelopment of an Ancient Gunditjmara Fish Trap over the Past 800 Years: Muldoons Trap Complex, Lake Condah, Southwestern Victoria.” Australian Archaeology 81 (1): 44–58.
Michel, Véronique, Hélène Valladas, Guanjun Shen, Wei Wang, Jian-xin Zhao, Chuan-Chou Shen, Patricia Valensi, and Christopher J. Bae. 2016. “The Earliest Modern Homo sapiens in China?” Journal of Human Evolution 101: 101–104.
Miller, D. Shane, Vance T. Holliday, and Jordon Bright. 2013. “Clovis across the Continent.” In Paleoamerican Odyssey, edited by Kelly E. Graf, Caroline V. Ketron, and Michael R. Waters, 207–220. College Station: Texas A&M University Press.
Neubauer, Simon, Jean-Jacques Hublin, and Philipp Gunz. 2018. “The Evolution of Modern Human Brain Shape.” Science Advances 4 (1): eaao5961. https://doi.org/10.1126/sciadv.aao5961.
Pearson, Osbjorn M. 2000. “Postcranial Remains and the Origin of Modern Humans.” Evolutionary Anthropology 9: 229–247.
Pearson, Osbjorn M. 2008. “Statistical and Biological Definitions of ‘Anatomically Modern’ Humans: Suggestions for a Unified Approach to Modern Morphology.” Evolutionary Anthropology: Issues, News, and Reviews 17 (1): 38–48.
Pietschnig, Jakob, Lars Penke, Jelte M. Wicherts, Michael Zeiler, and Martin Voracek. 2015. “Meta-Analysis of Associations between Human Brain Volume and Intelligence Differences: How Strong Are They and What Do They Mean?” Neuroscience & Biobehavioral Reviews 57: 411–432.
Posth, Cosimo, Nathan Nakatsuka, Iosif Lazaridis, Pontus Skoglund, Swapan Mallick, Thiseas C. Lamnidis, Nadin Rohland, et al. 2018. “Reconstructing the Deep Population History of Central and South America.” Cell 175 (5): 1185–1197.
Potter, Ben A., James F. Baichtal, Alwynne B. Beaudoin, Lars Fehren-Schmitz, C. Vance Haynes, Vance T. Holliday, Charles E. Holmes, et al. 2018. “Current Evidence Allows Multiple Models for the Peopling of the Americas.” Science Advances 4 (8): eaat5473. https://doi.org/10.1126/sciadv.aat5473.
Reich, David, Richard E. Green, Martin Kircher, Johannes Krause, Nick Patterson, Eric Y. Durand, Bence Viola, et al. 2010. “Genetic History of an Archaic Hominin Group from Denisova Cave in Siberia.” Nature 468 (7327): 1053–1060.
Reich, David, Nick Patterson, Martin Kircher, Frederick Delfin, Madhusudan R. Nandineni, Irina Pugach, Albert Min-Shan Ko, et al. 2011. “Denisova Admixture and the First Modern Human Dispersals into Southeast Asia and Oceania.” American Journal of Human Genetics 89 (4): 516–528.
Richter, Daniel, Rainer Grün, Renaud Joannes-Boyau, Teresa E. Steele, Fethi Amani, Mathieu Rué, Paul Fernandes, et al. 2017. “The Age of the Hominin Fossils from Jebel Irhoud, Morocco, and the Origins of the Middle Stone Age.” Nature 546 (7657): 293–296.
Roberts, Patrick, and Brian A. Stewart. 2018. “Defining the ‘Generalist-Specialist’ Niche for Pleistocene Homo sapiens.” Nature Human Behaviour 2: 542–550.
Rougier, Helene, Ştefan Milota, Ricardo Rodrigo, Mircea Gherase, Laurenţiu Sarcinǎ, Oana Moldovan, João Zilhão, et al. 2007. “Peştera Cu Oase 2 and the Cranial Morphology of Early Modern Europeans.” Proceedings of the National Academy of Sciences 104 (4): 1165–1170.
Sahlins, Marshall. 1968. “Notes on the Original Affluent Society.” In Man the Hunter, edited by R. B. Lee and I. DeVore, 85–89. New York: Aldine Publishing Company.
Sawyer, G. J., and Blaine Maley. 2005. “Neanderthal Reconstructed.” The Anatomical Record (Part B: New Anat.) 283 (1): 23–31.
Scerri, Eleanor M. L., Mark G. Thomas, Andrea Manica, Philipp Gunz, Jay T. Stock, Chris Stringer, Matt Grove, et al. 2018. “Did Our Species Evolve in Subdivided Populations Across Africa, and Why Does It Matter?” Trends in Ecology & Evolution 33 (8): 582–594.
Shea, John J. 2011. “Refuting a Myth about Human Origins.” American Scientist 99 (2): 128–135.
Shea, John J., and Ofer Bar-Yosef. 2005. “Who Were the Skhul/Qafzeh People? An Archaeological Perspective on Eurasia’s Oldest Modern Humans.” Journal of the Israel Prehistoric Society 35: 451–468.
Slatkin, Montgomery, and Fernando Racimo. 2016. “Ancient DNA and Human History.” Proceedings of the National Academy of Sciences 113 (23): 6380–6387.
Smith, Fred H., James C. M. Ahern, Ivor Janković, and Ivor Karavanić. 2017. “The Assimilation Model of Modern Human Origins in Light of Current Genetic and Genomic Knowledge.” Quaternary International 450: 126–136.
Smith, Michael. 2009. “V. Gordon Childe and the Urban Revolution: A Historical Perspective on a Revolution in Urban Studies.” Town Planning Review 80 (1): 3–29.
Stock, Jay T. 2008. “Are Humans Still Evolving?” EMBO Reports 9 (Suppl 1): S51–S54.
Swisher, Mark E., Dennis L. Jenkins, Lionel E. Jackson Jr., and Fred M. Phillips. 2013. “A Reassessment of the Role of the Canadian Ice-Free Corridor in Light of New Geological Evidence.” Poster Symposium 5B: Geology, Geochronology and Paleoenvironments of the First Americans at the Paleoamerican Odyssey Conference, Santa Fe, New Mexico, October 16–19.
Thorne, A. G., and P. G. Macumber. 1972. “Discoveries of Late Pleistocene Man at Kow Swamp, Australia.” Nature 238 (5363): 316–319.
Trinkaus, Erik, Ştefan Milota, Ricardo Rodrigo, Gherase Mircea, and Oana Moldovan. 2003a. “Early Modern Human Cranial Remains from the Peştera Cu Oase, Romania.” Journal of Human Evolution 45 (3): 245–253.
Trinkaus, Erik, Oana Moldovan, Adrian Bîlgăr, Laurenţiu Sarcina, Sheela Athreya, Shara E Bailey, Ricardo Rodrigo, Gherase Mircea, Thomas Higham, and Christopher Bronk Ramsey. 2003b. “An Early Modern Human from the Peştera Cu Oase, Romania.” Proceedings of the National Academy of Sciences 100 (20): 11231–11236.
Velemínská, J., J. Brůzek, P. Velemínský, L. Bigoni, A. Sefcáková, and S. Katina. 2008. “Variability of the Upper-Palaeolithic Skulls from Predmostí Near Prerov (Czech Republic): Craniometric Comparison with Recent Human Standards.” Homo 59 (1): 1–26.
Vidal, Céline M., Christine S. Lane, Asfawossen Asrat, Dan N. Barfod, Darren F. Mark, Emma L. Tomlinson, Ambdemichael Zafu Tadesse, et al. (2022). “Age of the Oldest Known Homo sapiens from Eastern Africa. Nature 601 (7894): 579–583.
Villa, Paola, Sylvain Soriano, Tsenka Tsanova, Ilaria Degano, Thomas F. G. Higham, Francesco d’Errico, Lucinda Backwell, Jeannette J. Lucejko, Maria Perla Colombini, and Peter B. Beaumont. 2012. “Border Cave and the Beginning of the Later Stone Age in South Africa.” Proceedings of the National Academy of Sciences 109 (33): 13208–13213.
Wall, Jeffrey D., and Deborah Yoshihara Caldeira Brandt. 2016. “Archaic Admixture in Human History.” Current Opinion in Genetics & Development 41: 93–97.
White, Tim D., Berhane Asfaw, David DeGusta, Henry Gilbert, Gary D. Richards, Gen Suwa, and F. Clark Howell. 2003. “Pleistocene Homo sapiens from Middle Awash, Ethiopia.” Nature 423 (6941): 742–747.
Woo, Ju-Kang. 1959. “Human Fossils Found in Liukiang, Kwangsi, China.” Vertebrata PalAsiatica 3 (3): 109–118.
Wu, XiuJie, Wu Liu, Wei Dong, JieMin Que, and YanFang Wang. 2008. “The Brain Morphology of Homo Liujiang Cranium Fossil by Three-Dimensional Computed Tomography.” Chinese Science Bulletin 53 (16): 2513–2519.
Acknowledgments
I could not have undertaken this project without the help of many who got me to where I am today. I extend sincere thank yous to the many colleagues and former students who have inspired me to keep learning and talking about anthropology. Thank you also to all who are involved in this textbook project. The anonymous reviewers truly sparked improvements to the chapter. Lastly, the staff of Starbucks #5772 also contributed immensely to this text.
Amanda Wolcott Paskey, M.A., Cosumnes River College
AnnMarie Beasley Cisneros, M.A., American River College
This chapter is a revision from "Chapter 11: Archaic Homo” by Amanda Wolcott Paskey and AnnMarie Beasley Cisneros. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Identify the main groupings of Archaic Homo sapiens, such as Neanderthals.
- Explain how shifting environmental conditions required flexibility of adaptations, both anatomically and behaviorally.
- Describe the unique anatomical and cultural characteristics of Archaic Homo sapiens, including Neanderthals, in contrast to other hominins.
- Articulate how Middle Pleistocene hominin fossils fit into evolutionary trends including cranial capacity (brain size) development, cultural innovations, and migration patterns.
- Identify the shared traits, regional variations, and local adaptations among Archaic Homo sapiens.
- Detail the increased complexity and debates surrounding the classification of hominins in light of transitional species, species admixture, etc.
Breaking the Stigma of the "Caveman"
What do you think of when you hear the word “caveman”? Perhaps you imagine a character from a film such as The Croods, Tarzan, and Encino Man or from the cartoon The Flintstones. Maybe you picture the tennis-playing, therapy-going hairy Neanderthals from Geico Insurance commercials. Or perhaps you imagine characters from The Far Side or B.C. comics. Whichever you picture, the character in your mind is likely stooped over with a heavy brow, tangled long locks and other body hair, and clothed in animal skins, if anything. They might be holding a club with a confused look on their face, standing at the entrance to a cave or dragging an animal carcass to a fire for their next meal (see Figure 11.1). You might have even signed up to take this course because of what you knew—or expected to learn—about “cavemen.”
These images have long been the stigma and expectation about our ancestors at the transition to modern Homo sapiens. Tracing back to works as early as Carl Linnaeus, scientists once propagated and advanced this imagery, creating a clear picture in the minds of early scholars that informed the general public, even

through today, that Archaic Homo sapiens, “cavemen,” were somehow fundamentally different and much less intelligent than we are now. Unfortunately, this view is overly simplistic, misleading, and incorrect. Understanding what Archaic Homo sapiens were actually like requires a much more complex and nuanced picture, one that comes into sharper focus as continuing research uncovers more about the lives of our not-too-distant (and not-too-different) ancestors.
The first characterizations of Archaic Homo sapiens were formed from limited fossil evidence in a time when ethnocentric and species-centric perspectives (anthropocentrism) were more widely accepted and entrenched in both society and science. Today, scientists are working from a more complete fossil record from three continents (Africa, Asia, and Europe), and genetic evidence informs their analyses and conclusions. The existence of Archaic Homo sapiens marks an exciting point in our lineage—a point at which many modern traits had emerged and key refinements were on the horizon. Anatomically, humans today are not that much different from Archaic Homo sapiens.
The Changing Environment
While modern climate change is of critical concern today due to its cause (human activity) and pace (unprecedentedly rapid), the existence of global climate change itself is not a recent phenomenon. The focus of this chapter, the Middle Pleistocene (roughly between 780 kya and 125 kya), is the time period in which Archaic Homo sapiens appears in the fossil record—a time that witnessed some of the most drastic climatic changes in human existence. During this time period, there were 15 major and 50 minor glacial events in Europe, alone.
What exactly is glaciation? When scientists talk about glacial events, they are referring to the climate being in an ice age. This means that the ocean levels were much lower than today, because much of the earth’s water was tied up in large glaciers or ice sheets. Additionally, the average temperature would have been much cooler, which would have better supported an Arctic or tundra-adapted plant-and-animal ecosystem in northern latitudes. The most interesting and relevant features of Middle Pleistocene glacial events are the sheer number of them and their repeated bouts: this era alternated between glacial periods and warmer periods, known as interglacials. In other words, the planet wasn’t in an ice age the whole time.
You can see the dramatic and increasing fluctuations in temperature, recorded through foraminifera, in Figure 11.2. The distance between highs and lows demonstrates the severity of temperature shifts. Much as the Richter scale represents more intense earthquakes with more dramatic peaks, so too does this chart, which uses dramatic peaks to demonstrate intense temperature swings.
Glacial periods are defined by Earth’s average temperature being lower. Worldwide, temperatures are reduced, with cold areas becoming even colder. Huge portions of the landscape may have become inaccessible during glacial events due to the formation of glaciers and massive ice sheets. In Europe, the Scandinavian continental glacier covered what is today Ireland, England, Sweden, Norway, Denmark, and some of continental Europe. Plant and animal communities shifted to lower latitudes along the periphery of ice sheets. Additionally, some new land was opened during glacials. Evaporation with little runoff reduced sea levels by as much as almost 150 meters, shifting coastlines outward by in some instances as much as almost 100 kilometers. Additionally, land became exposed that connected what were previously unconnected continents such as Africa into Yemen at the Gulf of Aden.
Glacial periods also affected equatorial regions and other regions that are today thought of as warmer or at least more temperate parts of the globe, including Africa. While these areas were not covered with glaciers, increased global glaciation resulted in lower sea levels and expanded coastlines. Cooler temperatures were accompanied by the drying of the climate, which caused significantly reduced rainfall, increased aridity, and the expansion of deserts. It is an interesting question to consider whether the same plants and animals that lived in these regions prior to the ice ages would be able to survive and thrive in this new climate. Plant and animal communities shifted in response to the changing climate, whenever possible.
Surviving During the Middle Pleistocene
Rather than a single selective force, the Middle Pleistocene was marked by periods of fluctuation, not just cold periods. Interglacials interrupted glaciations, reversing trends in sea level, coastline, temperature, precipitation, and aridity, as well as glacier size and location. Interglacials are marked by increased rainfall and a higher temperature, which causes built-up ice in glaciers to melt. This leads to glacial retreat, which is the shrinking of glaciers and the movement of the glaciers back toward the poles, as we’ve seen in our lifetime. During interglacials, sea levels increase, flooding some previously exposed coastlines and continental connections. In addition, plant and animal communities shift accordingly, often finding more temperate climates to the north and less arid and more humid climates in the tropics (Van Andel and Tzedakis 1996).
Scientists have found that the Olorgesailie region in southern Kenya was at various times in the Middle Pleistocene a deep lake, a drought-dried lakebed with an area criss-crossed by small streams, and a grassland. While various animal species would have moved in and out of the area as the climate shifted, some animal species went extinct, and new, often related, species took up residence. The trend, scientists noted, was that animals with more specialized features went extinct and animals with more generalized features, such as animals we see today, survived in this changing climatic time period. For example, a zebra with specialized teeth for eating grass was ultimately replaced by a zebra that could eat both grass and other types of vegetation. If this small, localized example shows such a dramatic change in terms of the environment and the plant and animal biocommunities, what would have been the impact on humans?
There is no way humans could have escaped the effects of Middle Pleistocene climate change, no matter what region of the world they were living in. As noted earlier, and as evidenced by what was seen in the other biotic communities, humans would have faced changing food sources as previous sources of food may have gone extinct or moved to a different latitude. Depending on where they were living, fresh water may have been limited. Durial glacials, lower sea levels would have given humans more land to live on, while the interglacials would have reduced the available land through the increase in rainfall and associated sea level rise. Dry land connections between the continents would have made movement from one continent to another by foot easier at times than today, although these passageways were not consistently available through the Middle Pleistocene due to the glacial/interglacial cycle. Finally, as evidenced by the Olorgesailie region in Kenya, during the Middle Pleistocene animal species that were overly specialized to one particular type of environment were less likely to survive when compared to their more generalized counterparts. Evidence suggests that this same pattern may have held true for Archaic Homo sapiens, in terms of their ability to survive this dramatic period of climate change.
Defining Characteristics of Archaic Homo sapiens
Archaic Homo sapiens share our species name but are distinguished by the term “Archaic” as a way of recognizing both the long period of time between their appearance and ours, as well as the way in which human traits have continued to evolve over time—making Archaic Homo sapiens look slightly different from us today, despite being considered the same species. Living throughout Africa, and the Middle East during the Middle Pleistocene, Archaic Homo sapiens are considered, in many ways, transitional between Homo erectus and modern Homo sapiens (see Figure 11.3). Archaic Homo sapiens share the defining trait of an increased brain size of at least 1,100 cc and averaging 1,200 cc, although there are significant regional and temporal (time) variations. Because of these variations, scientists disagree on whether these fossils represent a single, variable species or multiple, closely related species (sometimes called Homo antecessor, Homo bodoensis, Homo heidelbergensis, Homo georgicus, Homo neanderthalensis, and Homo rhodesiensis).
An active area of scholarship in the discipline involves reconciling the diversity of species from this time period and establishing the phylogenetic relationships between them. The term “Archaic Homo sapiens” can mean different things to different scholars within the discipline. The intent of this chapter is to provide an understanding of the diversity of this time period and provide data used to make interpretations from among the most likely possibilities. Although we recognize that some anthropologists split many of these fossils into separate species, until the issue is resolved at the discipline level, this chapter will rely on the widely used naming conventions that refer to many fossils from this time period as Archaic Homo sapiens. We will discuss these contemporaneous fossils as a unit, with the exception of a particularly unique population living in Europe and West Asia known as the Neanderthals, which we will examine separately.
|
Trait |
Homo erectus |
Archaic Homo sapiens (including Neaderthals) |
Anatomically Modern Homo sapiens |
|
Time |
1.8 mya–200,000 ya |
600,000–40,000 ya |
315,000 ya–today |
|
Brain size |
900 cc |
1,200 cc (1,500 cc when including Neanderthals) |
1,400 cc |
|
Skull Shape |
Long and low, angular |
Intermediate |
Short and high, globular |
|
Forehead |
Absent |
Emerging |
Present |
|
Nasal Region |
Projecting nasal bones (bridge of the nose), no midfacial prognathism |
Wider nasal aperture and some midfacial prognathism, particularly pronounced among Neanderthals |
Narrower nasal aperture, no midfacial prognathism |
|
Chin |
Absent |
Absent |
Present |
|
Other Facial Features |
Large brow ridge and large projecting face |
Intermediate |
Small brow ridge and retracted face |
|
Other Skull Features |
Nuchal torus, sagittal keel, thick cranial bone |
Projecting occipital bone, often called occipital bun in Neanderthals; intermediate thickness of cranial bone |
Small bump on rear of skull, if anything; thin cranial bone |
|
Dentition |
Large teeth, especially front teeth |
Slightly smaller teeth; front teeth still large; retromolar gap in Neanderthals |
Smaller teeth |
|
Postcranial Features |
Robust bones of skeleton |
Robust bones of skeleton |
More gracile bones of skeleton |
When comparing Homo erectus, Archaic Homo sapiens, and anatomically modern Homo sapiens, one can see that Archaic Homo sapiens are intermediate in their physical form. For some features, this follows the trends first seen in Homo erectus with other features having early, less developed forms of traits seen in modern Homo sapiens. For example, Archaic Homo sapiens trended toward less angular and higher skulls than Homo erectus. However, the archaic skulls were not as short and globular and had less developed foreheads compared to anatomically modern Homo sapiens. Archaic Homo sapiens had smaller brow ridges and a less-projecting face than Homo erectus and slightly smaller teeth, although incisors and canines were often about as large as those of Homo erectus. Archaic Homo sapiens also had a wider nasal aperture, or opening for the nose, and a forward-projecting midfacial region, which is later seen more fully developed among Neanderthals and is known as midfacial prognathism. The occipital bone often projected and the cranial bone was of intermediate thickness, somewhat reduced from Homo erectus but not nearly as thin as that of anatomically modern Homo sapiens. The postcrania remained fairly robust. Identifying a set of features that is unique to Archaic Homo sapiens is a challenging task, due to both individual and geographic variation—these developments were not all present to the same degree in all individuals. Neanderthals are the exception, as they had several unique traits that made them notably different from modern Homo sapiens as well as their closely related Archaic cousins.
The one thing that is clear about Archaic Homo sapiens is that, despite general features, there is a lot of regional variation, which is first seen in the different Homo erectus specimens across Asia and Africa. While the general features of Archaic Homo sapiens, identified earlier, are present in the fossils of this time period, there are significant regional differences. The majority of this regional variation lies in the degree to which fossils have features more closely aligned with Homo erectus or with anatomically modern Homo sapiens.
To illustrate this point, we will examine three exemplary specimens, one from each of the three continents on which Archaic Homo sapiens lived. First, in Africa, a specimen from Broken Hill is one of several individuals found in the Kabwe lead mine in Zambia. It had a large brain (1,300 cc) and taller cranium as well as many Homo erectus-like skull features, including massive brow ridges, a large face, and thick cranial bones (Figure 11.4). Second, one partial crania from Dali, China, is representative of Archaic Homo sapiens in Asia, with large and robust features with heavy brow ridges, akin to what is seen in Homo erectus, and a large cranial capacity intermediate between Homo erectus and anatomically modern Homo sapiens (Figure 11.5). Third, an almost-complete skeleton was found in northern Spain at Atapuerca. Atapuerca 5 (Figure 11.6) has thick cranial bone, an enlarged cranial capacity, intermediate cranial height, and a more rounded cranium than seen previously. Additionally, Atapuerca 5 demonstrates features that foreshadow Neanderthals, including increased midfacial prognathism. After examining some of the fossils such as those from Kabwe, Dali, and Atapuerca, the transitional nature of Archaic Homo sapiens is clear: their features place them squarely between Homo erectus and modern Homo sapiens.
Due to the transitional nature of Archaic Homo sapiens, identifying the time period with which they are associated is problematic and complex. Generally, it is agreed that Archaic Homo sapiens lived between 600,000 and 200,000 years ago, with regional variation and overlap between Homo erectus on the early end of the spectrum and modern Homo sapiens and Neanderthals on the latter end. The earliest-known Archaic Homo sapiens fossils tentatively date to about 600,000 years ago in Africa, to around 300,000 years ago in Asia, and to about 350,000 years ago in Europe (and potentially as early as 600,000 years ago). Determining the end point of Archaic Homo sapiens is also problematic since it largely depends upon when the next subspecies of Homo sapiens appears and the classification of highly intermediate specimens. For example, in Africa, the end of Archaic Homo sapiens is met with the appearance of modern Homo sapiens, while in Europe it is the appearance of Neanderthals that is traditionally seen as marking the transition from other Archaic Homo sapiens.
It is important to remember that this time period is represented by many branching relationships and assuming an evolutionary trajectory that follows a single linear path would not be correct. Even still, Archaic Homo sapiens mark an important chapter in the human lineage, connecting more ancestral forms, such as Homo erectus, to modern Homo sapiens. During this period of climatic transition and fluctuation, Archaic Homo sapiens mirror the challenges of their environments. Showing increasing regional variation due to the need for local adaptation, there is no single archetype for this group; the defining characteristic seems to be variability.
Neanderthals
One well-known population of Archaic Homo sapiens are the Neanderthals, named after the site where they were first discovered in the Neander Valley, or “thal” in German, located near Dusseldorf, Germany. Popularly known as the stereotypical “cavemen” examined at the outset of this chapter, recent research is upending long-held beliefs about this group of Archaics. Neanderthal behavior was increasingly complex, far beyond what was exhibited by even other Archaic Homo sapiens discussed throughout this chapter. We implore you to forget the image of the iconic caveman and have an open mind when exploring the fossil evidence of the Neanderthals.
It is important to understand why Neanderthals are separated from other Archaic Homo sapiens. Unlike the rest of Archaic Homo sapiens, Neanderthals are easily defined and identified in many ways. Evidence suggests the time period when Neanderthals lived was between 150,000 and 40,000 years ago. There is a clear geographic boundary of where Neanderthals lived: western Europe, the Middle East, and western Asia. No Neanderthal fossils have ever been discovered outside of this area, including Africa. This is a bit curious, as other Archaics seem to have adapted in Africa and then migrated elsewhere, but Neanderthals’ regional association makes sense in light of the environment to which they were best adapted: namely, extreme cold weather. Additionally, Neanderthals have a unique and distinct cluster of physical characteristics. While a few aspects of Neanderthals are shared among some Archaic Homo sapiens, such as the types of tools, most Neanderthal anatomical and behavioral attributes are unique to them.
Neanderthals lived during some of the coldest times during the last Ice Age and at far northern latitudes. This means Neanderthals were living very close to the glacial edge, rather than in a more temperate region of the globe like some of their Archaic Homo sapiens relatives. While able to survive in arctic conditions, most Neanderthal sites dating to the glacial periods were found farther away from the severe cold, in a steppe tundra-like environment, which would have been more hospitable to Neanderthals, and their food sources, both flora and fauna (Ashton 2002; Nicholson 2017; Richter 2006). Their range likely expanded and contracted along with European glacial events, moving into the Middle East during glacial events when Europe became even cooler, and when the animals they hunted would have moved for the same reason. During interglacials, when Europe warmed a bit, Neanderthals and their prey would have been able to move back into Western Europe. Clearly, the true hallmark of Neanderthals is their adaptation to an unstable environment, shifting between warm and cold, as the climate was in constant flux throughout their existence (Adler et al. 2003; Boettger et al. 2009).
Many of the Neanderthals’ defining physical features are more extreme and robust versions of traits seen in other Archaic Homo sapiens, clustered in this single population. Brain size, namely an enlargement of the cranial capacity, is one such trait. The average Neanderthal brain size is around 1,500 cc, and the range for Neanderthal brains can extend to upwards of 1,700 cc. The majority of the increase in the brain occurs in the occipital region, or the back part of the brain, resulting in a skull that has a large cranial capacity with a distinctly long and low shape that is slightly wider than previous forms at the far back of the skull. Modern humans have a brain size comparable to that of Neanderthals; however, our brain expansion occurred in the frontal region of the brain, not the back, as in Neanderthal brains. This difference is also the main reason why Neanderthals lack the vertical forehead that modern humans possess. They simply did not need an enlarged forehead, because their brain expansion occurred in the rear of their brain. Due to cranial expansion, the back of the Neanderthal skull is less angular (as compared to Homo erectus), but not as rounded as Homo sapiens, producing an elongated shape, akin to a football.
Another feature that continues the trend noted in previous hominins is the enlargement of the nasal region, or the nose. Neanderthal noses are large and have a wide nasal aperture, which is the opening for the nose. While the nose is only made up of two bones, the nasals, the true size of the nose can be determined by looking at other facial features, including the nasal aperture, and the angle of the nasal and maxillary, or facial bones. In Neanderthals, these indicate a large, forward-projecting nose that appears to be pulled forward away from the rest of the face. This feature is further emphasized by the backward-sloping nature of the cheekbones, or the zygomatic arches. The unique shape and size of the Neanderthal nose is often characterized by the term midfacial prognathism—a jutting out of the middle portion of the face, or nose. This is in sharp contrast to the prognathism exhibited by other hominins, who exhibited prognathism, or the jutting out, of their jaws.
The teeth of the Neanderthals follow a similar pattern seen in the Archaic Homo sapiens, which is an overall reduction in size, especially as compared to the extremely large teeth seen in the genus Australopithecus. However, while the teeth continued to reduce, the jaw size did not keep pace, leaving Neanderthals with an oversized jaw for their teeth, and a gap between their final molar and the end of their jaw. This gap is called a retromolar gap.
The projecting occipital bone present in other Archaic Homo sapiens is also more prominent in Neanderthals, extending the trend found in Archaics. Among Neanderthals, this projection of bone is easily identified by its bun shape on the back of the skull and is known as an occipital bun. This projection appears quite similar to a dinner roll in size and shape. Its purpose, if any, remains unknown.
Continuing the Archaic Homo sapiens trend, Neanderthal brow ridges are prominent but somewhat smaller in size than those of Homo erectus and earlier Archaic Homo sapiens. In Neanderthals, the brow ridges are also often slightly less arched than those of other Archaic Homo sapiens.
In addition to extending traits present in Archaic Homo sapiens, Neanderthals possess several distinct traits. Neanderthal infraorbital foramina, the holes in the maxillae or cheek bones through which blood vessels pass, are notably enlarged compared to other hominins. The Neanderthal postcrania are also unique in that they demonstrate increased robusticity in terms of the thickness of bones and body proportions that show a barrel-shaped chest and short, stocky limbs, as well as increased musculature. These body portions are seen across the spectrum of Neanderthals—in men, women, and children.
Traditionally, many of the unique traits that Neanderthals possess were seen as adaptations to the extreme cold, dry environments in which they often lived and which exerted strong selective forces. For example, Bergmann’s and Allen’s Rules dictate that an increased body mass and short, stocky limbs are common in animals that live in cold conditions. Neanderthals were said to have matched the predictions of Bergmann’s and Allen’s Rules perfectly (Churchill 2006). In addition, the Neanderthal skull also exhibits adaptations to the cold. Neanderthals’ large infraorbital foramina allow for larger blood vessels, increasing the volume of blood that is found closest to the skin, which helps to keep the skin warmer. Their enlarged noses resulted in longer nasal passages and mucus membranes that warmed and moistened cold air before it reached the lungs. The Neanderthals’ larger nose has long been thought to have acted as a humidifier, easing physical exertion in their climate, although research on this particular trait continues to be studied and debated (Rae et al. 2011).
New research, however, seems to suggest that these unique skeletal adaptations might not have been strict adaptations to cold weather (Evteev et al. 2017; Pearce et al. 2013). For example, large brow ridges might have served as a way to shade the face from the sun. The increased occipital portion of the brain, some researchers state, was to support a larger visual system present in Neanderthals. This visual system would have given them increased light sensitivity, which would have been useful in higher latitudes that had dark winters. And, while recent modeling of nostril airflow on reconstructed Neanderthal specimens supports the notion that Neanderthals had extensive mucus membranes inside their noses, the data shows that modern Homo sapiens are superior to Neanderthals in our ability to use our noses as a way to warm and cool air. However, Neanderthals were able to snort air through their noses better than we can. Why is this important? When combined with the fact that Neanderthals tended to prefer a more temperate, tundra-like environment, and that other physical traits suggest that Neanderthals had huge bodies that needed massive amounts of calories to sustain them, the picture gets clearer. Massive amounts of energy would have been required to power a Neanderthal body, and anything that might have made them more calorically efficient would have been favored. Efficient breathing, through larger noses into large lungs, meaning deeper breaths, would have been favored. To further save energy expenditure, body sizes might have been sacrificed as well. These same types of adaptations are similar to ones seen in children today who are born in high altitudes, not cold climates. Figure 11.7 provides a summary of these unique features of the Neanderthal.
|
Distinct Neanderthal Anatomical Features |
|
|
Brain Size |
1,500 cc average |
|
Skull Shape |
Long and low |
|
Brow Ridge Size |
Large |
|
Nose Size |
Large, with midfacial prognathism |
|
Dentition |
Reduced, but large jaw size, creating retromolar gap |
|
Occipital Region |
Enlarged occipital region, occipital bun |
|
Other Unique Cranial Features |
Large infraorbital foramina |
|
Postcranial Features |
Short and stocky body, increased musculature, barrel-shaped chest |
A classic example of a Neanderthal with all of the characteristics mentioned above is the nearly complete La Ferrassie 1 Neanderthal, from France. This is a male skeleton, with a brain size of around 1640cc, an extremely large nose and infraorbital foramina, brow ridges that are marked in size, and an overall robust skeleton (Figure 11.8).
Neanderthal Culture: Tool Making and Use
One key Neanderthal adaptation was their cultural innovations, which are an important way that hominins adapt to their environment. As you recall, Homo erectus's tools, Acheulean handaxes, represented an increase in complexity over Oldowan tools, allowing more efficient removal of meat and possibly calculated scavenging. In contrast, Neanderthal tools mark a significant innovation in tool-making technique and use with Mousterian tools (named after the Le Moustier site in southwest France). These tools were significantly smaller, thinner, and lighter than Acheulean handaxes and formed a true toolkit. The materials used for Mousterian tools were of higher quality, which allowed for both more precise toolmaking and tool reworking when the tools broke or dulled after frequent reuse. The use of higher-quality materials is also indicative of required forethought and planning to acquire them for tool manufacture. It has been suggested that the Neanderthals, unlike Homo erectus, saved and reused their tools, rather than making new ones each time a tool was needed.
Mousterian tools are constructed in a very unique manner, utilizing the Levallois technique (Figure 11.9), named after the first finds of tools made with this technique, which were discovered in the Levallois-Perret suburb of Paris, France. The Levallois technique is a multistep process that requires preparing the core, or raw material, in a specific way that will yield flakes that are roughly uniform in dimension. The flakes are then turned into individual tools. The preparation of the core is akin to peeling a potato or carrot with a vegetable peeler—when peeling vegetables, you want to remove the skin in long, regular strokes, so that you are taking off the same amount of the vegetable all the way around. In the same way, the Levallois technique requires removing all edges of the cortex, or outside surface of the raw material, in a circle before removing the lid. The flakes, which will eventually be turned into the individual tools, can then be removed from the core. The potential yield of tools from one core would be many, as seen in Figure 11.10, compared to all previous tool-making processes, in which one core yielded a single tool. This manufacturing process might be considered the ultimate zero-waste tool-making technique (Delpiano et al. 2018).

It is suggested that Neanderthal tools were used for a variety of purposes, including cutting, butchering, woodworking or antler working, and hide working. Additionally, because the Mousterian tools were lighter than previous stone tools, Neanderthals could haft, or attach the tool onto a handle, as the stone would not have been too heavy (Degano et al. 2019). Neanderthals attached small stone blades onto short wood or antler handles to make knives or other small weapons, as well as attached larger blades onto longer shafts to make spears. New research examining tar-covered stones and black lumps at several Neanderthal sites in Europe suggests that Neanderthals may have been making tar by distilling it from birch tree bark, which could have been used to glue the stone tool onto its handle. If Neanderthals were, in fact, manufacturing tar to act as glue, this would predate modern humans in Africa using tree resin or similar adhesives by nearly 100,000 years.
Evidence shows that raw materials used by Neanderthals came from distances as far away as 100 km. This could indicate a variety of things regarding Neanderthal behavior, including a limited trade network with other Neanderthal groups or simply a large area scoured by Neanderthals when collecting raw materials. While research on specific applications continues, it should be clear from this brief discussion that Neanderthal tool manufacturing was much more complex than previous tool-making efforts, requiring technical expertise, patience, and skills beyond toolmaking to carry out.
Neanderthal Culture: Hunting and Diet
With their more sophisticated suite of tools and robust muscular bodies, Neanderthals were better armed for hunting than previous hominins. The animal remains in Neanderthal sites show that, unlike earlier Archaic Homo sapiens, Neanderthals were very effective hunters who were able to kill their own prey, rather than relying on scavenging. Though more refined than the tools of earlier hominins, the Neanderthal spear was not the kind of weapon that would have been thrown; rather, it would have been used in a jabbing fashion (Churchill 1998; Kortlandt 2002). This may have required Neanderthals to hunt in groups rather than individually and made it necessary to approach their prey quite closely (Gaudzinski-Windheuser et al. 2018). Remember, the animals living with Neanderthals were very large-bodied due to their adaptations to cold weather; this would have included species of deer, horses, and bovids (relatives of the cow).
Isotopes from Neanderthal bones show that meat was a significant component of their diet, similar to that seen in carnivores like wolves (Bocherens et al. 1999; Jaouen et al. 2019; Richards et al. 2000). In addition to large prey, their diet included ibex, seals, rabbits, and pigeons. Though red meat was a critical component of the Neanderthal diet, evidence shows that at times they also ate limpets, mussels, and pine nuts. Tartar examined from Neanderthal teeth in Iraq and Belgium reveal that they also ate plant material including wheat, barley, date palms, and tubers, first cooking them to make them palatable (Henry et al. 2010). While Neanderthals’ diet varied according to the specific environment in which they lived, meat comprised up to 80% of their diet (Wiẞin et al. 2015).
Neanderthal Culture: Caring for the Injured and Sick
While the close-range style of hunting used by Neanderthals was effective, it also had some major consequences. Many Neanderthal skeletons have been found with significant injuries, which could have caused paralysis or severely limited their mobility. Many of the injuries are to the head, neck, or upper body. Thomas Berger and Erik Trinkaus (1995) conducted a statistical comparative analysis of Neanderthal injuries compared to those recorded in modern-day workers’ compensation reports and found that the closest match was between Neanderthal injuries and those of rodeo workers. Rodeo professionals have a high rate of head and neck injuries that are similar to the Neanderthals’ injuries. What do Neanderthals and rodeo workers have in common? They were both getting very close to large, strong animals, and at times their encounters went awry.
The extensive injuries sustained by Neanderthals are evident in many fossil remains. Shanidar 1 (Figure 11.11), an adult male found at the Shanidar site in northern Iraq and dating to 45,000 ya, has a lifetime of injuries recorded in his bones (Stewart 1977). Shanidar 1 sustained—and healed from—an injury to the face that would have likely caused blindness. His lower right arm was missing and the right humerus shows severe atrophy, likely due to disuse. This pattern has been interpreted to indicate a substantial injury that required or otherwise resulted in amputation or wasting away of the lower arm. Additionally, Shanidar 1 suffered from bony growths in the inner ear that would have significantly impaired his hearing and severe arthritis in the feet. He also exhibited extensive anterior tooth wear, matching the pattern of wear found among modern populations who use their teeth as a tool. Rather than an anomaly, the type of injuries evident in Shanidar 1 are similar to those found in many other Neanderthal fossils, revealing injuries likely sustained from hunting large mammals as well as demonstrating a long life of physical activity.

The pattern of injuries is as significant as the fact that Shanidar 1 and other injured Neanderthals show evidence of having survived their severe injuries. One of the earliest-known Neanderthal discoveries—the one on whom misinformed analysis shaped the stereotype of the species for nearly a century—is the La Chapelle-aux-Saints Neanderthal (Trinkaus 1985). The La Chapelle Neanderthal had a damaged eye orbit that likely caused blindness and suffered arthritis of the spine (Dawson and Trinkaus 1997). He had also lost most of his teeth, many of which he had lived without for so long that the mandibular and maxillary bones were partially reabsorbed due to lack of use. The La Chapelle Neanderthal was also thought to be at least in his mid-forties at death, an old age for the rough life of the Late Pleistocene—giving rise to his nickname, “the Old Man.” To have survived so long with so many injuries that obviously precluded successful large game hunting, he must have been taken care of by others. Such caretaking behavior is also evident in the survival of other seriously injured Neanderthals, such as Shanidar 1. Long thought to be a hallmark modern human characteristic, taking care of the injured and elderly, for example preparing or pre-chewing food for those without teeth, indicates strong social ties among Neanderthals.
Neanderthal Culture: Ritual Life
Such care practices may have been expressed upon death as well. Nearly complete Neanderthal skeletons are not uncommon in the fossil record, and most are well preserved within apparently deliberate burials that involve deep graves and bodies found in specific, often fetal or flexed positions (Harrold 1980). Discoveries of pollen in a grave at the Shanidar site in the 1960s led scientists to think that perhaps Neanderthals had placed flowering plants in the grave, an indication of ritual ceremony or spirituality so common in modern humans. But more recent investigations have raised some doubt about this conclusion (Pomeroy et al. 2020). The pollen may have been brought in by burrowing rodents. Claims of grave goods or other ornamentation in burials are similarly debated, although possible.
Some tantalizing evidence for symbolism, and debatably, ritual, is the frequent occurrence of natural pigments, such as ochre (red) and manganese dioxide (black) in Neanderthal sites that could have been used for art. However, the actual uses of pigments are unclear, as there is very little evidence of art or paintings in Mousterian sites. One exception may be the recent discovery in Spain of a perforated shell that appears to be painted with an orange pigment, which may be evidence of Neanderthal art and jewelry. However, many pigments also have properties that make them good emulsifiers in adhesive (like for attaching a stone tool to a wooden handle) or useful in tanning hides. So the presence of pigment may or may not be associated with symbolic thought; however, it definitely does show a technological sophistication beyond that of earlier Archaic hominins.
Dig Deeper: Evidence of Endocannibalism Among the Neanderthals
Krapina, a Neanderthal site in Croatia, has recently sparked new archeological discourse as many investigations upon fossilized remains show evidence of post-mortem modifications and manipulations of limbs and bones through the use of tools (Rougier et al., 2016). These findings provide compelling evidence of Neanderthals engaging in cannibalism as part of their post-mortem practices. Additionally, these uncovered remains were found to have died of natural causes as opposed to being killed, showing that even the earliest humans may have had some sort of morality or ethics surrounding the cannibalizing of their kin, meaning they were aware of death in a social context as opposed to merely a physical one.
While the original evidence in Krapina was uncovered in 1901, Croatian geologist and paleontologist Dragutin Gorjanović-Kramberger’s discovery of fragmented and burned human bones (Ullrich, 2005) was not yet confirmed to be linked to endocannibalism until much later. Whereas the discovery of burned bones does not mean they were being prepared for consummation, due to its context among other findings, this information supports the hypothesis that early hominids conducted post-mortem rituals and practices with their dead. Building on Gorjanović-Kramberger’s research, Herbert Ullrich wrote in Anthropologie (2005) that broken bones—resulting from post-mortem bodily manipulations—were “defleshed in preparation for secondary burial” (2005, 251) and intentionally left outside rock shelters, while selectively chosen bones were seemingly brought inside for use in mortuary practices.
For nearly 150 years, since the first Neanderthal skeletal remains were discovered, anthropologists and researchers have continued to debate the cognitive, social, and physical abilities of this species. In 2016, Rougier and colleagues wrote in Scientific Reports, furthering the research, presenting 99 new Neanderthal remains found in Goyet, Belgium. Among these remains, similar evidence of human-induced alterations was identified, including signs of butchering, consumption, and the use of bones to modify stone tools (Rougier et al., 2016). This discovery provides significant support for the presence of cannibalistic behaviour among Northern European Neanderthals, contributing to the growing body of evidence that Neanderthals engaged with death in ways that reflect social awareness, ritual behaviour, and complex cultural practices.
Contemporary Cases of Prion Disease Related to Endocannibalism
The evidence of endocannibalism does not end with early hominids; with Australian medical anthropologists recording thousands of cannibalism-related prion disease occurrences present in populations up until 2009 (Radford & Scragg, 2013). Following a mysterious epidemic of a new form of spongiform encephalopathy–a “progressive degenerative disease of the central nervous system” (2013, p.29)–anthropological research regarding the the cultural mortuary rites within the Okapa region of Papua New Guinea have linked the newfound disease ‘Kuru’ to post-mortem consumption of human remains (Radford & Scragg, 2013; Collinge et al., 2006).
Local oral histories collected during the first investigations by these researchers in the 1950s traced the earliest cases back to the 1920s, with detailed case histories. Epidemiological data revealed a strong correlation between the spread of Kuru and participation in mortuary feasts, in which the deceased were ritually consumed as part of funerary rites (2006, p. 2070). From 1957 to 2004, over 2,700 cases were reported, with mortality peaking at over 200 deaths annually in the late 1950s (2006, p.2070); however, following the cessation of cannibalism in the easly 1960s due to governmental efforts, natural transmission of the disease has stopped, dropping the death toll dramatically, with the “last three single cases reported in 2005, 2007, and 2009” (Radford & Scragg, p.48).
The Lasting Gift of Neanderthals: Tantalizing New Directions for Research
Examining the more recent time period in which Neanderthals lived and the extensive excavations completed across Europe allows for a much more complete archaeological record from this time period. Additionally, the increased cultural complexity such as complex tools and ritual behaviors expressed by Neanderthals left a more detailed record than previous hominins. Intentional burials enhanced preservation of the dead and potentially associated ritual behaviors. Such evidence allows for a more complete and nuanced picture of this species.

Additional analyses are possible on many Neanderthal finds, due to increased preservation of bone, the amount of specimens that have been uncovered, and the recency in which Neanderthals lived. We should be cautious, however, to consider the potential bias of many Neanderthal sites. Overwhelmingly, Neanderthal skeletons are found complete, with injuries or evidence of disease in caves. Does this mean all Neanderthals lived a tough, disease-wrought life? Probably not. It does, however, indicate that the sick were cared for by others, and that they lived in environments that preserved their bodies incredibly well. These additional studies include the examination of dental calculus and even DNA analysis. While limited, samples of Neanderthal DNA have been successfully extracted and analyzed. Research thus far has identified specific genetic markers that show some Neanderthals were light-skinned and probably red-haired with light eyes. Genetic analyses, different from the typical hominin reconstruction done with earlier species, allow scientists to further investigate soft tissue markers of Neanderthals and other more recent hominin species. These studies offer striking conclusions regarding Neanderthal traits, their physical appearance, and their culture, as reflected in these artists’ reconstructions (Figure 11.12).

Dr. Svante Pääbo (Figure 11.13), of the Max Planck Institute for Evolutionary Anthropology, has been at the forefront of much of this new research, largely in the form of genomic studies (The Nobel Prize 2022). Awarded the Nobel Prize for Physiology or Medicine in 2022, Pääbo is known primarily for his work with ancient DNA. He has successfully sequenced mitochondrial DNA (mtDNA) as well as the entire Neanderthal genome from nuclear DNA. His genomic work has led to the realization that Denisovans are genetically distinct from Neanderthals, as well as the recent identification of a Neanderthal father and teenage daughter, which he discovered by looking for unique DNA markers in the fossil record. Additionally, Pääbo’s genomic work has provided researchers with additional lines of evidence regarding the connections between hominin fossils (such as Neanderthals) and modern people, their time of divergence, and current genetic overlap. The work of Pääbo has even formalized a new field of study within anthropology—paleogenomics. To stay up to date with Dr. Svante Pääbo’s work, be sure to follow his lab’s website.
Neanderthal Culture: Communicating through Speech
To successfully live in groups and to foster cultural innovations, Neanderthals would have required at least a basic form of communication in order to function, possibly using a speech-based communication system. The challenge with this line of research is that speech, of course, is not preserved, so indirect evidence must be used to support this conclusion. It is thought that Neanderthals would have possessed some basic speech, as evidenced from a variety of sources, including throat anatomy and genetic evidence (Lieberman 1971). There is only one bone in the human body that could demonstrate if a hominin was able to speak, or produce clear vocalizations like modern humans, and that is the hyoid, a U-shaped bone that is found in the throat and is associated with the ability to precisely control the vocal cords. Very few hyoid bones have been found in the archaeological record; however, a few have been uncovered in Neanderthal burials. The shape of the Neanderthal hyoid is nearly identical to that of modern humans, pointing to the likelihood that they had the same vocal capabilities as modern humans. In addition, geneticists have uncovered a mutation present in both modern humans and Neanderthals—the FOXP2 gene—that is possibly linked to the ability to speak. However, other scientists argue that we cannot make sweeping conclusions that the FOXP2 gene accounts for speech due to small sample size. Finally, scientists have also pointed to the increasingly complex cultural behavior of Neanderthals as a sign that symbolic communication, likely through speech, would have been the only way to pass down the skills needed to make, for example, a Levallois blade or to position a body for intentional burial.
Neanderthal Intelligence
One of the enduring questions about Neanderthals centers on their intelligence, specifically in comparison to modern humans. Brain volume indicates that Neanderthals certainly had a large brain, but it continues to be debated if Neanderthals were of equal intelligence to modern humans. Remember, creatures with larger body sizes tend to have larger brains; however, scaling of the brain is not always associated with greater intelligence (Alex 2018). Brain volume (cranial capacity), cultural complexity, tool use, and compassion toward their kind all point to an increase in intellect among Neanderthals when compared to previous hominins.
Yet, new research is suggesting additional differences between Neanderthal brains and our own. For example, Euluned Pearce and colleagues (2013), from the University of Oxford, noted the frontal lobes of Neanderthals and modern humans are almost identical. However, Neanderthals had a larger visual cortex—the portion of the brain involved in processing visual information. This would have left Neanderthals with less brain tissue for other functions, including those that would have aided them in dealing with large social groupings, one of the differences that has been suggested to exist between Neanderthals and modern humans. Other differences were found when geneticist John Blangero, from the Texas Biomedical Research Institute, compared data from the Neanderthal genome against data from modern study participants. Blangero and his colleagues (Blangero et al. 2014) discovered that some Neanderthal brain components were very different, and smaller, than those in the modern sample. Differences were found in areas associated with the processing of information and controlling emotion and motivation, as well as overall brain connectivity. In short, as Blangero stated, “Neanderthals were certainly cognitively adept,” although their specific abilities may have differed from modern humans’ in key areas (qtd. in Wong 2015). This point has been echoed in other recent genetic studies comparing Neanderthal and anatomically modern human brains (el-Showk 2019).
Finally, scientists are fairly certain that Neanderthal brain development after birth was not the same as that of modern humans. After birth, anatomically modern Homo sapiens babies go through a critical period of brain expansion and cognitive development. It appears that Neanderthal babies’ brains and bodies did not follow the same developmental pattern (Smith et al. 2010; Zollikofer and Ponce de León 2013). Modern humans enjoy an extended period of childhood, which allows children to engage in imaginative play and develop creativity that fosters cognitive skills. Neanderthals had a more limited childhood, with less development of the creative mind that may have affected their species’ success (Nowell 2016).
The exact nature of Neanderthal intelligence remains under investigation, however. Some studies disagree with the idea that Neanderthal intelligence had limitations compared to our own, noting the extensive evidence of Neanderthals having limb asymmetry. Their tools also have wear marks indicating that they were hand-dominant. This is further supported by marks on Neanderthal teeth that demonstrate hand dominance. The Neanderthal “stuff-and-cut method” of eating, noted by David Frayer and colleagues (Frayer et al. 2012), would have seen Neanderthals hold a piece of meat in their teeth, while pulling it taut with one hand, and then using the other hand, their dominant one, to cut the meat off of the larger slab being held in their teeth. When looking at 17 Neanderthals and their tooth wear, only two do not show markings associated with a right-hand dominant individual eating in this manner. Further, it has been established that favoring the right hand is a key marker between modern humans and chimpanzees, and that handedness also relates to language development, in the form of bilateral brain development. That Neanderthals likely were hand-dominant suggests they had an indicator of bilateral brain development and a precondition for human speech.
The Middle Stone Age: Neanderthal Contemporaries in Africa
While Neanderthals made their home on and adapted to the European and Asian continents, evidence of fossil humans in Africa show they were also adapting to their local environments. These populations in Africa exhibit many more similarities to modern humans than Neanderthals, as well as overall evolutionary success. While the African fossil sample size is smaller and more fragmentary than the number of Neanderthal specimens across Europe and Asia, the African sample is interesting in that it represents a longer time period and larger geographical area. This group of fossils—often represented by the name “Middle Stone Age,” or MSA—dates to between 300,000 and 30,000 years ago across the entire continent of Africa. As with Archaic Homo sapiens, there is much variability seen in this African set of fossils. There are also a few key consistent elements: none of them exhibit Neanderthal skeletal features; instead, they demonstrate features that are increasingly consistent with anatomically modern Homo sapiens.
Similarities to Neanderthals and MSA contemporaries in Africa are seen, however, in their behavioral adaptations, including stone tools and other cultural elements. The tools associated with the specimens living in Africa during this time period are, like their physical features, varied. In some parts of Africa, namely Northern Africa, stone tools from this time so closely resemble Neanderthal tools that they are classified as Mousterian. In sub-Saharan Africa, the stone tools associated with these specimens are labeled as MSA. Some scholars argue that these could also be a type of Mousterian tools, but they are still typically subdivided based on geographical location.
Recall that Mousterian tools were much more advanced than their Acheulean predecessors in terms of how the stone tools were manufactured, the quality of the stones used, and the ultimate use of the tools that were made. In addition, recent evidence suggests that MSA tools may also have been heat treated—to improve the quality of the stone tool produced (Stolarczyk and Schmidt 2018). Evidence for heat treating is seen not only through advanced analysis of the tool itself but also through the residue of fires from this time period. Fire residues show a shift over time from small, short fires fueled by grasses (probably intended for cooking) to larger, more intensive fires that required the exploitation of dry wood, exactly the type of fire that would have been needed for heat treating stone tools (Esteban et al. 2018).
Other cultural elements seen with MSA specimens include the use of marine (sea-based) resources for their diet (Parkington 2003), manufacture of bone tools, use of adhesive and compound tools (e.g., hafted tools), shell bead production, engraving, use of pigments (such as ochre), and other more advanced tool-making technology (e.g., microlithics). While many of these cultural elements are also seen to a limited extent among Neanderthals, developments at MSA sites appear more complex. This MSA cultural expansion may have been a response to climate change or an increased use of language, complex communication, and/or symbolic thought. Others have suggested that the MSA cultural expansion was due to the increase of marine resources in their diet, which included more fatty acids that may have aided their cognitive development. Still others have suggested that the increased cultural complexity was due to increased interaction among groups, which spurred competition to innovate. Recent studies suggest that perhaps the best explanation for the marked cultural complexity of MSA cultural artifacts is best explained by the simple fact that they lived in diverse habitats (Kandel et al. 2015). This would have necessitated a unique set of cultural adaptations for each habitat type (for example, specialized marine tools would have been needed along coastal sites but not at inland locations). Simply put, the most useful adaptation of MSA was their flexibility of behavior and adaptability to their local environment. As noted previously in this chapter, flexibility of behavior and physical traits, rather than specialization, seems to be a feature that was favored in hominin evolution at this time.
Where Did They Go? The End of Neanderthals
While MSA specimens were increasingly successful and ultimately transitioned into modern Homo sapiens, Neanderthals disappeared from the fossil record by around 40,000 years ago. What happened to them? We know, based on genetics, that modern humans come largely from the modern people who occupied Africa around 300,000 to 100,000 years ago, at the same time that Neanderthals were living in northern Europe and Asia. As you will learn in Chapter 12, modern humans expanded out of Africa around 150,000 years ago, rapidly entering areas of Europe and Asia inhabited by Neanderthals and other Archaic hominins. Despite intense interest and speculation in fictional works about possible interactions between these two groups, there is very little direct evidence of either peaceful coexistence or aggressive encounters. It is clear, though, that these two closely related hominins shared Europe for thousands of years, and recent DNA evidence suggests that they occasionally interbred (Fu et al. 2015). Geneticists have found traces of Neanderthal DNA (as much as 1% to 4%) in modern humans of European and Asian descent not present in modern humans from sub-Saharan Africa. This is indicative of limited regional interbreeding with Neanderthals.
While some interbreeding likely occurred, as a whole, Neanderthals did not survive. What is the cause for their extinction? This question has fascinated many researchers and several possibilities (theories) have been suggested, including:
- At the time that Neanderthals were disappearing from the fossil record, the climate went through both cooling and warming periods—each of which posed challenges for Neanderthal survival (Defleur and Desclaux 2019; Staubwasser et al. 2018). It has been argued that as temperatures warmed, large-bodied animals, well adapted to cold weather, moved farther north to find colder environments or faced extinction. A shifting resource base could have been problematic for continued Neanderthal existence, especially as additional humans, in the form of modern Homo sapiens, began to appear in Europe and compete for a smaller pool of available resources.
- It has been suggested that the eruption of a European volcano 40,000 years ago could have put a strain on available plant resources (Golovanova et al. 2010). The eruption would have greatly affected local microclimates, reducing the overall temperature enough to alter the growing season.
- Possible differences in cognitive development may have limited Neanderthals in terms of their creative problem solving. As much as they were biologically specialized for their environment, the nature of their intelligence might not have offered them the creative problem-solving skills to innovate ways to adapt their culture when faced with a changing environment (Pearce et al. 2013).
- CRISPR gene-editing technology has been used in studies to evaluate potential differences between human and Neanderthal brains, based on differences in the genetic code. Potential differences include a Neanderthal propensity for mutations related to brain development that could account for more rapid brain development, maturation, synapse misfires, and less-orderly neural processes (Mora-Bermúdez et al. 2022; Trujillo et al. 2021). Fundamental differences in brain function at the cellular level may account for the differential survival rates of Neanderthal and modern human populations.
- There is evidence that suggests reproduction may have posed challenges for Neanderthals. Childbirth was thought to have been at least as difficult for female Neanderthals as anatomically modern Homo sapiens (Weaver and Hublin 2009). Female Neanderthals may have become sexually mature at an older age, even older than modern humans. This delayed maturation could have kept the Neanderthal population size small. A recent study has further suggested that male Neanderthals might have had a genetic marker on the Y chromosome that could have caused incompatibility between the fetus and mother during gestation; this would have had severe consequences for birth rate and survival (Mendez et al. 2016). Even a small but continuous decrease in fertility would have been enough to result in the extinction of Neanderthals (Degioanni et al. 2019).
- As mentioned above, the end of Neanderthal existence overlaps with modern human expansion into northern Europe and Asia. There is no conclusive direct evidence to indicate that Neanderthals and modern humans lived peacefully side by side, nor that they engaged in warfare, but by studying modern societies and the tendencies of modern humans, it has been suggested that modern humans may not have warmly embraced their close but slightly odd-looking cousins when they first encountered them (Churchill et al. 2009). Nevertheless, direct competition with modern humans for the same resources may have contributed to the Neanderthals’ decline (Gilpin et al. 2016); it may also have exposed them to new diseases, brought by modern humans (Houldcroft and Underdown 2016), which further decimated their population. Estimates of energy expenditures suggest Neanderthals had slightly higher caloric needs than modern humans (Venner 2018). When competing for similar resources, the slightly greater efficiency of modern humans might have helped them experience greater success in the face of competition—at a cost to Neanderthals.
It is suggested that the Neanderthal populations were fairly small to begin with (estimated between 5,000 and 70,000 individuals; Bocquet-Appel and Degioanni 2013), one or a combination of these factors could have easily led to their demise. As more research is conducted, we will likely get a better picture of exactly what led to Neanderthal extinction.
Denisovans

While Neanderthals represent one regionally adapted branch of the Archaic Homo sapiens family tree, recent discoveries in Siberia and the Tibetan Plateau surprised paleoanthropologists by revealing yet another population that was contemporary with Archaic Homo sapiens, Neanderthals, and modern Homo sapiens. The genetic analysis of a child’s finger bone (Figure 11.14) and an adult upper third molar (Figure 11.15) from Denisova Cave in the Altai Mountains in Siberia by a team including Svante Pääbo discovered that the mitochondrial and nuclear DNA sequences reflected distinct genetic differences from all known Archaic populations. Dubbed “Denisovans” after the cave in which the bones were found, this population is more closely related to Neanderthals than modern humans, suggesting the two groups shared an ancestor who split from modern humans first, then the Neanderthal-Denisovan line diverged more recently (Reich et al. 2010).
Denisovans share up to 5% of their DNA with modern Melanesians, aboriginal Australians, and Polynesians, and 0.2% of their DNA with other modern Asian populations and Native Americans. Additional studies have suggested one (Vernot et al. 2018) or two (Browning et al. 2018) separate points of time when interbreeding occurred between modern humans and Denisovans.
Genetic analysis reveals that Denisovans (potentially three distinct populations) had adaptations for life at high altitudes that prevented them from developing altitude sickness and hypoxia in extreme environments such as Tibet, where the average annual temperature is close to 0℃ and the altitude is more than a kilometer (about 4,000 feet) above sea level. Through protein analysis of a jawbone, one study (Chen et al. 2019) has placed Denisovans in Tibet as early as 160,000 years ago. Genetic evidence of interbreeding has linked modern Tibetan populations with Denisovans 30,000 to 40,000 years ago, which implies that the unique high-altitude adaptations seen in modern Tibetans may have originated with Denisovans (Huerta-Sanchez et al. 2014).
Other research suggests tantalizing new directions regarding Denisovans. Stone tools similar to those found in Siberia have been uncovered in the Tibetan plateau suggesting a connection between the Denisovan populations in those two areas (Zhang et al. 2018). The molar of a young girl, possibly Denisovan, has been found in Laos and shows strong similarities to specimens from China (Demeter et al. 2022). And DNA sequencing from discoveries in the Denisova Cave have yielded a genome that has been interpreted as the first-generation offspring of a Denisovan father and Neanderthal mother (Slon et al. 2018). While this research is not yet conclusive and is still being interpreted, exciting new possibilities are being revealed. To stay up-to-date with new discoveries, consider following organizations such as the Smithsonian’s Human Origins Program on social media.
How Do These Fit In? Homo naledi and Homo floresiensis
Recently, some fossils have been unearthed that have challenged our understanding of the hominin lineage. The fossils of Homo naledi and Homo floresiensis are significant for several reasons but are mostly known for how they don’t fit the previously held patterns of hominin evolution. While we examine information about these species, we ask you to consider the evidence presented in this chapter and others to draw your own conclusions regarding the significance and placement of these two unusual fossil species in the hominin lineage.
Homo naledi
In 2013 recreational spelunkers uncovered a collection of bones deep in a cave network in Johannesburg, South Africa. The cave system, known as Rising Star, had been well documented by other cavers; however, it appears few people had ever gone as far into the cave as these spelunkers did. Lee Berger, paleoanthropologist at University of Witwatersrand, in Johannesburg, immediately put out a call for what he termed “underground astronauts” to begin recovery and excavation of the fossil materials. Unlike other excavations, Berger and most other paleoanthropologists would not be able to access the elusive site, as it was incredibly difficult to reach, and at some points there was only eight inches of space through which to navigate. The underground astronauts, all petite, slender female anthropologists, were the only ones who were able to access this remarkable site. Armed with small excavation tools and a video camera, which streamed the footage up to the rest of the team at the surface, the team worked together and uncovered a total of 1,550 bones, representing at least 15 individuals, as seen in Figure 11.16. Later, an additional 131 bones, including an almost-complete cranium, were found in a nearby chamber of the cave, representing three more individuals (Figure 11.17). Berger called in a team of specialists to participate in what was dubbed “Paleoanthropology Summer Camp.” Each researcher specialized in a different portion of the hominin skeleton. With various specialists working simultaneously, more rapid analysis was possible of Homo naledi than most fossil discoveries.
While access to the site, approximately 80 m from any known cave entrance or opening, was treacherous for researchers, it must have been difficult for Homo naledi as well. The route included moving through a portion that is just 25 cm wide at some points, known as “Superman’s Crawl.” The only way to get through this section is by crawling on your stomach with one arm by your side and the other raised above your head. Past Superman’s Crawl, a jagged wall known as the Dragon’s Back would have been very difficult to traverse. Below that, a narrow vertical chute would have eventually led down to the area where the fossils were discovered. While geology changes over time and the cave system likely has undergone its fair share, it is not likely that these features arose after Homo naledi lived (Dirks et al. 2017). This has made scientists curious as to how the bones ended up in the bottom of the cave system in the first place. It has been suggested that Homo naledi deposited the bones there, one way or another. If Homo naledi did deposit the bones, either through random disposal or intentional burial, this raises questions regarding their symbolic behavior and other cultural traits, including the use of fire, to access a very dark cave system. Another competing idea is that a few individuals may have entered the cave system to escape a predator and then got stuck. To account for the sheer number of fossils, this would have had to happen multiple times.
The features of Homo naledi are well-documented due to the fairly large sample, which represents individuals of all sexes and a wide range of ages. The skull shape and features are very much like other members of the genus Homo—including a sagittal keel and large brow, like Homo erectus, and a well-developed frontal lobe, similar to modern humans—yet the brain size is significantly smaller than its counterparts, at approximately 500 cc (560 cc for males and 465 cc for females). The teeth also exhibit features of later members of the genus Homo, such as Neanderthals, including a reduction in overall tooth size. Homo naledi also had unique shoulder anatomy and curved fingers, indicating similarities to tree-dwelling primates, which is very different from any other hominin yet found. Perhaps the greatest shock of all is that Homo naledi has been dated to 335,000 to 236,000 years ago, placing it as a contemporary to modern Homo sapiens, despite its very primitive features. An additional specimen of a child, found in 2021, not only shares many of the unique features found in the adult specimen but will also add insight into the growth and development of individuals of this species (Brophy et al. 2021).
Homo floresiensis
In a small cave called Liang Bua, on the island of Flores, in Indonesia, a small collection of fossils were discovered beginning in 2003 (Figure 11.18). The fossil fragments represent as many as nine individuals, including a nearly complete female skeleton. The features of the skull are very similar to that of Homo erectus, including the presence of a sagittal keel, an arching brow ridges and nuchal torus, and the lack of a chin (Figure 11.19). Homo floresiensis, as the new species is called, had a brain size that was remarkably small at 400 cc, and recent genetic studies suggest a common ancestor with modern humans that predates Homo erectus.

The complete female skeleton, who was an adult, was approximately a meter tall and would have weighed just under 30 kg, which is significantly shorter and just a few kilograms more than the average, modern, young elementary-aged child. A reconstructed comparison between an anatomically modern human and Homo floresiensis can be seen in Figure 11.20. The small size of the fossil has earned the species the nickname “the Hobbit.” Many questions have been asked about the stature of this species, as all of the specimens found also show evidence of diminutive stature and small brain size. Some explanations include pathology; however, this seems unlikely as all fossils found thus far demonstrate the same pattern. Another possible explanation lies in a biological phenomena seen in other animal species also found on the island, which date to a similar time period. This phenomenon, called insular dwarfing, is due to limited food resources on an island, which can create a selective pressure for large-bodied species to be selected for smaller size, as an island would not have been able to support their larger-bodied cousins for a long period of time. This phenomenon is the cause of other unique species known to have lived on the island at the same time, including the miniature stegodon, a dwarf elephant species.
There is ongoing research and debate regarding Homo floresiensis’ dates of existence, with some researchers concluding that they lived on Flores until perhaps as recently as 17,000 years ago, although they are more often dated to 100,000 to 60,000 years ago. Stone tools from that time period uncovered at the site are similar to other hominin stone tools found on the island of Flores. Homo floresiensis would have hunted a wide range of animals, including the miniature stegodon, giant rats, and other large rodents. Other animals on the island that could have threatened them include the giant komodo dragon. An interesting note about this island chain is that ancestors of Homo floresiensis would have had to traverse the open ocean in order to get there, as the nearest island is almost 10 km away, and there is little evidence to support that a land bridge connecting mainland Asia or Australia to the island would have been present. This separation from the mainland would also have limited the number of other animals, including predators and human species, that would have had access to the island. Anatomically modern Homo sapiens arrived on the island around 30,000 years ago and, if some researchers’ later dates for Homo floresiensis are correct, both species may have lived on Flores at the same time. The modern population living on the island of Flores today believes that their ancestors came from the Liang Bua cave; however, recent genetic studies have determined they are not related to Homo floresiensis (Tucci et al. 2018).
Summary
Research presented in the chapter contributes to why scientists have taken to nicknaming this time period “the muddle in the middle.” We know that the Middle Pleistocene picks up from Homo erectus and ends with the appearance of anatomically modern Homo sapiens. While the start and the end are clear, it’s the middle that is messy. As more research is conducted and more data is collected, rather than clarifying our understanding of the hominin lineage during this time period, it only inspires more questions, particularly about the relationships between hominins during this time period, including the oft-misunderstood Neanderthal. Research is painting a more detailed picture of Neanderthal intelligence and both biological and behavioral adaptations. At the same time, their relationship to other Middle Pleistocene hominins, including Denisovans, as well as modern humans, remains unclear.
Homo naledi and Homo floresiensis are clear outliers when compared to their contemporary hominin species. Each has surprised paleoanthropologists for both their archaic traits in relatively modern times and their unique combination of traits seen in archaic species and modern humans. While these finds have been exciting, they have also completely upended the assumed trajectory of the human lineage, causing scientists to re-examine assumptions about hominin evolution and what it means to be modern. Add this to the developments being made using ancient DNA, other new fossil discoveries, and other innovations in paleoanthropology, and you see that our understanding of Archaic Homo sapiens and others living during this time period is rapidly developing and changing. This is a true testament to the nature of science and the scientific method.
Clearly, hominins of the Middle Pleistocene are distinct from our species today. Yet, understanding the hominins that directly preceded our species and clarifying the evolutionary relationships between us is important to better understanding our own place in nature.
Hominin Species Summaries
|
Hominin |
Archaic Homo sapiens |
|
Dates |
600,000–200,000 years ago (although some regional variation) |
|
Region(s) |
Africa, Europe, and Asia |
|
Famous discoveries |
Broken Hill (Zambia), Atapuerca (Spain) |
|
Brain size |
1,200 cc average |
|
Dentition |
Slightly smaller teeth in back of mouth, larger front teeth |
|
Cranial features |
Emerging forehead, no chin, projecting occipital region |
|
Postcranial features |
Robust skeleton |
|
Culture |
Varied regionally, but some continue to use Acheulean handaxe, others adopt Mousterian tool culture |
|
Other |
Lots of regional variation in this species |
|
Species |
Homo naledi |
|
Dates |
335,000–236,000 years ago |
|
Region(s) |
South Africa |
|
Famous discoveries |
Rising Star Cave |
|
Brain size |
500 cc average |
|
Dentition |
Reduced tooth size |
|
Cranial features |
Sagittal keel, large brow, well-developed frontal region |
|
Postcranial features |
Suspensory shoulder |
|
Culture |
unknown |
|
Other |
N/A |
|
Hominin |
Neanderthals |
|
Dates |
150,000–40,000 years ago |
|
Region(s) |
Western Europe, Middle East, and Western Asia only |
|
Famous discoveries |
Shanidar (Iraq), La Chapelle-aux-Saints (France) |
|
Brain size |
1500 cc average |
|
Dentition |
Retromolar gap |
|
Cranial features |
Large brow ridge, midfacial prognathism, large infraorbital foramina, occipital bun |
|
Postcranial features |
Robust skeleton with short and stocky body, increased musculature, barrel chest |
|
Culture |
Mousterian tools often constructed using the Levallois technique |
|
Other |
N/A |
|
Species |
Homo floresiensis |
|
Dates |
100,000–60,000 years ago, perhaps as recently as 17,000 years ago |
|
Region(s) |
Liang Bua, island of Flores, Indonesia |
|
Famous discoveries |
“The Hobbit” |
|
Brain size |
400 cc average |
|
Dentition |
unknown |
|
Cranial features |
Sagittal keel, arching brow ridges, nuchal torus, no chin |
|
Postcranial features |
Very short stature (approximately 3.5 ft.) |
|
Culture |
Tools similar to other tools found on the island of Flores |
|
Other |
N/A |
|
Hominin |
Denisovans |
|
Dates |
100,000–30,000 years ago |
|
Region(s) |
Siberia |
|
Famous discoveries |
Child’s finger bone and adult molar |
|
Brain size |
unknown |
|
Dentition |
Large molars (from limited evidence) |
|
Cranial features |
unknown |
|
Postcranial features |
unknown |
|
Culture |
unknown |
|
Other |
Closely related to Neanderthals (genetically) |
Review Questions
- What physical and cultural features are unique to Archaic Homo sapiens? How are Archaic Homo sapiens different in both physical and cultural characteristics from Homo erectus?
- Describe the specific changes to the brain and skull first seen in Archaic Homo sapiens. Why does the shape of the skull change so dramatically from Homo erectus?
- What role did the shifting environment play in the adaptation of Archaic Homo sapiens, including Neanderthals? Discuss at least one physical feature and one cultural feature that would have assisted these groups in surviving the changing environment.
- What does the regional variation in Archaic Homo sapiens represent in terms of the broader story of our species’ evolution?
- Describe the issues raised by the discoveries of Homo naledi and Homo floresiensis in the understanding of the story of the evolution of Homo sapiens.
Key Terms
Allele: Each of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome.
Anthropocentrism: A way of thinking that assumes humans are the most important species and leads to interpreting the world always through a human lens. Species-centric science and thought.
Cortex: The outside, or rough outer covering, of a rock. Usually the cortex is removed during the process of stone tool creation.
Ethnocentric: Applying negative judgments to other cultures based on comparison to one’s own.
Exogenous DNA: DNA that originates from sources outside of the specimen you are trying to sequence.
Flexed position: Fetal position, in which the legs are drawn up to the middle of the body and the arms are drawn toward the body center. Intentional burials are often found in the flexed body position.
Foraminifera: Microscopic single-celled organisms with a shell that are common in all marine environments. The fossil record of foraminifera extends back well over 500 million years.
Glaciation: A glacial period, or time when a large portion of the world is covered by glaciers and ice sheets.
Globular: Round-shaped, like a globe.
Grave goods: Items included with a body at burial. Items may signify occupation or hobbies, social status, or level of importance in the community, or they may be items believed necessary for the afterlife.
Haft: A handle. Also used as a verb—to attach a handle to an item, such as a stone tool.
Infraorbital foramina: Small holes on the maxilla bone of the face that allows nerves and blood to reach the skin.
Insular dwarfing: A form of dwarfism that occurs when a limited geographic region, such as an island, causes a large-bodied animal to be selected for a smaller body size.
Interglacial: A warmer period between two glacial time periods.
Levallois technique: A distinctive technique of stone tool manufacturing used by Archaic Homo sapiens, including Neanderthals. The technique involves the preparation of a core and striking edges off in a regular fashion around the core. Then a series of similarly sized pieces can be removed, which can then be turned into different tools.
Midfacial prognathism: A forward projection of the nose or the middle facial region. Usually associated with Neanderthals.
Mousterian tools: The stone tool industry of Neanderthals and their contemporaries in Africa and Western Asia. Mousterian tools are known for a diverse set of flake tools, which is different from the large bifacial tools of the Acheulean industry.
Nasal aperture: The opening for the nose visible on a skull. Often pear- or heart-shaped.
Occipital bun: A prominent bulge or projection on the back of the skull, specifically the occipital bone. This is a feature present only on Neanderthal skulls.
Ochre: A natural clay pigment mixed with ferric oxide and clay and sand. Ranges in color from brown to red to orange.
Retracted face: A face that is flatter.
Retromolar gap: A space behind the last molar and the end of the jaw. This is a feature present only on Neanderthals. It also occurs through cultural modification in modern humans who have had their third molars, or wisdom teeth, removed.
For Further Exploration
Anne and Bernard Spitzer Hall of Human Origins—American Museum of Natural History.
“Dawn of Humanity,” PBS documentary, 2015
“DNA Clues to Our Inner Neanderthal,” TED Talk by Svante Pääbo, 2011.
“The Dirt” Podcast, Episode 30, “The Human Family Tree (Shrub? Crabgrass? Tumbleweed?), Part 3: Very Humany Indeed”.
Frank, Rebecca. 2021. “The Genus Homo.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Hobbits on Flores, Indonesia - Smithsonian Human Origins.
Lumping or Splitting in the Fossil Record - UC Berkeley Understanding Evolution.
Neandertals and More - Max Planck Institute for Evolutionary Anthropology.
Neanderthals: Body of Evidence - SAPIENS.
Perash, Rose L., and Kristen A. Broehl. 2021. “Hominin Review: Evolutionary Trends.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Perkl, Bradley. “Brain, Language, Lithics.” In Explorations: Lab and Activity Manual, edited by. Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. CC BY-NC. Arlington, VA: American Anthropological Association.
Shanidar 3 - Neanderthal Skeleton - Smithsonian Human Origins.
Species - Smithsonian Human Origins.
Smithsonian Human Origins Program Facebook page (@smithsonian.humanorigins).
Paleoartist Brings Human Evolution to Life - Elisabeth Daynés.
References
Adler, Daniel S., Timothy J. Prindiville, and Nicholas J. Conard. 2003. “Patterns of Spatial Organization and Land Use During the Eemian Interglacial in the Rhineland: New Data from Wallertheim, Germany.” Eurasian Prehistory 1(2): 25–78.
Alex, Bridget. 2018. “Neanderthal Brains: Bigger, Not Necessarily Better.” Discover, September 21, 2018. https://www.discovermagazine.com/planet-earth/neanderthal-brains-bigger-not-necessarily-better.
Ashton, Nick M. 2002. “Absence of Humans in Britain during the Last Interglacial Period (Oxygen Isotope Stage 5e).” Publications du CERP 8: 93–103.
Berger, Lee R., John Hawks, Darryl J. de Ruiter, Steven E. Churchill, Peter Schmid, Lucas K. Delezene, Tracy L. Kivell, et al. 2015. “Homo naledi, a New Species of the Genus Homo from the Dinaledi Chamber, South Africa.” eLife 4:e09560. https://doi.org/10.7554/eLife.09560.
Berger, Thomas D., and Erik Trinkaus. 1995. “Patterns of Trauma among the Neanderthals.” Journal of Archaeological Science 22 (6): 841–852.
Blangero, J., E.E. Quillen, M.A. Almeida, D.R. McKay, J.M. Peralta, S. Williams-Blangero, J.E. Curran, R. Duggirala, D.C. Glahn. 2014. “Genomic Reconstruction of Neandertal Brain Structure and Function.” Paper presented at the American Association of Physical Anthropologists Annual Meeting, Calgary, Alberta, Canada, 12 April 2014.
Bocherens, Hervé, Daniel Billiou, André Mariotti, Marylène Patou-Mathis, Marcel Otte, Dominique Bonjean, and Michel Toussaint. 1999. “Palaeoenvionmental and Palaeodietary Implications of Isotopic Biogeochemistry of Last Interglacial Neanderthal and Mammal Bones in Scladina Cave (Belgium).” Journal of Archaeological Science 26 (6): 599–607.
Bocquet-Appel, Jean-Pierre and Anna Degioanni. 2013. “Neanderthal Demographic Estimates.” Current Anthropology 54 (S8): S202–S213.
Boettger, Tatjana, Elena Yu. Novenko, Andrej A. Velichko, Olga K. Borisova, Konstantin V. Kremenetski, Stefan Knetsch, and Frank W. Junge. 2009. “Instability of Climate and Vegetation Dynamics in Central and Eastern Europe during the Final Stage of the Last Interglacial (Eemian, Mikulino) and Early Glaciation.” Quaternary International 207 (1-2): 137–144. https://doi.org/10.1016/j.quaint.2009.05.006.
Brophy, Juliet K., Marina C. Elliott, Darryl J. De Ruiter, Debra R. Bolter, Steven E. Churchill, Christopher S. Walker, John Hawks, and Lee R. Berger. 2021. “Immature Hominin Craniodental Remains from a New Locality in the Rising Star Cave System, South Africa.” PaleoAnthropology 1: 1–14. https://doi.org/10.48738/2021.iss1.64.
Browning, Sharon R., Brian L. Browning, Ying Zhou, Serena Tucci, and Josh M. Akey. 2018. “Analysis of Human Sequence Data Reveals Two Pulses of Archaic Denisovan Admixture.” Cell 173 (1): 53–61.
Chen, Fahu, Frido Welker, Chuan-Chou Shen, Shara E. Bailey, Inga Bergmann, Simon Davis, Huan Xia, et al. 2019. “A Late Middle Pleistocene Denisovan Mandible from the Tibetan Plateau.” Nature 569: 409–412. https://doi.org/10.1038/s41586-019-1139-x.
Churchill, Steven E. 1998. “Cold Adaptations, Heterochrony, and Neanderthals.” Evolutionary Anthropology 7 (2): 46–60.
Churchill, Steven E. 2006. “Bioenergetic Perspective on Neanderthal Thermoregulatory and Activity Budgets.” In Neanderthals Revisited: New Approaches and Perspectives, edited by K. Havarti and T. Harrison, pp. 113–134. Dordrecht, Germany: Springer.
Churchill, Steven E., Robert G. Franciscus, Hilary A. McKean-Peraza, Julie A. Daniel, and Brittany R. Warren. 2009. “Shanidar 3 Neanderthal Rib Puncture Wound and Paleolithic Weaponry.” Journal of Human Evolution 57 (2): 163–178.
Collinge, J., Whitfield, J., McKintosh, E., Beck, J., Mead, S., Thomas, D. J., & Alpers, M. P. (2006). Kuru in the 21st century--an acquired human prion disease with very long incubation periods. Lancet (London, England), 367(9528), 2068–2074. https://doi.org/10.1016/S0140-6736(06)68930-7
Cook, Rebecca W., Antonio Vazzana, Rita Sorrentino, Stefano Benazzi, Amanda L. Smith, David S. Strait, and Justin A. Ledogar. 2021. “The Cranial Biomechanics and Feeding Performance of Homo floresiensis.” Interface Focus 11 (5). https://doi.org/10.1098/rsfs.2020.0083.
Dawson, James E., and Erik Trinkaus. 1997. “Vertebral Osteoarthritis of La Chapelle-Aux-Saints Neanderthal.” Journal of Archaeological Science 24 (11): 1015-1021. https://doi.org/10.1006/jasc.1996.0179.
Defleur, Alban R., and Emmanuel Desclaux. 2019. “Impact of the Last Interglacial Climate Change on Ecosystems and Neanderthals Behavior at Baume Moula-Guercy, Ardèche, France.” Journal of Archaeological Science 104: 114–124. https://doi.org/10.1016/j.jas.2019.01.002.
Degano, Ilaria, Sylvain Soriano, Paola Villa, Luca Pollarolo, Jeannette J. Lucejko, Zenobia Jacobs, Katerina Douka, Silvana Vitagliano, and Carlo Tozzi. 2019. “Hafting of Middle Paleolithic Tools in Latium (Central Italy): New Data from Fossellone and Sant’Agostino Caves.” PLoS ONE 14(10): 1–29. https://doi.org/10.1371/journal.pone.0213473.
Degioanni Anna, Chritophe Bonenfant, Sandrine Cabut, and Silvana Condemi. 2019. Living on the Edge: Was Demographic Weakness the Cause of Neanderthal Demise? PLoS ONE 14 (5): e0216742.
https://doi.org/10.1371/journal.pone.0216742.
Delpiano, Davide, Kristin Heasley, and Marci Peresani. 2018. “Assessing Neanderthal Land Use and Lithic Raw Material Management in Discoid Technology.” Journal of Anthropological Sciences 96: 89–110. https://doi.org/10.4436/jass.96006.
Demeter, Fabrice, Clément Zanolli, Kira E. Westaway, Renaud Joannes-Boyau, Phillippe Duringer, Mike W. Morley, Frido Welker, et al. 2022. “A Middle Pleistocene Denisovan Molar from the Annamite Chain of Northern Laos.” Nature Communications 13 (1): 2557. https://doi.org/10.7554/eLife.24231.
Dirks, Paul H. G. M., Eric M. Roberts, Hannah Hilbert-Wolf, Jan D. Kramers, John Hawks, Anthony Dosseto, Mathieu Duval, et al. 2017. “The Age of Homo naledi and Associated Sediments in the Rising Star Cave, South Africa.” eLife 6: e24231. https://doi.org/10.7554/eLife.24231.
el-Showk, Sedeer. 2019. “Neanderthal Clues to Brain Evolution in Humans.” Nature 571: S10–S11. https://doi.org/10.1038/d41586-019-02210-6.
Esteban, Irene, Curtis W. Marean, Erich C. Fisher, Panagiotis Karkanas, Dan Carbanes, and Rosa M. Albert. 2018. “Phytoliths as an Indicator of Early Modern Humans’ Plant-Gathering Strategies, Fire, Fuel, and Site-Occupation Intensity During the Middle Stone Age at Pinnacle Point 5-6 (South Coast, South Africa).” PLoS One 13 (6): 1–33.
Evteev, Andrej A., Alla A. Movsesian, and Alexandra N. Grosheva. 2017. “The Association between Mid-facial Morphology and Climate in Northeast Europe Differs from That in North Asia: Implications for Understanding the Morphology of Late Pleistocene Homo sapiens.” Journal of Human Evolution 107: 36–48. https://doi.org/10.1016/j.jhevol.2017.02.008.
Frayer, David W., Marina Lozano, Jose M. Bermudez de Castro, Eudald Carbonell, Juan-Luis Arsuaga, Jakov Radovcic, Ivana Fiore, and Luca Bondioli. 2012. “More Than 500,000 Years of Right-handedness in Europeans.” Laterality 17 (1): 51–69. https://doi.org/10.1080/1357650X.2010.529451.
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216. https://doi.org/10.1038/nature14558.
Gaudzinski-Windheuser, Sabine, Elizabeth S. Noack, Eduard Pop, Constantin Herbst, Johannes Pfleging, Jonas Buchli, Arne Jacob, et al. 2018. “Evidence for Close-Range Hunting by Last Interglacial Neanderthals.” Nature Ecology and Evolution 2: 1087–1092. https://doi.org/10.1038/s41559-018-0596-1.
Gilpin, William., Marcus W. Feldman, and Kenichi Aoki. 2016. “An Ecocultural Model Predicts Neanderthal Extinction through Competition with Modern Humans.” Proceedings of the National Academy of Sciences 113 (8): 2134–2139. https://doi.org/10.1073/pnas.1524861113.
Golovanova, Liubov. Vitaliena, Vladimir B. Doronichev, Naomi E. Cleghorn, Marianna A. Koulkova, Tatiana V. Sapelko, and M. Steven Shackley. 2010. “Significance of Ecological Factors in the Middle to Upper Paleolithic Transition.” Current Anthropology 51 (5): 655–691. https://doi.org.10.1086/656185.
Harrold, Francis B. 1980. “A Comparative Analysis of Eurasian Paleolithic Burials.” World Archaeology 12 (2): 195–211.
Hawks, John, Marina Elliott, Peter Schmid, Steven E. Churchill, Darryl J. de Ruiter, Eric M. Roberts, Hannah Hilbert-Wolf, et al. 2017. "New Fossil Remains of Homo naledi from the Lesedi Chamber, South Africa.” eLife 6: e24232. https://doi.org/10.7554/eLife.24232.
Henry, Amanda G., Alison S. Brooks, and Dolores R. Piperno. 2010. “Microfossils and Calculus Demonstrate Consumption of Plants and Cooked Foods in Neanderthal Diets (Shanidar III, Iraq; Spy I and II, Belgium).” Proceedings of the National Academy of Sciences USA 108 (2): 486–491. https://doi.org/10.1073/pnas.1016868108.
Houldcroft, Charlotte J., and Simon J. Underdown. 2016. “Neanderthal Genomics Suggests a Pleistocene Time Frame for the First Epidemiologic Transition.” American Journal of Physical Anthropology 160 (3): 379–388.
Huerta-Sánchez, Emilia, Xin Jin, Asan, Zhuoma Bianba, Benjamin M. Peter, Nicolas Vinckenbosch, Yu Liang, et al. 2014. “Altitude Adaptation in Tibetans Caused by Introgression of Denisovan-like DNA.” Nature 512 (7513): 194-197.
Jaouen, Klervia, Michael P. Richards, and Adeline Le Cabec. 2019. “Exceptionally High δ15N Values in Collagen Single Amino Acids Confirm Neanderthals as High-Trophic Level Carnivores.” Proceedings of the National Academy of Sciences 116 (11): 4928–4933. https://doi.org/10.1073/pnas.1814087116.
Kandel, Andrew W., Michael Bolus, Knut Bretzke, Angela A. Bruch, Miriam N. Haidle, Christine Hertler, and Michael Märker. 2015. “Increasing Behavioral Flexibility? An Integrative Macro-Scale Approach to Understanding the Middle Stone Age of Southern Africa.” Journal of Archaeological Method and Theory 23: 623–668. https://doi.org/10.1007/s10816-015-9254-y.
Kortlandt, Adriaan. 2002. “Neanderthal Anatomy and the Use of Spears.” Evolutionary Anthropology 11 (5): 183–184.
Kruger, A., P. Randolph-Quinney, and M. Elliott. 2016. “Multimodal Spatial Mapping and Visualization of Dinaledi Chamber and Rising Star Cave.” South African Journal of Science 112 (5–6). https://doi.org/10.17159/sajs.2016/20160032.
Lieberman, Philip, and Edmund S. Crelin. 1971. “On the Speech of Neanderthal Man.” Linguistic Inquiry 2 (2): 203–222.
Mendez, Fernando L., G. David Poznik, Sergi Castellano, and Carlos D. Bustamante. 2016. “The Divergence of Neanderthal and Modern Human Y Chromosomes.” American Journal of Human Genetics 98 (4): 728–734.
Mora-Bermúdez, Felipe, Philipp Kanis, Dominik Macak, Jula Peters, Ronald Naumann, Lei Xing, Mihail Sarov, et al. 2022. “Longer Metaphase and Fewer Chromosome Segregation Errors in Modern Human Than Neanderthal Brain Development.” Science Advances 8 (30). https://doi.org/10.1126/sciadv.abn7702.
Nicholson, Christopher M. 2017. “Eemian Paleoclimate Zones and Neanderthal Landscape Use: A GIS Model of Settlement Patterning during the Last Interglacial.” Quaternary International 438 (B): 144–157. https://doi.org/10.1016/j.quaint.2017.04.023.
Nobel Prize, The. 2022. “Press Release: The Nobel Prize in Physiology or Medicine 2022.” Nobelförsamlingen The Nobel Assembly at Karolinska Institutet. Press release, November 3, 2022. https://www.nobelprize.org/prizes/medicine/2022/press-release/.
Nowell, April. 2016. “Childhood, Play and the Evolution of Cultural Capacity in Neanderthals and Modern Humans.” In The Nature of Culture: Based on an Interdisciplinary Symposium “The Nature of Culture,” Tübingen, Germany, edited by M. N. Haidle, N. J. Conard, and M. Bolus, 87–97. Heidelberg, Germany: Springer. https://doi.org.10.1007/978-94-017-7426-0_9.
Parkington, John. 2003. “Middens and Moderns: Shellfishing and the Middle Stone Age of the Western Cape, South Africa.” South African Journal of Science 99 (5–6): 243–274.
Pearce, Eiluned, Chris Stringer, and R. I. M. Dunbar. 2013. “New Insights into Differences in Brain Organizations between Neanderthals and Anatomically Modern Humans.” Proceedings of the Royal Society B: Biological Sciences 280 (1758): 20130168. https://doi.org/10.1098/rspb.2013.0168.
Pomeroy, Emma, Paul Bennett, Chris O. Hunt, Tim Reynolds, Lucy Farr, Marine Frouin, James Holman, Ross Lane, Charles French, and Graeme Barker. 2020. “New Neanderthal Remains Associated with the ‘Flower Burial’ at Shanidar Cave.” Antiquity 94 (373): 11–26. https://doi.org/10.15184/aqy.2019.207.
Radford, A., & Scragg, R. (2013). Discovery of Kuru Revisited: How Anthropology Hindered Then Enhanced Kuru Research. Health and History, 15(2), 29–52. https://doi.org/10.5401/healthhist.15.2.0029
Rae, Todd, Thomas Koppe, and Chris B. Stringer. 2011. “The Neanderthal Face Is Not Cold-Adapted.” Journal of Human Evolution 60 (2): 234–239. https://doi.org/10.1016/j.jhevol.2010.10.003.
Reich, David, Richard E. Green, Martin Kircher, Johannes Krause, Nick Patterson, Eric Y. Durand, Bence Viola, et al. 2010. “Genetic History of an Archaic Hominin Group from Denisova Cave in Siberia.” Nature 468: 1053–1060. https://doi.org/10.1038/nature09710.
Richards, Michael P., Paul B. Pettit, Erik Trinkaus, Fred H. Smith, Maja Paunović, and Ivor Karavanić. 2000. “Neanderthal Diet at Vindija and Neanderthal Predation: The Evidence from Stable Isotopes.” Proceedings of the National Academy of Sciences 97 (13): 7663–7666.
https://doi.org/10.1073/pnas.120178997.
Richter, Jürgen, 2006. “Neanderthals in Their Landscape: Neanderthals in Europe.” In Proceedings of the International Conference held in the Gallo-Roman Museum in Tongeren, ERAUL 117, edited by B. Demarsin and M. Otte, 17–32. Köln: Universität zu Köln.
Roksandic, Mirjana, Predrag Radovic, Xiu-Jie Wu, and Christopher J. Bae. 2021. “Resolving the ‘Muddle in the Middle’: The Case for Homo bodoensis.” Evolutionary Anthropology: Issues, News, and Reviews, 31 (1). https://doi.org/10.1002/evan.21929.
Rougier, H., Crevecoeur, I., Beauval, C., Posth, C., Flas, D., Wißing, C., Furtwängler, A., Germonpré, M., Gómez-Olivencia, A., Semal, P., van der Plicht, J., Bocherens, H., & Krause, J. (2016). Neandertal cannibalism and Neandertal bones used as tools in Northern Europe. Scientific Reports, 6(1). https://doi.org/10.1038/srep29005
Slon, Viviane, Fabrizio Mafessoni, Benjamin Vernot, Cesare de Filippo, Steffi Grote, Bence Viola, Mateja Hajdinjak, et al. 2018. “The Genome of the Offspring of a Neanderthal Mother and a Denisovan Father.” Nature 561 (7721): 113–116.
Smith, Tanya M., Paul Tafforeau, Donald J. Reid, Joanne Pouech, Vincent Lazzari, John P. Zermeno, Debbi Guatelli-Steinberg, et al. 2010. “Dental Evidence for Ontogenetic Differences Between Modern Humans and Neanderthals.” Proceedings for the National Academy of Sciences 107 (49): 20923–20928.
Staubwasser, Michael, Virgil Drăguʂin, Bogdan P. Onac, Sergey Assonov, Vasile Ersek, Dirk L. Hoffman, and Daniel Veres. 2018. “Impact of Climate Change on the Transition of Neanderthals to Modern Humans in Europe.” Proceedings of the National Academy of Sciences 115 (37): 9116–9121. https://doi.org/10.1073/pnas.1808647115.
Stewart, T. D. 1977. “The Neanderthal Skeletal Remains from Shanidar Cave, Iraq: A Summary of Findings to Date.” Proceedings of the American Philosophical Society 121 (2): 121–165.
Stolarczyk, Regine E., and Patrick Schmidt. 2018. “Is Early Silcrete Heat Treatment a New Behavioural Proxy in the Middle Stone Age?” PLoS One 13 (10): 1–21.
Trinkaus, E. 1985. “Pathology and Posture of the La-Chapelle-aux-Saints Neanderthal.” American Journal of Physical Anthropology 67 (1): 19–41.
Trujillo Cleber A., Edward S. Rice, Nathan K. Schaefer, Isaac A. Chaim, Emily C. Wheeler, Assael A. Madrigal, Justin Buchanan, et al. 2021. “Reintroduction of the Archaic Variant of NOVA1 in Cortical Organoids Alters Neurodevelopment.” Science 371 (6530): eaax2537. https://doi.org/10.1126/science.aax2537.
Tucci, Serena, Samuel H. Vohr, Rajiv C. McCoy, Benjamin Vernot, Matthew R. Robinson, Chiara Barbieri, Brad J. Nelson, et al. 2018. “Evolutionary History and Adaptation of a Human Pygmy Population of Flores Island, Indonesia.” Science 361 (6401): 511–516.
Ullrich H, 2005: Cannibalistic Rites within Mortuary Practices from the Paleolithic to Middle Ages in Europe. Anthropologie (Brno) 43, 2-3: 249-261.
Van Andel, T. H., and P. C. Tzedakis. 1996. “Paleolithic Landscapes of Europe and Environs, 150,000–25,000 Years Ago: An Overview.” Quaternary Science Reviews 15 (5–6): 481–500.
Venner, Stephen J. 2018. “A New Estimate for Neanderthal Energy Expenditure.” CUNY Academic Works.
Vernot, Benjamin, Serena Tucci, Janet Kelso, Joshua G. Schraiber, Aaron B. Wolf, Rachel M. Gittelman, Michael Danneman, et al. 2016. “Excavating Neanderthal and Denisovan DNA from the Genomes of Melanesian Individuals.” Science 352 (6282): 235–239.
Weaver, T. D., and J. Hublin. 2009. “Neanderthal Birth Canal Shape and the Evolution of Human Childbirth.” Proceedings of the National Academy of Sciences 106 (20): 8151–8156.
Wiẞing, Christoph, Hélène Rougier, Isabelle Crevecoer, Mietje Germonpré, Yuichi Naito, Patrick Semal, and Hervé Bocherens. 2015. “Isotopic Evidence for Dietary Ecology of Late Neanderthals in Northwestern Europe.” Quaternary International 411 (A): 327–345. https://doi.org/10.1016/j.quaint.2015.09.091.
Wong, Kate. 2015. “Neanderthal Minds.” Scientific American (January): 312(2): 36-43. https://doi.org/10.1038/scientificamerican0215-36.
Zhang, X. L., B. B. Ha, S. J. Wang, Z. J. Chen, J. Y. Ge, H. Long, W. He, et al. 2018. “The Earliest Human Occupation of the High-Altitude Tibetan Plateau 40 Thousand to 30 Thousand Years Ago.” Science 362 (6418): 1049–1051. https://doi.org/10.1126/sciadv.add5582.
Zilhão João, Diego E. Angelucci, Ernestina Badal-García, Francesco d'Errico, Floréal Daniel, Laure Dayet, Katerina Douka, et al. 2010. “Symbolic Use of Marine Shells and Mineral Pigments by Iberian Neandertals.” Proceedings of the National Academy of Sciences 107 (3): 1023–1028. https://doi.org/10.1073/pnas.0914088107.
Zollikofer, Christopher Peter Edwards, and Marcia Silvia Ponce de León. 2013. “Pandora’s Growing Box: Inferring the Evolution and Development of Hominin Brains from Endocasts.” Evolutionary Anthropology 22 (1): 20–33. https://doi.org/10.1002/evan.21333.
Acknowledgments
The authors would like to extend their thanks to Cassandra Gilmore and Anna Goldfield for thoughtful and insightful suggestions on the first edition of this chapter.
Michael B. C. Rivera, Ph.D., University of Cambridge
This chapter is a revision from "Chapter 13: Race and Human Variation” by Michael B. C. Rivera. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Illustrate the troubling history of “race” concepts.
- Explain human variation and evolution as the thematic roots of biological anthropology as a discipline.
- Critique earlier “race” concepts based on a contemporary understanding of the apportionment of human genetic variation.
- Explain how biological variation in humans is distributed clinally and in accordance with both isolation-by-distance and Out-of-Africa models.
- Identify phenotypic traits that reflect selective and neutral evolution.
- Extend this more-nuanced view of human variation to today’s research, the implications for biomedical studies, applications in forensic anthropology, and other social/cultural/political concerns.
Humans exhibit biological variation. Humans also have a universal desire to categorize other humans in order to make sense of the world around them. Since the birth of the discipline of biological anthropology, we have been interested in studying how humans vary biologically and what the sources of this variation are. Before we tackle these big problems, first consider this question: Why should we study human variation?

There are certainly academic reasons for studying human variation. First, it is highly interesting and important to consider the evolution of our species (see Chapters 9–12) and how our biological variation may be similar to (or different from) that of other species (e.g., other primates and apes; see Chapters 5 and 6). Second, anthropologists study modern human variation to understand how different biological traits developed over evolutionary time (see Figure 13.1). Suppose we are able to grasp the evolutionary processes that produce the differences in biology, physiology, body chemistry, behavior, and culture (human variation). In that case, we can make more accurate inferences about evolution and adaptation among our hominin ancestors, complementing our study of fossil evidence and the archaeological record. Third, as will be discussed in more detail later on, it is important to consider that biological variation among humans has biomedical, forensic, and sociopolitical implications. For these reasons, the study of human variation and evolution has formed the basis of anthropological inquiry for centuries and continues to be a major source of intrigue and inspiration for scientific research conducted today.
An even-more-important role of the biological anthropologist is to improve public understanding of human evolution and variation—outside of academic circles. Terms such as race and ethnicity are used in everyday conversations and in formal settings within and outside academia. The division of humankind into smaller, discrete categories is a regular occurrence in day-to-day life. This can be seen regularly when governments acquire census data with a heading like “geographic origin” or “ethnicity.” Furthermore, such checkboxes and drop-down lists are commonly seen as part of the identifying information required for surveys and job applications.
According to professors of anthropology, ethnic studies, and sociology, race is often understood as rooted in biological differences, ranging from such familiar traits as skin color or eye shape to variations at the genetic level. However, race can also be studied as an “ideological construct” that goes beyond biological and genetic bases (Fuentes et al. 2019), at different times relating to our ethnicities, languages, religious beliefs, and cultural practices. Sometimes people associate racial identity with the concept of socioeconomic status or position, or they link ideas about race to what passport someone has, how long they have been in a country, or how well they have “integrated” into a population.
Some of these ideas about ethnicity have huge social and political impacts, and notions of race have been part of the motivation behind various forms of racism and prejudice today, as well as many wars and genocides throughout history. Racism manifests in many ways—from instances of bullying between kids on school playgrounds to underpaid minorities in the workforce, and from verbal abuse hurled at people of color to violent physical behaviors against those of a certain race. Prejudice can be characterized as negative views toward another group based on some perceived characteristic that makes all members of that group worthy of disdain, disrespect, or exclusion (not solely along racial lines but also according to [dis]ability, gender, sexual orientation, or socioeconomic background, for example). According to Shay-Akil McLean (2014), “Racism is not something particular to the United States and race is not the same everywhere in the world. Racial categories serve particular contextual purposes depending on the society they are used in, but generally follow the base logic of the supremacy of one type of human body over all others (ordering these human bodies in a hierarchical fashion).” Choosing which biological or nonbiological features to use when discussing race is always a social process (Omi and Winant 2014). Race concepts have no validity to them unless people continue to use them in their daily lives—and, in the worst cases, to use them to justify racist behaviors and problematic ideas about racial difference or superiority/inferiority. Recent work in anthropological genetics has revealed the similarities amongst humans on a molecular level and the relatively few differences that exist between populations (Omi and Winant 2014).
The role of the biological anthropologist becomes crucial in the public sphere, because we may be able to debunk myths surrounding human variation and shed light, for the nonanthropologists around us, on how human variation is actually distributed worldwide (see Figure 13.1). Rooted in scientific observations, our work can help nonanthropologists recognize how common ideas about “race” often have no biological or genetic basis. Many anthropologists work hard to educate students on the history of where race concepts come from, why and how they last in public consciousness, and how we become more conscious of racial issues and the need to fight against racism in our societies. Throughout this chapter, I will highlight how humans cannot actually be divided into discrete “races,” because most traits vary on a continuous basis and human biology is, in fact, very homogenous compared to the greater genetic variation we observe in closely related species. Molecular anthropology, or anthropological genetics, continues to add new layers to our understanding of human biological variation and the evolutionary processes that gave rise to the contemporary patterns of human variation. The study of human variation has not always been unbiased, and thinkers and scientists have always worked in their particular sociohistorical context. For this reason, this chapter opens with a brief overview of race concepts throughout history, many of which relied on unethical and unscientific notions about different human groups.
Special Topic: My Experiences as an Asian Academic

My name is Michael, and I am a biological anthropologist and archaeologist (Figure 13.2). What strikes me as most interesting to investigate is human biological variation today and the study of past human evolution. For instance, some of my research on ancient coastal populations has revealed positive effects of coastal living on dietary health and many unique adaptations in bones and teeth when living near rivers and beaches. I love talking to students and nonscientists about bioanthropologists’ work, through teaching, science communication events, and writing book chapters like this one. I grew up in Hong Kong, a city in southern China. My father is from the Philippines and my mother is from Hong Kong, which makes me a mixed Filipino-Chinese academic. Growing up, I noticed that people came in all shapes, sizes, and colors. My life is very different now in that I have gained the expertise to explain those differences, and I feel a great responsibility to guide those new to anthropology toward their own understandings of diversity.
Biological anthropology is not taught extensively in Hong Kong, so I moved to the United Kingdom to earn my bachelor’s, master’s, and doctorate degrees. It was fascinating to me that we could answer important questions about human variation and history using scientific methods. However, I did not have many minority academic role models to look up to while I was at university. My department was made up almost exclusively of white westerner faculty, and it was hard to imagine I could one day get a job at these western institutions. While pursuing my degrees, I also remember several instances of my research contributions being overlooked or dismissed. Sometimes professors and fellow students would make racist comments toward Asian scholars (including me) and other Black, Indigenous and researchers of color, making us greatly uncomfortable in those spaces. When one of us would work up the courage to tell university leaders our experiences of being stereotyped, dismissed, or insulted, we received little support and were further excluded from research and teaching activities. This is a common experience for Black, Indigenous, and other people of color who pursue biological anthropology, and we face the difficult choice between leaving the field or bearing with such unsafe spaces.
It became important to me at that time to find other academics of color with whom to share experiences and form community. I feel inspired by all my colleagues who advocate for greater representation and diversity in universities (whether they are minority academics or not). I admire many of my fellow researchers who are underrepresented and do a great job of representing minority groups through their cutting-edge research and quality teaching at the undergraduate and graduate levels. Although I no longer work in the West, it has remained my great hope that those in the West and the “Global North” will continue to improve university culture, and I support any efforts there to welcome all scholars.
My current work is based in Hong Kong, where I am deeply dedicated to helping develop biological anthropology in East and Southeast Asia and promoting research from our home regions on the international scene. The study of anthropology really highlights how we share a common humanity and history. Being somebody who is “mixed race” and Asian likely played a key role in steering me toward the study of human variation. As this chapter hopefully shows, there is a lot to discuss about race and ethnicity regarding the discipline’s history and current understandings of human diversity. Some scientific and technological advancements today are misused for reasons to do with money, politics, or the continuation of antiquated ideas. It is my belief, alongside many of my friends and fellow anthropologists, that science should be more about empathy toward all members of our species and contributing to the intellectual and technological nourishment of society. After speaking to many members of the public, as well as my own undergraduate students, I have received lovely messages from other individuals of color expressing thanks and appreciation for my presence and understanding as a minority scientist and mentor figure. Anthropology needs much more diversity as well as to make room for those who have traveled different routes into the discipline. All paths taken into anthropology are valid and valuable. I would encourage everyone to study anthropology—it is a field for understanding and celebrating the intricacies of human diversity.
The History of "Race" Concepts
“Race” in the Classical Era
The earliest classification systems used to understand human variation are evidenced by ancient manuscripts, scrolls, and stone tablets recovered through archaeological, historical, and literary research. The Ancient Egyptians had the Book of Gates, dated to the New Kingdom between 1550 B.C.E. and 1077 B.C.E (Figure 13.3). In one part of this tome dedicated to depictions of the underworld, scribes used pictures and hieroglyphics to illustrate a division of Egyptian people into the four categories known to them at the time: the Aamu (Asiatics), the Nehesu (Nubians), the Reth (Egyptians), and the Themehu (Libyans). Though not rooted in any scientific basis like our current understandings of human variation today, the Ancient Egyptians believed that each of these groups were made of a distinctive category of people, distinguishable by their skin color, place of origin, and even behavioral traits.
Another well-known early document is the Bible, where it is written that all humankind descends from one of three sons of Noah: Shem (the ancestor to all olive-skinned Asians), Japheth (the ancestor to pale-skinned Europeans), and Ham (the ancestor to darker-skinned Africans). Similar to the Ancient Egyptians, these distinctions were based on behavioral traits and skin color. More recent work in historiography and linguistics suggest that the branches of “Hamites,” “Japhethites,” and “Shemites” may also relate to the formation of three independent language groups some time between 1000 and 3000 B.C.E. With the continued proliferation of Christianity, this concept of approximately three racial groupings lasted until the Middle Ages and spread as far across Eurasia as crusaders and missionaries ventured at the time.
There is also the “Great Chain of Being,” conceived by ancient Greek philosophers like Plato (427‒348 B.C.E.) and Aristotle (384‒322 B.C.E.). They played a key role in laying the foundations of empirical science, whereby observations of everything from animals to humans were noted with the aim of creating taxonomic categories. Aristotle describes the Great Chain of Being as a ladder along which all objects, plants, animals, humans, and celestial bodies can be mapped in an overall hierarchy (in the order of existential importance, with humans placed near the top, just beneath divine beings; see Figure 13.4). When he writes about humans, Aristotle expressed the belief that certain people are inherently (or genetically) more instinctive rulers, while others are more natural fits for the life of a worker or enslaved person. Based on research by biological anthropologists, we currently recognize that these early systems of classification and hierarchization are unhelpful in studying human biological variation. Both behavioral traits and physical traits are coded for by multiple genes each, and how we exhibit those traits based on our genetics can vary significantly even between individuals of the same population.
“Race” during the Scientific Revolution
The 1400s to 1600s saw the beginnings of the Scientific Revolution in Western societies, with thinkers like Nicholas Copernicus, Galileo Galilei, and Leonardo Da Vinci publishing some of their most important findings. While by no means the first or only scholars globally to use observation and experimentation to understand the world around them, early scientists living at the end of the medieval period in Europe increasingly employed more experimentation, quantification, and rational thought in their work. This is the main difference between the work of the ancient Egyptians, Romans, and Greeks and that of later scientists such as Isaac Newton and Carl Linnaeus in the 1600s and 1700s.

Linnaeus is the author of Systema Naturae (1758), in which he classified all plants and animals under the formalized naming system known as binomial nomenclature (Figure 13.5). This system is typological, in that organisms are placed into groups according to how they are similar or different to others under study. What was most anthropologically notable about Linnaeus’s typological system was that he was one of the first to group humans with apes and monkeys, based on the anatomical similarities between humans and nonhuman primates. However, Linnaeus viewed the world in line with essentialism, a problematic concept that dictates that there are a unique set of characteristics that organisms of a specific kind must have and that would remove organisms from taxonomic categorizations if they lacked any of the required criteria.
Linnaeus subdivided the human species into four varieties, with overtly racist categories based on skin color and “inherent” behaviors. Some European scientists during this period were not aware of their own biases skewing their interpretations of biological variation, while others deliberately worked to shape public perceptions of human variation in ways that established “otherness” and enforced European domination and the subordination of non-European people. The conclusions and claims at which they arrived, consciously or subconsciously, often fit the times they were living through—the so-called Age of Discovery, when the superiority of European cultures over others was a pervasive idea throughout people’s social and political lives. Although much of Eurasia was linked by spice- and silk-trading routes, the European colonial period between the 1400s and 1700s was marked by many new and unfortunately violent encounters overseas (Figure 13.6). When Europeans arrived by ship on the shores of continents that were already inhabited, it was their first meeting with the Indigenous peoples of the Americas and Australasia, who looked, spoke, and behaved differently from peoples with whom they were familiar. Building on the idea of species and “subspecies,” natural historians of this time invented the term race, from the French rasse meaning “local strain.”

Another scientist of the times, Johann Friedrich Blumenbach (1752‒1840), classified humans into five races based on his observations of cranial form variation as well as skin color. He thus dubbed the “original” form of the human cranium the “Caucasian” form, with the idea that the ideal climate conditions for early humans would have been in the Caucasus region near the Caspian Sea. The key insight Blumenbach presented was that human variation in any particular trait should be more accurately viewed as falling along a gradation (Figure 13.7). While some of his theories were correct according to what we observe today with more knowledge in genetics, they erroneously believed that human “subspecies” were “degenerated” or “transformed” varieties of an ancestral Caucasian or European race. According to them, the Caucasian cranial dimensions were the least changed over human evolutionary time, while the other skull forms represented geographic variants of this “original.” As will be discussed in greater detail later in this chapter, we have genetic and craniometric evidence for sub-Saharan Africa being the origin of the human species instead (see Chapter 12 on the fossil record that places the origins of modern Homo sapiens in north and east Africa). Based on work that shows how most biological characteristics are coded for by nonassociated genes, it is not reasonable to draw links between individuals’ personalities and their skull shapes.
“Race” and the Dawn of Scientific Racism
Between the 1800s and mid-1900s, and contrary to what you might expect, an increased use of scientific methods to justify racial schemes developed in scholarship. Differing from earlier views, which saw all humans as environmentally deviated from one “original” humankind, classification systems after 1800 became more polygenetic (viewing all people as having separate origins) rather than monogenetic (viewing all people as having a single origin). Instead of moving closer to our modern-day understandings of human variation, there was increased support for the notion that each race was created separately and with different attributes (intelligence, temperament, and appearance).
The 1800s were an important precursor to modern biological anthropology as we know it, given that this was when the scientific measurement of human physical features (anthropometry) truly became popularized. However, empirical studies in the 1800s pushed even further the idea that Europeans were culturally and biologically superior to others. While considered one of the pioneers of American “physical” anthropology, Samuel George Morton (1799‒1851) was a scholar who had a large role in perpetuating 1800s scientific racism. By measuring cranial size and shape, he calculated that “Caucasians,” on average, have greater cranial volumes than other groups, such as the Indigenous peoples of the Americas and peoples Morton referred to collectively as “Negros.” Today, we know that cranial size variation depends on such factors as Allen’s and Bergmann’s rules, which give a more likely explanation: in colder environments, it is advantageous for those living there to have larger and rounder heads because they conserve heat more effectively than more slender heads (Beals et al. 1984). The leading figures in craniometry during the 1800s instead were linked heavily with powerful individuals and wealthy sociopolitical institutions and financial bodies. Theories in support of hierarchical racial schemes using “scientific” bases certainly helped continue the exploitative and unethical trafficking and enslavement of Africans between the 1500s and 1800s.
Morton went on to write in his publication Crania Americana (1839) a number of views that fit with a concept called biological determinism. The idea behind biological determinism is that an association exists between people’s physical characteristics and their behavior, intelligence, ability, values, and morals. If the idea is that some groups of people are essentially superior to others in cognitive ability and temperament, then it is easier to justify the unequal treatment of certain groups based on outward appearances. Another such problematic thinker was Paul Broca (1824‒1880), after whom a region of the frontal lobe related to language use is named (Broca’s area). Influenced by Morton, Broca likewise claimed that internal skull capacities could be linked with skin color and cognitive ability. He went on to justify the European colonization of other global territories by purporting it was noble for a biologically more “civilized” population to improve the “humanity” of more “barbaric” populations. Today, these theories of Morton, Broca, and others like them are known to have no scientific basis. If we could speak with them today, they would likely try to emphasize that their conclusions were based on empirical evidence and not a priori reasoning. However, we now can clearly see that their reasoning was biased and affected by prevailing societal views at the time.
“Race” and the Beginnings of Physical Anthropology
In the early 20th century, we saw a number of new figures coming into the science of human variation and shifting the theoretical focus within. Most notably, these included Aleš Hrdlička and Franz Boas.

Aleš Hrdlička (1869‒1943) was a Czech anthropologist who moved to the United States. In 1903, he established the physical anthropology section of the National Museum of Natural History (Figure 13.8). In 1918, he founded the American Journal of Physical Anthropology, which remains one of the foremost scientific journals disseminating bioanthropological research. As part of his work and the scope of the journal, he differentiated “physical anthropology” from other kinds of anthropology: he wrote that physical anthropology is “the study of racial anatomy, physiology, and pathology” and “the study of man’s variation” (Hrdlička 1918). In some ways, although the scope and technological capabilities of biological anthropologists have changed significantly, Hrdlička established an area of inquiry that has continued and prospered for over a hundred years.
Franz Boas (1858‒1942) was a German American anthropologist who established the four-field anthropology system in the United States and founded the American Anthropological Association in 1902. He argued that the scientific method should be used in the study of human cultures and the comparative method for looking at human biology worldwide. One of Boas’s significant contributions to biological anthropology was the study of skull dimensions with respect to race. After a long-term research project, he demonstrated how cranial form was highly dependent on cultural and environmental factors and that human behaviors were influenced primarily not by genes but by social learning. He wrote in one essay for the journal Science: “While individuals differ, biological differences between races are small. There is no reason to believe that one race is by nature so much more intelligent, endowed with great willpower, or emotionally more stable than another, that the difference would materially influence its culture” (Boas 1931:6). This conclusion directly contrasted with the theories of the past that relied on biological determinism. Biological anthropologists today have found evidence that corroborates Boas’s explanations: societies do not exist on a hierarchy or gradation of “civilizedness” but instead are shaped by the world around them, their demographic histories, and the interactions they have with other groups.
The first half of the 1900s still involved some research that was essentialist and focused on proving racial determinism. Anthropologists like Francis Galton (1822‒1911) and Earnest A. Hooton (1887‒1954) created the field of eugenics as an attempt to formalize the social scientific study of “fitness” and “superiority” among members of 19th-century Europe. As a way of “dealing with” criminals, diseased individuals, and “uncivilized” people, eugenicists recommended prohibiting parts of the population from being married or sterilizing these members of society so they could no longer procreate (Figure 13.9). They instead encouraged “reproduction in individual families with sound physiques, good mental endowments, and demonstrable social and economic capability” (Hooton 1936). In the 1930s, Nazi Germany used this false idea of there being “pure races” to highly destructive effect. The need to be protected against admixture from “unfit” groups was their justification for their blatant racism and purging of citizens that fell under their subjective criteria.
It should be noted that eugenicist ideas were popularly discussed and debated in many non-European contexts, as in the U.S., China, and South Africa, too. The Immigration Restriction Act of 1924 was passed in the United States, with the explicit aim of reducing the country’s “burden” of people considered inferior by restricting immigration of eastern European Jews, Italians, Africans, Arabs, and Asians. In the early 1900s, Chinese scientists and politicians showed great interest in eugenic ideologies, which came to dictate decisions in law-making, family life, and the medical field. Noted American anthropologist Ruth Benedict wrote extensively on Japanese culture and society during and after World War II. Her essentialist portrayals of Japanese people were heavily cited in popular discourse at the time. In 1950s South Africa, interracial marriages and sexual relations were banned by law; antimiscegenation became one of the huge focuses of apartheid resistance activists in later years. We still see the continuation of eugenics-based logic today around the world—in exclusionary immigration laws, cases of incarcerated prison inmates being forcibly sterilized, and the persistence of intelligence testing as a form of measuring people’s “fitness” in a society.
Shortly after World War II and the Nazi Holocaust, the full extent of essentialist, eugenicist thinking became clear. Social constructions of race, and the notion that one can predict psychological or behavioral traits based on external appearance, had become unpopular both within and outside the discipline. It was up to those in the field of physical anthropology at the time to separate physical anthropology from race concepts that supported unscientific and socially damaging agendas. This does not mean that there are no physiological or behavioral differences between different members of the human species. However, going forward, a number of physical anthropologists saw human biological variation as more complicated than simple typologies could describe.
“The New Physical Anthropology”

After 1950, focus steered away from the concept of “race” as a unit of variation and toward understanding why variation exists in populations from an evolutionary perspective. This was outlined by those pioneering the “new physical anthropology,” such as Sherwood Washburn, Theodosius Dobzhansky (Figure 13.10), and Julian Huxley, who borrowed this approach from contemporary population geneticists. Whether using genetic or phenotypic markers as the units of study, “groups” or “clusters” of humans differentiated by these became defined as populations that differ in the frequency of some gene or genes. Anthropologists consider what “makes” a population—a group of individuals potentially capable of or actually interbreeding due to shared geographic proximity, language, ethnicity, culture, and/or values. Put another way, a population is a local interbreeding group with reduced gene flow between themselves and other groups of humans. Members of the same population may be expected to share many genetic traits (and, as a result, many phenotypic traits that may or may not be visible outwardly).

Thinking of humans in terms of populations was part of Julian Huxley’s (1942) “Modern Synthesis”—so named because it helped to reconcile two fundamental principles about evolution that had not been made sense of together before (Figure 13.11). As discussed in Chapter 3, Gregor Mendel (1822‒1884) was able to show that inheritance was mediated by discrete particles (or genes) and not blended in the offspring. However, it was difficult for some 19th-century scientists to accept this model of genetic inheritance at the time because much of biological variation appeared to be continuous and not particulate (take skin color or height as examples). In the 1930s, it was demonstrated that traits could be polygenic and that multiple alleles could be responsible for any one phenotypic trait, thus producing the continuous variation in traits such as eye color that we see today. Thus, Huxley’s “Modern Synthesis” outlines not only how human populations are capable of exchanging genes at the microevolutionary level but also how multiple alleles for one trait (polygenic exchanges) can cause gradual macroevolutionary changes.
Human Variation in Biological Anthropology Today
Many Human Traits Are Nonconcordant
In our studies of human (genetic) variation today, we understand most human traits to be nonconcordant (Figure 13.12). “Nonconcordance” is a term used to describe how biological traits vary independent of each other—that is, they do not get inherited in a correlative manner with other genetically controlled traits. For example, if you knew an individual had genes that coded for tall height, you would not be able to predict if they are lighter-skinned or have red hair. This is different from earlier essentialist views of human variation: the idea that skin color could predict one’s brain function or even “temperament” and tendencies toward criminal behavior.
Human Variation Is Clinal/Continuous (Not Discrete)
Frank B. Livingstone (1928‒2005) wrote: “There are no races, only clines” (1962: 279). A cline is a gradation in the frequency of an allele/trait between populations living in different geographic regions. Human variation cannot be broken into discrete “races,” because most physical traits vary on a continuous or “clinal” basis. One obvious example of this is how human height does not only come in three values (“short,” “medium,” and “tall”) but instead varies across a spectrum of vertical heights achievable by humans all over the world. On the one hand, we can describe human height as exhibiting continuous variation, forming a continuous pattern, but height does not vary according to where people live across the globe and does not exhibit a clinal pattern. On the other hand, skin color variation between populations does show patterning that fits quite well on to how near or far they are from each other on a world map. This makes a trait like skin color clinally distributed worldwide. When large numbers of genetic loci for large numbers of samples were sampled from human populations distributed worldwide during the 1960s and 1970s, the view that certain facets of human diversity were clinally distributed was further supported by genetic data.
To study human traits that are clinally distributed, genetic tests must be performed to uncover the true frequencies of an allele or trait across a certain geographic space. One easily visible example of a clinal distribution seen worldwide is the patterning of human variation in skin color. Whether in southern Asia, sub-Saharan Africa, or Australia, dark brown skin is found. Paler skin tones are found in higher-latitude populations such as those who have lived in areas like Europe, Siberia, and Alaska for millennia. Skin color is easily observable as a phenotypic trait exhibiting continuous variation.
A clinal distribution still derives from genetic inheritance; however, clines often correspond to some gradually changing environmental factor. Clinal patterns arise when selective pressures in one geographic area differ from those in another as well as when people procreate and pass on genes together with their most immediate neighbors. There are several mechanisms, selective and neutral, that can lead to the clinal distribution of an allele or a biological trait. Natural selection is the mechanism that produced a global cline of skin color, whereby darker skin color protects equatorial populations from high amounts of UV radiation; there is a transition of lessening pigmentation in individuals that reside further and further away from the tropics (Jablonski 2004; Jablonski and Chaplin 2000; see Figure 13.13). The ability and inability to digest lactose (milk sugar) among different world communities varies according to differential practices and histories of milk and dairy-product consumption (Gerbault et al. 2011; Ingram et al. 2009). Where malaria seems to be most prevalent as a disease stressor on human populations, a clinal gradient of increasing sickle cell anemia experience toward these regions has been studied extensively by genetic anthropologists (Luzzatto 2012). Sometimes, culturally defined mate selection based on some observable trait can lead to clinal variation between populations as well.
Two neutral microevolutionary processes that may produce a cline in a human allele or trait are gene flow and genetic drift (see Chapter 4). The ways in which neutral processes can produce clinal distributions is seen clearly when looking at clinal maps for different blood groups in the human ABO blood group system (Figure 13.14). For instance, scientists have identified an East-to-West cline in the distribution of the blood type B allele across Eurasia. The frequency of B allele carriers decreases gradually westward when we compare the blood groups of East and Southeast Asian populations with those in Europe. This shows how populations residing nearer to one another are more likely to interbreed and share genetic material (i.e., undergo gene flow). We also see 90%‒100% of native South American individuals, as well as between 70%‒90% of Aboriginal Australian groups, carrying the O allele (Mourant, Kopeć, and Domaniewska-Sobczak 1976). These high frequencies are likely due to random genetic drift and founder effects, in which population sizes were severely reduced by the earliest O allele-carrying individuals migrating into those areas. Over time, the O blood type has remained predominant.
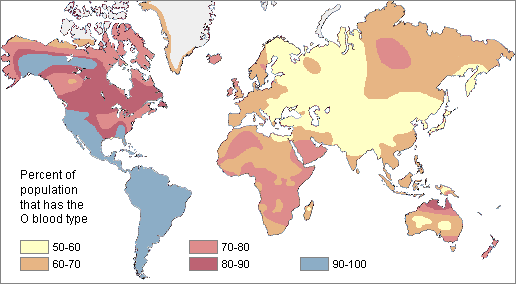
Genetic Variation Is Greater Within Group than Between Groups
One problem with race-based classifications is they relied on an erroneous idea that individuals with particular characteristics would share more similar genes with each other within a particular “race” and share less with individuals of other “races” possessing different traits and genetic makeups. However, since around 50 years ago, scientific studies have shown that the majority of human genetic differences worldwide exist within groups (or “races”) individually rather than between groups. Indeed, most genetic variation we see occurs in Africa, and many variants are shared among individuals on all continents (Figure 13.15).
In 2002, a landmark article by Noah Rosenberg and colleagues explored worldwide human genetic variation using an even-greater genetic data set. They used 377 highly variable markers in the human genome and sampled from 1,056 individuals representative of 52 populations. The markers chosen for study were not ones that code for any expressed genes. Because these regions of the human genome were made of unexpressed genes, we may understand these markers as neutrally derived (as opposed to selectively derived) because they do not code for functional advantages or disadvantages. These neutral genetic markers likely reflect an intricate combination of regional founder effects and population histories. Analyses of these neutral markers allowed scientists to identify that 93%‒95% of global genetic differences, referred to as variance, can be accounted for by within-population differences, while only a small proportion of genetic variance (3%‒5%) can be attributed to differences among major groups (Rosenberg et al. 2002). This research supports the theory that distinct biological races do not exist, even though misguided concepts of race may still have real social and political consequences.
Biological Data Fit Isolation-By-Distance and Out-of-Africa Models
One further note is that the world’s population may be genetically divided into “groups,” “subsets,” “clumps,” or “clusters” that reflect some degree of genetic similarity. These identifiable clusters reflect genetic or geographic distances—either with gene flow facilitated by proximity between populations or impeded by obstacles like oceans or environmentally challenging habitats (Rosenberg et al. 2005). Sometimes, inferred clusters using multiple genetic loci are interpreted by nongeneticists literally as “ancestral populations.” However, it would be wrong to assume from these genetic results that highly differentiated and “pure” ancestral groups ever existed. These groupings reflect differences that have arisen over time due to clinal patterning, genetic drift, and/or restricted or unrestricted gene flow (Weiss and Long 2009). The clusters identified by scientists are arbitrary and the parameters used to split up the global population into groups is subjective and dependent on the particular questions or distinctions being brought into focus (Relethford 2009).
Additionally, research on worldwide genetic variation has shown that human variation decreases with increasing distance from sub-Saharan Africa, where there is evidence for this vast region being the geographical origin of anatomically modern humans (Liu et al. 2006; Prugnolle, Manica, and Balloux 2005; see Figures 13.16 and 13.17). Genetic differentiation decreases in human groups the further you sample data from relative to sub-Saharan Africa because of serial founder effects (Relethford 2004). Over the course of human colonization of the rest of the world outside Africa, populations broke away in expanding waves across continents into western Asia, then Europe and eastern Asia, followed by Oceania and the Americas. As a result, founder events occurred whereby genetic variation was lost, as the colonization of each new geographical region involved a smaller number of individuals moving from the original larger population to establish a new one (Relethford 2004). The most genetic variation is found across populations residing in different parts of sub-Saharan Africa, while other current populations in places like northern Europe and the southern tip of South America exhibit some of the least genetic differentiation relative to all global populations (Campbell and Tishkoff 2008).
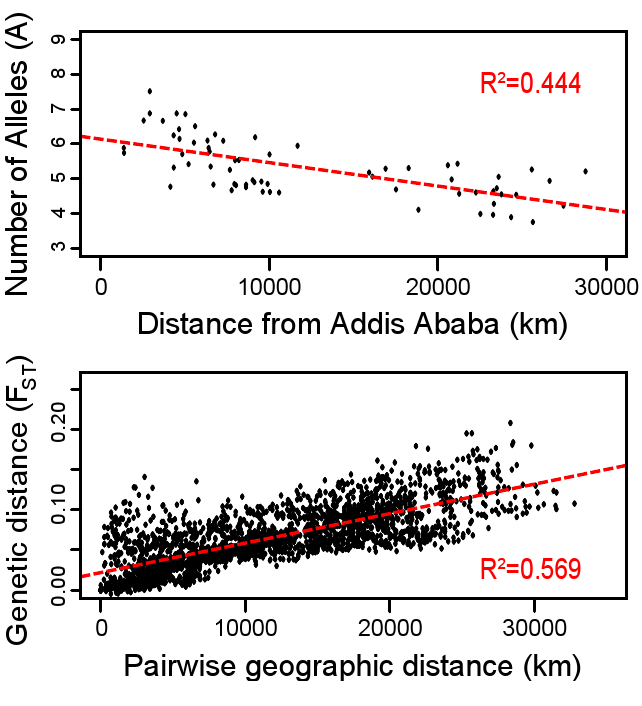
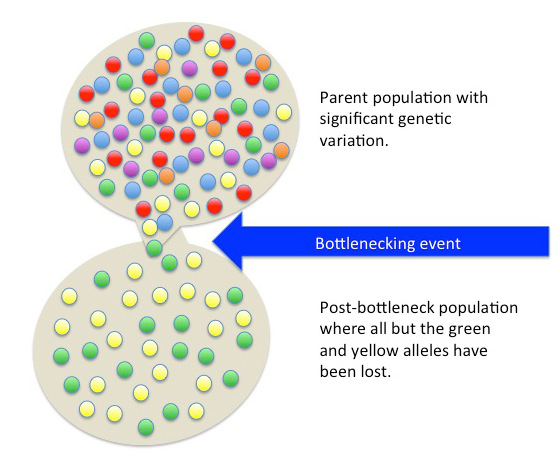
Besides fitting nicely into the Out-of-Africa model, worldwide human genetic variation conforms to an isolation-by-distance model, which predicts that genetic similarity between groups will decrease exponentially as the geographic distance between them increases (Kanitz et al. 2018). This is because of the greater and greater restrictions to gene flow presented by geographic distance, as well as cultural and linguistic differences that occur as a result of certain degrees of isolation. Since genetic data conform to isolation-by-distance and Out-of-Africa models, these findings support the abolishment of “race” groupings. This research demonstrates that human variation is continuous and cannot be differentiated into geographically discrete categories. There are no “inherent” or “innate” differences between human groups; instead, variation derives from some degree of natural selection, as well as neutral processes like population bottle-necking (Figure 13.18), random mutations in the DNA, genetic drift, and gene flow through between-mate interbreeding.
Humans Have Higher Homogeneity Compared to Many Other Species
An important fact to bear in mind is that humans are 99.9% identical to one another. This means that the apportionments of human variation discussed above only concern that tiny 0.1% of difference that exists between all humans globally. Compared to other mammalian species, including the other great apes, human variation is remarkably lower. This may be surprising given that the worldwide human population has already exceeded seven billion, and, at least on the surface level, we appear to be quite phenotypically diverse. Molecular approaches to human and primate genetics tells us that external differences are merely superficial. For a proper appreciation of human variation, we have to look at our closest relatives in the primate order and mammalian class. Compared to chimpanzees, bonobos, gorillas and other primates, humans have remarkably low average genome-wide heterogeneity (Osada 2005).
When we look at chimpanzee genetic variation, it is fascinating that western, central, eastern, and Cameroonian chimpanzee groups have substantially more genetic variation between them than large global samples of human DNA (Bowden et al. 2012; Figure 13.19). This is surprising given that all of these chimpanzee groups live relatively near one another in Africa, while measurements of human genetic variation have been conducted using samples from entirely different continents. First, geneticists suppose that this could reflect differential experiences of the founder effect between humans and chimpanzees. As it has been argued that all non-African human populations descended from a small number of anatomically modern humans who left Africa, it would be expected that all groups descended from that smaller ancestral group would be similar genetically. Second, our species is really young, given that we have only existed on the planet for around 150,000 to 300,000 years. This gave humans little time for random genetic mutations to occur as genes get passed down through genetic interbreeding and meiosis. Chimpanzees, however, have inhabited different ecological niches, and less interbreeding has occurred between the four chimpanzee groups over the past six to eight million years compared to the amount of gene flow that occurred between worldwide human populations (Bowden et al. 2012).
Recent advances have now enabled the attainment of genetic samples from the larger family of great apes and the evaluation of genetic variation among bonobos, orangutans, and gorillas alongside that of chimpanzees and humans (Prado-Martinez et al. 2013). Collecting such data and analyzing primate genetic variation has been important not only to elucidate how different ecological, demographic, and climatic factors have shaped our evolution but also to inform upon conservation efforts and medical research. Genes that may code for genetic susceptibilities to tropical diseases that affect multiple primates can be studied through genome-wide methods. Species differences in the genomes associated with speech, behavior, and cognition could tell us more about how human individuals may be affected by genetically derived neurological or speech-related disorders and conditions (Prado-Martinez et al. 2013; Staes et al. 2017). In 2018, a great ape genomic study also reported genetic differences between chimpanzees and humans related to brain cell divisions (Kronenberg et al. 2018). From these results, it may be inferred that cognitive or behavioral variation between humans and the great apes might relate to an increased number of cortical neurons being formed during human brain development (Kronenberg et al. 2018). Comparative studies of human and nonhuman great ape genetic variation highlight the complex interactions of population histories, environmental changes, and natural selection between and within species. When viewed in the context of overall great ape variation, we may reconsider how variable the human species is relatively and how unjustified previous “race” concepts really were.
Phenotypic Traits That Reflect Neutral Evolution
Depending on the trait being observed, different patterns of phenotypic variation may be found within and among groups worldwide. In this subsection, some phenotypic traits that reflect the aforementioned patterns of genetic variation will be discussed.

Looking beyond genetic variation briefly, recent studies have revisited biological anthropology’s earlier themes of externally observable traits, such as skull shape. In the last 20 or so years, anthropologists have evaluated the level to which human cranial shape variation reflects the results from genetic markers, such as those used previously to fit against Out-of-Africa models (Relethford 2004) or those used in the apportionment of human variation between and within groups (Lewontin 1972; Rosenberg et al. 2002). Using larger sample sizes of cranial data collected from thousands of skulls worldwide and a long list of cranial measurements, studies demonstrate a similar decrease in variation with distance from Africa and show that a majority of cranial variation occurs within populations rather than between populations (Betti et al. 2009; Betti et al. 2010; Manica et al. 2007; Relethford 2001; von Cramon-Taubadel and Lycett 2008; see Figure 13.20). The greatest cranial variation is found among skulls of sub-Saharan African origin, while the least variation is found among populations inhabiting places like Tierra del Fuego at the southern tip of Argentina and Chile. While ancient and historical thinkers previously thought “race” categories could reasonably be determined based on skull dimensions, modern-day analyses using more informative sets of cranial traits simply show that migrations out of Africa and the relative distances between populations can explain a majority of worldwide cranial variation (Betti et al. 2009).
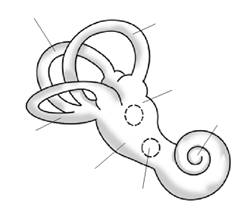
This same patterning in phenotypic variation has even been found in studies examining shape variation of the pelvis (Betti et al. 2013; Betti et al. 2014), the teeth (Rathmann et al. 2017), and the human bony labyrinth of the ear (Ponce de León et al. 2018;Figure 13.21). The skeletal morphology of these bones still varies worldwide, but a greater proportion of that variation can still be attributed to the ways in which human populations migrated across the world and exchanged genes with those closer to them rather than those further away. Human skeletal variation in these parts of the body is continuous and nondiscrete. Given the important functions of the cranium and these other skeletal parts, we may infer that the genes that underpin their development have been relatively conserved by neutral evolutionary processes such as genetic drift and gene flow. It is also important to note that while some traits such as height, weight, cranial dimensions, and body composition are determined, in part, by genes, the underlying developmental processes behind these traits are underpinned by complex polygenic mechanisms that have led to the continuous spectrum of variation in such variables among modern-day human populations.
Phenotypic Traits That Reflect Natural Selection
Even though 99.9% of our DNA is the same across all humans worldwide, and many traits reflect neutral processes, there are parts of that remaining 0.1% of the human genome that code for individual and regional differences. Similarly to craniometric analyses that have been conducted in recent decades, human variation in skin color has also been reassessed using new methods and in light of greater knowledge of biological evolution.
New technologies allow scientists to use color photometry to sample and quantify the visible wavelength of skin color, in a way 19th- and 20th-century readers could not. In one report, it was found that 87.9% of global skin color variation can be attributed to genetic differences between groups, 3.2% to those among local populations within regions, and 8.9% within local populations (Relethford 2002). This apportionment differs significantly and is the reverse situation found in the distribution of genetic differences we see when we examine genetic markers such as blood type–related alleles. However, this pattern of human skin color worldwide is not surprising, given that we now understand that past selection has occurred for darker skin near the equator and lighter skin at higher latitudes (Jablonski 2004; Jablonski and Chaplin 2000). While most genetic variation reflects neutral variation due to population migrations, geographic isolation, and restricted gene flow dynamics, some human genetic/phenotypic variation is best explained as local adaptation to environmental conditions (i.e., selection). Given that skin color variation is atypical compared to other genetic markers and biological traits, this, in fact, goes against earlier “race” typologies. This is because recent studies ironically show how so much of genetic variation relates to neutral processes, while skin color does not. It follows that skin color cannot be viewed as useful in making inferences about other human traits.

It is also true that some populations have not been studied extensively in skin pigmentation genetics (e.g., African, Austronesian, Melanesian, Southeast Asian, Indigenous American, and Pacific Islander populations, according to Lasisi and Shriver 2018). Earlier dispersals of these populations, and their local genetic varition, will have contributed to worldwide genetic variation, inclusive of skin pigmentation variation. Gene loci we did not previously appreciate as being linked to pigmentation are now being recognized thanks to better tools, more diverse genetic samples, and more accessible datasets (Quillen et al. 2018). Biological anthropologists look forward to further discoveries elucidating the different selective pressures and population dynamics that influence skin pigmentation evolution.
Social Implications
To finish this chapter, we will consider the social, economic, political, and biological implications of poor understandings of race and the deliberate perpetuation of social and medical racism.
The Black Lives Matter movement (BLM) of 2013 began with the work of racial justice activists and community organizers Alicia Garza, Opal Tometi, and Patrissa Cullors. First incited by the murder of Trayvon Martin, a 17-year-old African American, and the acquittal of the man who shot him, BLM went on to protest against the deaths of numerous Black individuals, most of whom were killed by police officers (for example, Ahmaud Arbery was killed in February of 2020 by two white non-police officers). Some key characteristics of BLM from the start were its decentralized grassroots structure, the role of university students and social media in spreading awareness of the movement, and its embrace of other movements (e.g., climate justice, ending police brutality, feminist campaigns, queer activism, immigration reform, etc.). When George Floyd was murdered by a white police officer on May 25, 2020, the BLM gained new momentum, across 2,000-plus cities in the United States, and among many protesting against historic racism and police brutality in other contexts around the globe. Many in the biological anthropology community have responded to these events with a great dedication to working against systemic racism in society and institutions (American Association of Biological Anthropologists 2020).
BLM continues to be an important movement, as is evidenced in the degree of community organizing, mutual aid efforts, calls for political reform, progress toward curriculum reform and equality, inclusion and diversity (EDI) work in businesses and universities, the removal of monuments honoring historical figures associated with slavery and racism, and many other important actions. Garza (2016) writes: “The reality is that race in the United States operates on a spectrum from black to white … the closer you are to white on that spectrum, the better off you are.” Tometi (2016) has stated: “We need [a human rights movement that challenges systemic racism] because the global reality is that Black people are subject to all sorts of disparities in most of our challenging issues of our day. I think about climate change, and how six of the ten worst impacted nations by climate change are actually on the continent of Africa.” In the words of Cullors (2016), “Black Lives Matter is our call to action. It is a tool to reimagine a world where Black people are free to exist, free to live. It is a tool for our allies to show up differently for us.” We gather from their words the importance of learning from the egregious role that anthropologists have played in the past, recognizing the legacies of “scientific” justifications for eugenics and racism in our society today, and proactively working toward environmental and social equity.
Another major industry that engages in the quantification and interpretation of human variation is medical and clinical work (National Research Council [U.S.] Committee on Human Genome Diversity 1997). Large-scale genomic studies sampling from human populations distributed worldwide have produced detailed knowledge on variation in disease resistance or susceptibility between and within populations. Let’s think about drug companies who develop medicines for Black patients particularly. The predispositions to particular diseases are higher among people of African descent than some pharmaceutical businesses have taken into account. Through targeted sampling of various world groups, clinical geneticists may also identify genetic risk factors of certain common disorders such as chronic heart disease, asthma, diabetes, autoimmune diseases, and behavioral disorders. Having an understanding of population-specific biology is crucial in the development of therapies, medicines, and vaccinations, as not all treatments may be suitable for every human, depending on their genotype. During diagnosis and treatment, it is important to have an evolutionary perspective on gene-environment relationships in patients. Typological concepts of “race” are not useful, given that most racial groups (whether self-identified or not) popularly recognized lack homogeneity and are, in fact, variable. Cystic fibrosis, for instance, occurs in all world populations but can often be underdiagnosed in populations with African ancestry because it is thought of as a “white” disease (Yudell et al. 2016).
Sociologists, law scholars, and professors of race studies have written extensively on how genetic/technological/medical revolutions impact people of color. In her book, Fatal Invention: How Science, Politics, and Big Business Re-create Race in the Twenty-First Century (2013), Professor Dorothy E. Roberts writes about how technological advances have been used in resuscitating race as a biological category for dividing humans in essentialist ways (Figure 13.23). She notes how members of law enforcement have engaged in racial profiling, sometimes with the use of machine-learning and facial-recognition technologies. Ancestry-testing services also purport to tell us “what” we are and to insist that this information is “written” in our genes. Such advertising campaigns obscure the nuances of genetic variation with the primary motive of tapping into people’s desire to “know themselves” and driving up profits for their businesses. Commercial genetic testing reinforces the idea that genes map neatly onto race, all while generating massive stores of data in DNA databases. In Roberts’s view, the myth of the biological concept of race being perpetuated in these ways undermines a just society and reproduces racial inequalities.

The COVID-19 pandemic has had a significant impact on the world’s population, particularly people living in the economic Global South and many Black, Indigenous and communities of color residing in the Global North. We have witnessed disproportionately high numbers of COVID-related deaths and infection cases among marginalized groups. Many immigrants and ethnic minorities in various societies have also experienced scapegoating and blame directed at them for being the source of COVID-19 spread.
To inform us on how to interpret this current worldwide pandemic, historians and anthropologists are looking back at the lessons learned from past instances of racist medicine (discriminatory practices based on broader social discrimination) and medical racism (application of discriminatory practices justified on medical grounds). Historically, who could become doctors and medical professionals was often racialized, gendered, and class specific. This made it difficult for many to overcome prejudices against women, Black people, Indigenous individuals, or other people of color from becoming doctors and clinical researchers in places such as South Africa and the United States. This, in turn, affects the sorts of information we know about health levels and health outcomes among these very groups. In the past decade, long-overdue attention is finally being paid to how race affects biological outcomes. For instance, researchers have focused on the negative legacies of racial discrimination and racism-induced stress on hormone (im)balances, mental health disorders, cardiovascular disease prevalence, and other health outcomes (Kuzawa and Sweet 2009; Shonkoff, Slopen, and WIlliams 2021; Williams 2018). The technology and standards of protocol in medical testing have been scrutinized (for more on how pulse oximeters were not designed with nonwhite patients in mind, for example, see Sjoding et al. 2020). Scholars of race and medicine have also written on how illness and disease spread have often been used to perpetuate societal prejudices. This manifests as xenophobic tendencies at a societal level, such as the blaming of “outgroups” and increased “in-group” protectiveness. Overreliance on the idea that people are “inherently” disease carriers due to genetic or biological reasons leads to improper accounting for socioeconomic or infrastructural issues that lead to differential disease prevalence amongst minority communities. (For more on race and COVID, see Tsai 2021 as well as this textbook’s Chapter 16: Contemporary Topics: Human Biology and Health.)
It is important to remember that while it is possible to look for clues about one’s ancestry or geographic origin based on skull morphology, again, the amount of distinctiveness in any given sample makes it impossible to distinguish whether a cranium belongs to one group (Relethford 2009). Individuals can vary in their skeletal dimensions by continental origin, country origin, regional origin, sex, age, environmental factors, and the time period in which they lived, making it difficult to assign individuals to particular categories in a completely meaningful way (Ousley, Jantz, and Freid 2009). When forensic reports and scientific journal articles give an estimation of ancestry, it is crucial to keep in mind that responsible assignments of ancestry will be done through robust statistical testing and stated as a probability estimate. Today, we also live in a more globalized world where a skeletal individual may have been born originally to parents of two separate traditional racial categories. In contexts of great heterogeneity within populations, this definitely adds difficulty to the work of forensic scientists and anthropologists preparing results for the courtroom (genetic testing may be comparatively more helpful in such situations).
Dig Deeper: Measuring FST
Richard Lewontin (1929‒) is a biologist and evolutionary geneticist who authored an article evaluating where the total genetic variation in humans lies. Titled “The Apportionment of Human Diversity” (Lewontin 1972), the article addressed the following question: On average, how genetically similar are two randomly chosen people from the same group when compared to two randomly chosen people from different groups?
Lewontin studied this problem by using genetic data. He obtained data for a large number of different human populations worldwide using 17 genetic markers (including alleles that code for various important enzymes and proteins, such as blood-group proteins). The statistical analysis he ran used a measure of human genetic differences in and among populations known as the fixation index (FST).
Technically, FST can be defined as the proportion of total genetic variance within a subpopulation relative to the total genetic variance from an entire population. Therefore, FST values range from 0 to 1 (or, sometimes you will see this stated as a percentage between 0% and 100%). The closer the FST value of a population (e.g., the world’s population) approaches 1, the higher the degree of genetic differentiation among subpopulations relative to the overall population (see Figure 13.24 for a detailed illustration).
In his article, Lewontin (1972) identified that most of human genetic differences (85.4%) were found within local subpopulations (e.g., the Germans or Easter Islanders), whereas 8.3% were found between populations within continental human groups, and 6.3% were attributable to traditional “race” groups (e.g., “Caucasian” or “Amerind”). These findings have been important for scientifically rejecting the existence of biological races (Long and Kittles 2003).
Talking About Human Biological Variation Going Forward
To conclude, utilizing the term races to describe human biological variation is not accurate or productive. Using a select few hundred genetic loci, or perhaps a number of phenotypic traits, it may be possible to assign individuals to a geographic ancestry, but what constitutes a bounded genetic or geographical grouping is both arbitrary and potentially harmful owing to ethical and historical reasons. The discipline of biological anthropology has moved past typological frameworks that shoehorn continuously variable human populations into discrete and socially constructed subsets. Improvements in the number of markers, the genetic technologies used to study variation, and the number of worldwide populations sampled have led to more nuanced understandings of human variation. It is of utmost importance that scientists make the following points clear to the public:
- Today, we refer to different local human groups as “populations.” What constitutes a population should be carefully defined in scientific reports based on some geographical, linguistic, or cultural criteria and some degree of relativity to other closely or distantly related human groups.
- Humans have significantly less genetic variation than other primates and mammals, and all human beings on Earth share 99.9% of their overall DNA. Some of the remaining 0.1% of human variation varies on a clinal or continuous basis, such as can be seen when looking at ABO blood-type polymorphisms worldwide.
- Many biological characteristics in humans are actually determined nonconcordantly and/or polygenically. Therefore, superiority or inferiority in human behavior or body form cannot justifiably be linked to fixed and innate differences between groups.
- Genetic distances are correlated with geographic distances among the global human population. This is especially apparent when we consider that genetic variation is highest in sub-Saharan Africa, and average genetic heterogeneity decreases in populations further away from the African continent in accordance with the migratory history of anatomically modern Homo sapiens.
- The effects of gene flow, genetic drift, and population bottlenecking are reflected in some phenotypic traits, such as cranial shape.
- We recognize other traits, like skin color and lactase persistence, to be the products of many millennia of natural selective pressures influencing human biology from the external environment.
Taken together, genetic analyses of human variation do not support 20th-century (or even-earlier) concepts of race. In discussions about human variation, these genomic results help clarify how biological variation is distributed across the human population today. Taking care to think about and debate the nature of human variation is important, because although the effects and events that produced genetic differences among groups occurred in the ancient past, sociocultural concepts about race and ethnicity continue to have real social, economic, and political consequences.
Beyond talking about variation in the university setting, it is important that teachers, researchers, and students of anthropology recognize and assume the responsibility of influencing public perspectives of human variation. Race-based classification systems were developed during the colonial era, the transatlantic trafficking of kidnapped Africans and the so-called “Scientific Revolution” by the first “anthropologists” and scholars of humankind’s variation. Unfortunately, some of their early ideas have persisted and evolved into present-day lived realities. Some of today’s politicians and socioeconomic bodies have racially charged agendas that promote racism or certain kinds of economic or racial inequalities. As anthropologists, we must acknowledge that while human “races” are not a biological reality, their status as a (misguided) social construction does have real consequences for many people (Antrosio 2011).
In other words, while “race” is a sociocultural invention, the treatment different individuals receive due to their perceived “race” can have significant financial, emotional, sociopolitical, and physiological costs. However—and importantly assuming a “color-blind” position when it comes to the topics of “race” and ethnicity (especially in political discussions) is actually counterproductive, because the negative social consequences of modern “race” ideas could be ignored, making it harder to examine and address instances of discrimination properly (Wise 2010). Rather than shy away from these topics, we can use our scientific findings to establish socially relevant and biologically accurate ideas concerning human diversity. Today, research into genetic and phenotypic differentiation among and within various human populations continues to expand in its scope, its technological capabilities, its sample sizes, and its ethical concerns. It is thanks to such scientific work done in the past few decades that we now have a deeper understanding not only of how humans vary but also of how we are biologically a rather homogenous, intermixing world population.
Review Questions
- How is the genetic variation of the human species distributed worldwide?
- What evolutionary processes are responsible for producing genotypic/phenotypic variation within and between human populations?
- Should we continue to attribute any value to “race” concepts older than 1950, based on our current understandings of human biological variation?
- How should we communicate scientific findings about human biological variation more accurately and responsibly to those outside the anthropological discipline?
Key Terms
Age of Discovery: A period between the late 1400s and late 1700s when European explorers and ships sailed extensively across the globe in pursuit of new trading routes and territorial conquest.
Ancestry: Biogeographical information about an individual, traced either through the study of an individual’s genome, skeletal characteristics, or some other form of forensic/archaeological evidence. Anthropologists carry out probabilistic estimates of ancestry. They attribute sets of human remains to distinctive “ancestral” groups using careful statistical testing and should report ancestry estimations with statistical probability values.
Binomial nomenclature: A system of naming living things developed by Linnaeus in the 1700s. It employs a scientific name made up of two italicized Latin or Greek words, with the first word capitalized and representative of an organism’s genus and the second word indicating an organism’s species (e.g., Homo sapiens, Australopithecus afarensis, Pongo tapanuliensis, etc.).
Biological anthropology: A branch of study under anthropology (the study of humankind) that focuses on when and where humans and our human ancestors first originated, how we have evolved and adapted globally over time, and the reasons why we see biological variation among humans worldwide today.
Biological determinism: The erroneous concept that an individual’s behavioral characteristics are innate and determined by genes, brain size, or other physiological attributes—and, notably, without the influence of social learning or the environment around the individual during development.
Bony labyrinth: A system of interconnected canals within the auditory (ear- or hearing-related) apparatus, located in the inner ear and responsible for balance and the reception of sound waves.
Cline: A gradient of physiological or morphological change in a single character or allele frequency among a group of species across environmental or geographical lines (e.g., skin color varies clinally, as, over many generations, human groups living nearer the equator have adapted to have more skin pigmentation).
Continuous variation: This term refers to variation that exists between individuals and cannot be measured using distinct categories. Instead, differences between individuals within a population in relation to one particular trait are measurable along a smooth, continuous gradient.
Cystic fibrosis: A genetic disorder in which one defective gene causes overproduction and buildup of mucus in the lungs and other bodily organs. It is most common in northern Europeans (but also occurs in other world populations).
Ecological niche: The position or status of an organism within its community and/or ecosystem, resulting from the organism’s structural and functional adaptations (e.g., bipedalism, omnivorous diets, lactose digestion, etc.).
Essentialism: A belief or view that an entity, organism, or human grouping has a specific set of characteristics that are fundamentally necessary to its being and classification into definitive categories.
Ethnicity: A term used commonly in an interchangeable way with the term race, complicated because how different people define this term depends on the qualities and characteristics they use to assign a label or identity to themselves and/or others (which may include aspects of family background, skin color, language(s) spoken, religion, physical proportions, behavior and temperament, etc.).
Eugenics: A set of beliefs and practices that involves the controlled selective breeding of human populations with the hope of improving their heritable qualities, especially through surgical procedures like sterilization and legal rulings that affect marriage rights for interracial couples.
Gene flow: A neutral (or nonselective) evolutionary process that occurs when genes get shared between populations.
Genetic drift: A neutral evolutionary process in which allele frequencies change from generation to generation due to random chance.
Heterogeneity: The quality of being diverse genetically.
Homogenous: The quality of being uniform genetically.
Human diversity: Human diversity is a measure of variation that may describe how many different forms of human there are, separated or clustered into groups according to some genetic, phenotypic, or cultural trait(s). The term can be applied to culture (in which case humans can be described as significantly diverse) or genetics (in which case humans are not diverse because all humans on Earth share a majority of their genes).
Human variation: Differences in biology, physiology, body chemistry, behavior, and culture. By measuring these differences, we understand the degrees of variation between individuals, groups, populations, or species.
Isolation-by-distance model: A model that predicts a positive relationship between genetic distances and geographical distances between pairs of populations.
Monogenetic: Pertaining to the idea that the origin of a species is situated in one geographic region or time (as opposed to polygenetic).
Mutation: A gene alteration in the DNA sequence of an organism. As a random, neutral evolutionary process that occurs over the course of meiosis and early cell development, gene mutations are possible sources of variation in any given human gene pool. Genetic mutations that occur in more than 1% of a population are termed polymorphisms.
Natural selection: An evolutionary process whereby certain traits are perpetuated through successive generations, likely owing to the advantages they give organisms in terms of chances of survival and/or reproduction.
Nonconcordance: The fact of genes or traits not varying with one another and instead being inherited independently.
Otherness: In postcolonial anthropology, we now understand “othering” to mean any action by someone or some group that establishes a division between “us” and “them” in relation to other individuals or populations. This could be based on linguistic or cultural differences, and it has largely been based on external characteristics throughout history.
Out-of-Africa model: A model that suggests that all humans originate from one single group of Homo sapiens in (sub-Saharan) Africa who lived between 100,000 and 315,000 years ago and who subsequently diverged and migrated to other regions across the globe.
Physical anthropology: This used to be the more common name given to the subdiscipline of anthropology centered upon the study of human origins, evolution and variation (also see biological anthropology above). This name for the field has gradually become less popular due to two reasons: first, it may not reflect our interests in other aspects of humankind that are not physical (such as those behavioral, cultural and spiritual), and second, using this term popular in the early decades of our field may be viewed by some as harkening back to a time when biological anthropologists conducted their work in unethical ways.
Polygenetic: Having many different ancestries, as in older theories about human origins that involved multiple traditional groupings of humans evolving concurrently in different parts of the world before they merged into one species through interbreeding and/or intergroup warfare. These earlier suggestions have now been overwhelmed by insurmountable evidence for a single origin of the human species in Africa (see the “Out-of-Africa model”).
Polymorphism: A genetic variant within a population (caused either by a single gene or multiple genes) that occurs at a rate of over 1% among the population. Polymorphisms are responsible for variation in phenotypic traits such as blood type and skin color.
Population: A group of humans living in a particular geographical area, with more local interbreeding within-group than interbreeding with other groups. A limited or restricted amount of gene flow between populations can occur due to geographical, cultural, linguistic, or environmental factors.
Population bottlenecking: An event in which genetic variation is significantly reduced owing to a sharp reduction in population size. This can occur when environmental disaster strikes or as a result of human activities (e.g., genocides or group migrations). An important example of this loss in genetic variation occurred over the first human migrations out of Africa and into other continental regions.
Prejudice: An unjustified attitude toward an individual or group that is not based on reason, whether positive (and showing preference for one group of people over another) or negative (and resulting in harm or injury to others).
Race: The identification of a group based on a perceived distinctiveness that makes that group more similar to each other than they are to others outside the group. This may be based on cultural differences, genetic parentage, physical characteristics, behavioral attributes, or something arbitrarily and socially constructed. As a social or demographic category, perceptions of “race” can have real and serious consequences for different groups of people. This is despite the fact that biological anthropologists and geneticists have demonstrated that all humans are genetically homogenous and that more differences can be found within populations than between them in the overall apportionment of human biological variation. This term is sometimes used interchangeably with ethnicity.
Racism: Any action or belief that discriminates against someone based on perceived differences in race or ethnicity.
Scientific Revolution: A period between the 1400s and 1600s when substantial shifts occurred in the social, technological, and philosophical sense, when a scientific method based on the collection of empirical evidence through experimentation was emphasized and inductive reasoning was used to test hypotheses and interpret their results.
Typological: Of or describing an assortment system that relies on the interpretation of qualitative similarities or differences in the study of variation among objects or people. The categorization of cultures or human groups according to “race” was performed with a typological approach in the earliest practice of anthropology, but this practice has since been discredited and abandoned.
Variance: In statistics, variance measures the dispersal of a set of data around the mean or average value.
For Further Exploration
Videos
American Medical Association (AMA). 2020. “Examining Race-Based Medicine.” YouTube, October 29. Accessed June 4, 2023.
Crenshaw, Kimberlé. 2016. “The Urgency of Intersectionality.” YouTube, December 7. Accessed June 4, 2023.
Golash-Boza, Tanya. 2018. “What Is Race? What Is Ethnicity? Is There a Difference?.” YouTube, October 28. Accessed June 4, 2023.
Lasisi, Tina. 2020. “How Hair Reveals the Futility of Race Categories.” National Museum of Natural History webinar, October 21.
Lasisi, Tina. 2022. “Where Does My Skin Color Come From?.” PBS Terra, August 18. Accessed June 4, 2023.
PBS Origins. 2018. “The Origin of Race in the USA.” YouTube, April 3. Accessed June 4, 2023.
Roberts, Dorothy. 2016. “The Problem with Race-Based Medicine.” YouTube, March 4. Accessed June 4, 2023.
Vox. 2015. “The Myth of Race, Debunked in 3 Minutes.” YouTube, January 13. Accessed June 4, 2023.
Podcast Episodes
Kwong, Emily, and Rebecca Ramirez. 2021. “Here’s a Better Way to Talk about Hair: A 16 Minute Listen with Tina, Lasisi” NPR Short Wave, October 6. Accessed June 4, 2023.
Speaking of Race. 2020. “Race and Health series.” Speaking of Race, April 10. Accessed June 4, 2023.
Websites
Choices Program. 2023. “An Interactive Timeline: Black Activism and the Long Fight for Racial Justice.” Choices Program, Brown University [Interactive Timeline], Updated February, 2023.
References
American Association of Biological Anthropologists. 2020. “An Open Letter to Our Community in Response to Police Brutality against African-Americans and a Call to Antiracist Action”. American Association of Biological Anthropologists, June 10, 2020. Accessed June 4, 2023.
Antrosio, Jason. 2011. “‘Race Reconciled’: Race Isn’t Skin Color, Biology, or Genetics.” Living Anthropologically (website), June 5, 2011; updated May 20, 2020. Accessed June 4, 2023.
Beals, Kenneth L., Courtland L. Smith, Stephen M. Dodd, J. Lawrence Angel, Este Armstrong, Bennett Blumenberg, Fakhry G. Girgis, et al. 1984. “Brain Size, Cranial Morphology, Climate, and Time Machines [and Comments and Reply].” Current Anthropology 25 (3): 301‒330.
Betti, Lia, François Balloux, Tsunehiko Hanihara, and Andrea Manica. 2010. “The Relative Role of Drift and Selection in Shaping the Human Skull.” American Journal of Physical Anthropology 141 (1): 76‒82. https://doi.org/10.1002/ajpa.21115.
Betti, Lia, François Balloux, William Amos, Tsunehiko Hanihara, and Andrea Manica. 2009. “Distance from Africa, Not Climate, Explains Within-Population Phenotypic Diversity in Humans.” Proceedings: Biological Sciences 276 (1658): 809‒814. https://doi.org/10.1098/rspb.2008.1563.
Betti, Lia, Noreen von Cramon-Taubadel, Andrea Manica, and Stephen J. Lycett. 2013. “Global Geometric Morphometric Analyses of the Human Pelvis Reveal Substantial Neutral Population History Effects, Even across Sexes.” PloS ONE 8 (2): e55909. https://doi.org/10.1371/journal.pone.0055909.
Betti, Lia, Noreen von Cramon-Taubadel, Andrea Manica, and Stephen J. Lycett. 2014. “The Interaction of Neutral Evolutionary Processes with Climatically Driven Adaptive Changes in the 3D Shape of the Human Os Coxae.” Journal of Human Evolution 73 (August): 64‒74. https://doi.org/10.1016/j.jhevol.2014.02.021.
Boas, Franz. 1931. “Race and Progress.” Science 74 1905): 1‒8.
Bowden, Rory, Tammie S. MacFie, Simon Myers, Garrett Hellenthal, Eric Nerrienet, Ronald E. Bontrop, Colin Freeman, Peter Donnelly, and Nicholas I. Mundy. 2012. “Genomic Tools for Evolution and Conservation in the Chimpanzee: Pan troglodytes ellioti Is a Genetically Distinct Population.” PLoS Genetics 8 (3): e1002504. https://doi.org/10.1371/journal.pgen.1002504.
Campbell, Michael C., and Sarah A. Tishkoff. 2008. “African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping.” Annual Review of Genomics and Human Genetics 9: 403‒433.
Clee, Paul R. Sesink, Ekwoge E. Abwe, Ruffin D. Ambahe, Nicola M. Anthony, Roger Forso, Sabrina Locatelli, Fiona Maisels, et al. 2015. “Chimpanzee Population Structure in Cameroon and Nigeria Is Associated with Habitat Variation That May Be Lost Under Climate Change.” BMC Evolutionary Biology 15: 2. https://doi.org/10.1186/s12862-014-0275-z.
Cullors, Patrisse. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Fuentes, Agustín, Rebecca Rogers Ackermann, Sheela Athreya, Deborah Bolnick, Tina Lasisi, Sang-Hee Lee, Shay-Akil McLean, and Robin Nelson. 2019. “AAPA Statement on Race and Racism.” American Journal of Physical Anthropology 169 (3): 400‒402.
Garza, Alicia. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Gerbault, Pascale, Anke Liebert, Yuval Itan, Adam Powell, Mathias Currat, Joachim Burger, Dallas M. Swallow, and Mark G. Thomas. 2011. “Evolution of Lactase Persistence: An Example of Human Niche Construction.” Philosophical Transactions of the Royal Society B 366 (1566): 863‒877. https://doi.org/10.1098/rstb.2010.0268.
Hooton, Earnest A. 1936. “Plain Statements about Race.” Science 83 (2161): 511‒513.
Hrdlička, Aleš. 1918. “Physical Anthropology: Its Scope and Aims; Its History and Present Status in America. A: Physical Anthropology; Its Scopes and Aims.” American Journal of Physical Anthropology 1 (1): 3‒23.
Huxley, Julian. 1942. Evolution: The Modern Synthesis. London: Allen and Unwin.
Ingram, Catherine J. E., Charlotte A. Mulcare, Yuval Itan, Mark G. Thomas, and Dallas M. Swallow. 2009. “Lactose Digestion and the Evolutionary Genetics of Lactase Persistence.” Human Genetics 124 (6): 579‒591. https://doi.org/10.1007/s00439-008-0593-6.
Jablonski, Nina G. 2004. “The Evolution of Human Skin and Skin Color.” Annual Review of Anthropology 33: 585‒623. https://doi.org/10.1146/annurev.anthro.33.070203.143955.
Jablonski, Nina G., and George Chaplin. 2000. “The Evolution of Human Skin Coloration.” Journal of Human Evolution 39 (1): 57‒106. https://doi.org/10.1006/jhev.2000.0403.
Kanitz, Ricardo, Elsa G. Guillot, Sylvain Antoniazza, Samuel Neuenschwander, and Jérôme Gedout. 2018. “Complex Genetic Patterns in Human Arise from a Simple Range-Expansion Model over Continental Landmasses.” PLoS ONE 13 (2): e0192460.
Kronenberg, Zev N., Ian T. Fiddes, David Gordon, Shwetha Murali, Stuart Cantsilieris, Olivia S. Meyerson, Jason G. Underwood, et al. 2018. “High-Resolution Comparative Analysis of Great Ape Genomes.” Science 360 (6393): eaar6343. https://doi.org/10.1126/science.aar6343.
Kuzawa, Christopher W., and Elizabeth Sweet. 2009. “Epigenetics and the Embodiment of Race: Development Origins of US Racial Disparities in Cardiovascular Health.” American Journal of Human Biology 21 (1) : 2‒15.
Lasisi, Tina, and Mark D. Shriver. 2018. “Focus on African Diversity Confirms Complexity of Skin Pigmentation Genetics.” Genomic Biology 19: 13.
Lewontin, Richard. 1972. “The Apportionment of Human Diversity.” In Evolutionary Biology, vol. 6, edited by Theodosius Dobzhansky, Max K. Hecht, and William C. Steere, 381‒398. New York: Springer.
Linnaeus, Carl. 1758. Systema Naturae. Stockholm: Laurentius Salvius. https://www.cabdirect.org/abstracts/20057000018.html.
Liu, Hua, Franck Prugnolle, Andrea Manica, and François Balloux. 2006. “A Geographically Explicit Genetic Model of Worldwide Human-Settlement History.” American Journal of Human Genetics 79 (2): 230‒237.
Livingstone, Frank B. 1962. “On the Nonexistence of Human Races.” Current Anthropology 3 (3): 279‒281.
Long, Jeffery C., and Rick A. Kittles. 2003. “Human Genetic Diversity and the Nonexistence of Biological Races.” Human Biology 75 (4): 449‒471.
Luzzatto, Lucio. 2012. “Sickle Cell Anaemia and Malaria.” Mediterranean Journal of Hematology and Infectious Diseases 4 (1). https://doi.org/10.4084/MJHID.2012.065.
Manica, Andrea, William Amos, François Balloux, and Tsunehiko Hanihara. 2007. “The Effect of Ancient Population Bottlenecks on Human Phenotypic Variation.” Nature 448 (7151): 346‒348. https://doi.org/10.1038/nature05951.
McLean, Shay-Akil. 2014. “‘Race, Ethnicity, & Racism.” Decolonize ALL The Things Website, Accessed January 10, 2023. https://decolonizeallthethings.com/learning-tools/race-ethnicity-racism/.
Morton, Samuel George. 1839. Crania Americana, or, A Comparative View of the Skulls of Various Aboriginal Nations of North and South America. Philadelphia: J. Dobson.
Mourant, A. E., Ada C. Kopeć, and Kazimiera Domaniewska-Sobczak. 1976. The Distribution of the Human Blood Groups and Other Polymorphisms, 2nd edition. Oxford: Oxford University Press.
National Research Council (U.S.) Committee on Human Genome Diversity. 1997. Evaluating Human Genetic Diversity. Washington, D.C.: National Academies Press.
Omi, Michael, and Howard Winant. 2014. “The Theory of Racial Formation.” In Racial Formation in the United States,3rd edition, edited by Michael Omi and Howard Winant, 105‒126. Routledge: New York.
Osada, Naoki. 2015. “Genetic Diversity in Humans and Non-Human Primates and Its Evolutionary Consequences.” Genes and Genetic Systems 90 (3): 133‒145.
Ousley, Stephen D., Richard L. Jantz, and Donna Freid. 2009. “Understanding Race and Human Variation: Why Forensic Anthropologists Are Good at Identifying Race.” American Journal of Physical Anthropology 139 (1): 68‒76. https://doi.org/10.1002/ajpa.21006.
Ponce de León, Marcia S., Toetik Koesbardiati, John David Weissmann, Marco Millela, Carlos S. Reyna-Blanco, Gen Suwa, Osamu Kondo, Anna-Sapfo Malaspinas, Tim D. White, and Christoph P. E. Zollikofer. 2018. “Human Bony Labyrinth Is an Indicator of Population History and Dispersal from Africa.” Proceedings of the National Academy of Sciences 115 (16): 4128‒4133. https://doi.org/10.1073/pnas.1808125115.
Prado-Martinez, Javier, Peter H. Sudmant, Jeffrey M. Kidd, Heng Li, Joanna L. Kelley, Belen Lorente-Galdos, Krishna R. Veeramah, et al. 2013. “Great Ape Genetic Diversity and Population History.” Nature 499 (7459): 471–475. https://doi.org/10.1038/nature12228.
Prugnolle, Franck, Andrea Manica, and François Balloux. 2005. “Geography Predicts Neutral Genetic Diversity of Human Populations.” Current Biology 15 (5): 159‒160.
Quillen, Ellen E., Heather L. Norton, Esteban J. Parra, Frida Lona-Durazo, Khai C. Ang, Florin Mircea Illiescu, Laurel N. Pearson, et al. 2018. “Shades of Complexity: New Perspectives on the Evolution and Genetic Architecture of Human Skin.” American Journal of Physical Anthropology 168 (S67): 4–26.
Rathmann, Hannes, Hugo Reyes-Centeno, Silvia Ghirotto, Nicole Creanza, Tsunehiko Hanihara, and Katerina Harvati. 2017. “Reconstructing Human Population History from Dental Phenotypes.” Scientific Reports 7: 12495. https://doi.org/10.1038/s41598-017-12621-y.
Relethford, John H. 2001. “Global Analysis of Regional Differences in Craniometric Diversity and Population Substructure.” Human Biology 73 (5): 629‒636. https://doi.org/10.1353/hub.2001.0073.
Relethford, John H. 2002. “Apportionment of Global Human Genetic Diversity Based on Craniometrics and Skin Color.” American Journal of Physical Anthropology 118 (4): 393‒398. https://doi.org/10.1002/ajpa.10079.
Relethford, John H. 2004. “Global Patterns of Isolation by Distance Based on Genetic and Morphological Data.” Human Biology 76 (4): 499‒513. https://doi.org/10.1353/hub.2004.0060.
Relethford, John H. 2009. “Race and Global Patterns of Phenotypic Variation.” American Journal of Physical Anthropology 139 (1): 16‒22. https://doi.org/10.1002/ajpa.20900.
Roberts, Dorothy. 2013. Fatal Invention: How Science, Politics, and Big Business Re-Create Race in the Twenty-First Century. New York: The New Press.
Rosenberg, Noah A., Saurabh Mahajan, Sohini Ramachandran, Chengfeng Zhao, Jonathan K. Pritchard, and Marcus W. Feldman. 2005. “Clines, Clusters, and the Effect of Study Design on the Inference of Human Population Structure.” PLoS Genetics 1 (6): e70. https://doi.org/10.1371 /journal.pgen.0010070.
Rosenberg, Noah A., Jonathan K. Pritchard, James L. Weber, Howard M. Cann, Kenneth K. Kidd, Lev A. Zhivotovsky, and Marcus W. Feldman. 2002. “Genetic Structure of Human Populations.” Science 298 (5602): 2381‒2385.
Sauer, Norman J. 1992. “Forensic Anthropology and the Concept of Race: If Races Don’t Exist, Why Are Forensic Anthropologists So Good at Identifying Them?” Social Science and Medicine 34 (2): 107‒111. https://doi.org/10.1016/0277-9536(92)90086-6.
Shonkoff, Jack P., Natalie Slopen, and David R. Williams. 2021. “Early Childhood Adversity, Toxic Stress, and the Impacts of Racism on the Foundations of Health.” Annual Review of Public Health 42: 115‒134.
Sjoding, Michael W., Robert P. Dickson, Theodore J. Iwashyna, Steven E. Gay, and Thomas S. Valley. 2020. “Racial Bias in Pulse Oximetry Measurement.” The New England Journal of Medicine 383: 2477-2478.
Staes, Nicky, Chet C. Sherwood, Katharine Wright, Marc de Manuel, Elaine E. Guevara, Tomas Marques-Bonet, Michael Krützen, et al. 2017. “FOXP2 Variation in Great Ape Populations Offers Insight into the Evolution of Communication Skills.” Scientific Reports 7 (1): 1‒10. https://doi.org/10.1038/s41598-017-16844-x.
Tomati, Opal. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Tsai, Jennifer. 2021. “COVID-19 Is Not a Story of Race, but a Record of Racism—Our Scholarship Should Reflect That Reality.” The American Journal of Bioethics 21 (2): 43‒47. https://doi.org/10.1080/15265161.2020.1861377.
von Cramon-Taubadel, Noreen, and Stephen J. Lycett. 2008. “Brief Communication: Human Cranial Variation Fits Iterative Founder Effect Model with African Origin.” American Journal of Physical Anthropology 136 (1): 108‒113. https://doi.org/10.1002/ajpa.20775.
Weiss, Kenneth M., and Jeffrey C. Long. 2009. “Non-Darwinian Estimation: My Ancestors, My Genes’ Ancestors.” Genome Research 19: 703‒710. https://doi.org/10.1101/gr.076539.108.19.
Williams, David W. 2018. “Stress and the Mental Health of Populations of Color: Advancing Our Understanding of Race-related Stressors.” Journal of Health and Social Behavior 59 (4): 466‒485.
Wise, Tim. 2010. Colorblind: The Rise of Post-Racial Politics and the Retreat from Racial Equity. San Francisco: City Lights.
Yudell, Michael, Dorothy Roberts, Rob DeSalle, and Sarah Tishkoff. 2016. “Taking Race out of Human Genetics.” Science 351 (6273): 564‒565. https://doi.org/10.1126/science.aac4951.

