10 Early Members of the Genus Homo
Beth Shook; Ph.D.; Lara Braff; Katie Nelson; Kelsie Aguilera; and M.A.
Bonnie Yoshida-Levine Ph.D., Grossmont College
This chapter is a revision from “Chapter 10: Early Members of the Genus Homo” by Bonnie Yoshida-Levine. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Describe how early Pleistocene climate change influenced the evolution of the genus Homo.
- Identify the characteristics that define the genus Homo.
- Describe the skeletal anatomy of Homo habilis and Homo erectus based on the fossil evidence.
- Assess opposing points of view about how early Homo should be classified.
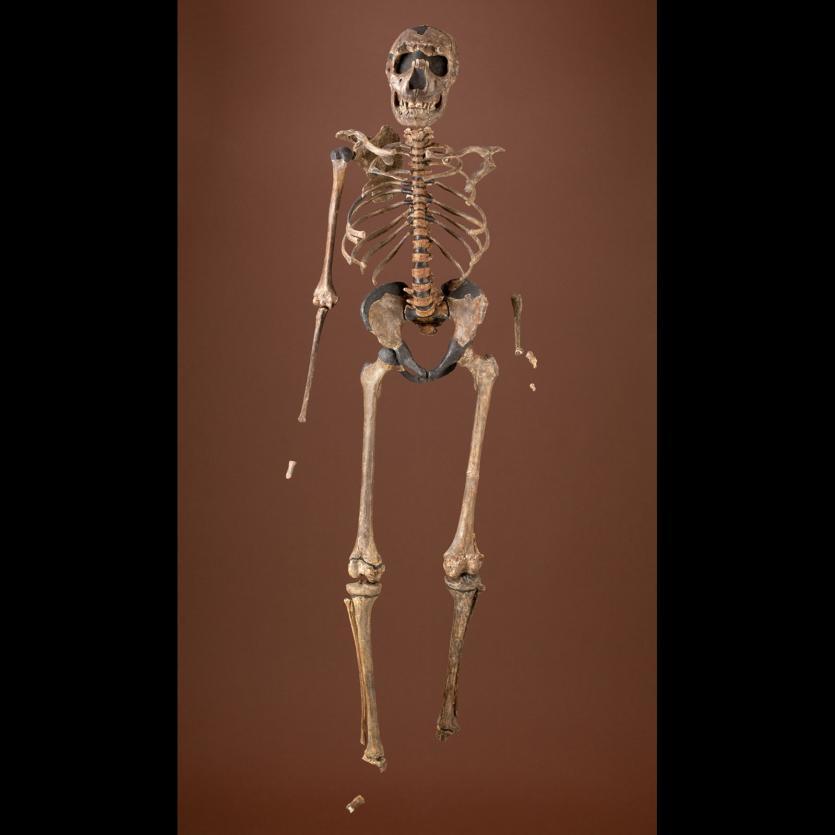
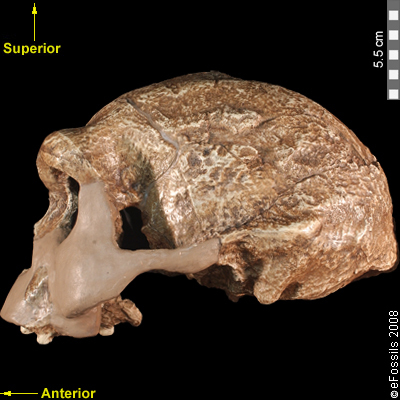
The boy was no older than nine years when he perished by the swampy shores of the lake. After death, his slender, long-limbed body sank into the mud of the lake shallows. His bones fossilized and lay undisturbed for 1.5 million years. In the 1980s, fossil hunter Kamoya Kimeu, working on the western shore of Lake Turkana, Kenya, glimpsed a dark-colored piece of bone eroding in a hillside. This small skull fragment led to the discovery of what is arguably the world’s most complete early hominin fossil—a youth identified as a member of the species Homo erectus. Now known as Nariokotome Boy, after the nearby lake village, the skeleton has provided a wealth of information about the early evolution of our own genus, Homo (see Figure 10.1). Today, a stone monument with an inscription in three languages—English, Swahili, and the local Turkana language—marks the site of this momentous fossil discovery.

Chapter 9 described our oldest human ancestors, primarily members of the genus Australopithecus, who lived between 2 million and 4 million years ago. This chapter introduces the earliest members of the genus Homo, focusing on Homo habilis and Homo erectus.
Defining the Genus Homo
Because Anthropology is fundamentally concerned with what makes us human, defining our own genus takes on special significance for anthropologists. Ever since scientists acknowledged the existence of extinct species of humans, they have debated which of them display sufficient “humanness” to merit classification in the genus Homo. When grouping species into a common genus, biologists consider criteria such as physical characteristics (morphology), evidence of recent common ancestry, and adaptive strategy (use of the environment). However, there is disagreement about which of those criteria should be prioritized, as well as how specific fossils should be interpreted in light of the criteria.
Nevertheless, there is general agreement that species classified as Homo should share characteristics that are broadly similar within our species. These include the following:
- a relatively large brain size,
indicating a high degree of intelligence; - a smaller and flatter face
- smaller jaws and teeth
- increased reliance on culture, particularly the use of stone tools, to exploit a greater diversity of environments (adaptive zone).
Some researchers would include larger overall body size and limb proportions (longer legs/shorter arms) in this list. While these criteria seem relatively clear-cut, evaluating them in the fossil record has proved more difficult, particularly for the earliest members of the genus. There are several reasons for this. First, many fossil specimens dating to this time period are incomplete and poorly preserved. Second, early Homo fossils appear quite variable in brain size, facial features, and teeth and body size, and there is not yet consensus about how to best make sense of this diversity. Finally, there is growing evidence that the evolution of the genus Homo proceeded in a mosaic pattern: in other words, these characteristics did not appear all at once in a single species; rather, they were patchily distributed in different species from different regions and time periods. Consequently, different researchers have come up with conflicting classification schemes depending on which criteria they think are most important.
In this chapter, we will take several pathways toward examining the origin and evolution of the genus Homo. First, we will explore the environmental conditions of the Pleistocene epoch in which the genus Homo evolved. Next we will examine the fossil evidence for the two principal species traditionally identified as early Homo: Homo habilis and Homo erectus. Then we will use data from fossils and archaeological sites to reconstruct the behavior of early members of Homo, including tool manufacture, subsistence practices, migratory patterns, and social structure. Finally, we will consider these together in an attempt to characterize the key adaptive strategies of early Homo and how they put our early ancestors on the trajectory that led to our own species, Homo sapiens.
Climate Change and Human Evolution
A key goal in the study of human origins is to learn about the environmental pressures that may have shaped human evolution. As indicated in Chapter 7, scientists use a variety of techniques to reconstruct ancient environments. These include stable isotopes, core samples from oceans and lakes, windblown dust, analysis of geological formations and volcanoes, and fossils of ancient plant and animal communities. Such studies have provided valuable information about the environmental context of early Homo.
The early hominin species covered in Chapter 9, such as Ardipithecus ramidus and Australopithecus afarensis, evolved during the late Pliocene epoch. The Pliocene (5.3 million to 2.6 million years ago) was marked by cooler and drier conditions, with ice caps forming permanently at the poles. Still, Earth’s climate during the Pliocene was considerably warmer and wetter than at present.
The subsequent Pleistocene epoch (2.6 million years to 11,000 years ago) ushered in major environmental change. The Pleistocene is popularly referred to as the Ice Age. Since the term “Ice Age” tends to conjure up images of glaciers and woolly mammoths, one would naturally assume that this was a period of uniformly cold climate around the globe. But this is not actually the case. Instead, climate became much more variable, cycling abruptly between warm/wet (interglacial) and cold/dry (glacial) cycles. These patterns were influenced by changes in Earth’s elliptical orbit around the sun. As is shown in Figure 10.2, each cycle averaged about 41,000 years during the early Pleistocene; the cycles then lengthened to about 100,000 years starting around 1.25 million years ago. Since mountain ranges, wind patterns, ocean currents, and volcanic activity can all influence climate patterns, there were wide-ranging regional and local effects.
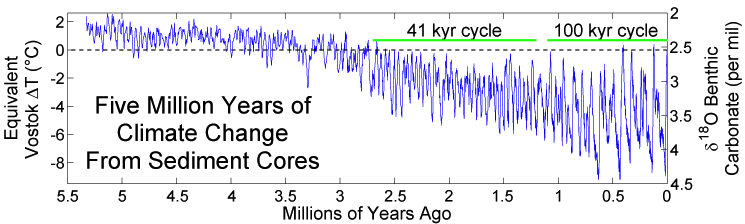
Data on ancient geography and climate help us understand how our ancestors moved and migrated to different parts of the world—as well as the constraints under which they operated. When periods of global cooling dominated, sea levels were lower as more water was captured as glacial ice. This exposed continental margins and opened pathways between land masses. During glacial periods, the large Indonesian islands of Sumatra, Java, and Borneo were connected to the Southeast Asian mainland, while New Guinea was part of the southern landmass of greater Australia. There was a land bridge connection between Britain and continental Europe, and an icy, treeless plain known as Beringia connected Northern Asia and Alaska. At the same time, glaciation made some northern areas inaccessible to human habitation. For example, there is evidence that hominin species were in Britain 950,000 years ago, but it does not appear that Britain was continuously occupied during this period. (It is speculated) These early humans may have died out or been forced to abandon the region during glacial periods.
In Africa, paleoclimate research has determined that grasslands (shown in Figure 10.3) expanded and shrank multiple times during this period, even as they expanded over the long term (deMenocal 2014). From studies of fossils, paleontologists have been able to reconstruct Pleistocene animal communities and to consider how they were affected by the changing climate. Among the African animal populations, the number of grazing animal species such as antelope increased. Although the African and Eurasian continents are connected by land, the Sahara desert and the mountainous topography of North Africa serve as natural barriers to crossing. But the fossil record shows that at different times animal species have moved back and forth between Africa and Eurasia. During the early Pleistocene, there is evidence of African mammal species such as baboons, hippos, antelope, and African buffalo migrating out of Africa into Eurasia during periods of aridity (Belmaker 2010).

This changing environment was undoubtedly challenging for our ancestors, but it offered new opportunities to make a living. One solution adopted by some hominins was to specialize in feeding on the new types of plants growing in this landscape. The robust australopithecines (described in Chapter 9) likely developed their large molar teeth with thick enamel in order to exploit this particular dietary niche.
Members of the genus Homo took a different route. Faced with the unstable African climate and shifting landscape, they evolved bigger brains that enabled them to rely on cultural solutions such as crafting stone tools that opened up new foraging opportunities. This strategy of behavioral flexibility served them well during this unpredictable time and led to new innovations such as increased meat-eating, cooperative hunting, and the exploitation of new environments outside Africa.
Homo habilis: The Earliest Members of Our Genus
Homo habilis has traditionally been considered the earliest species placed in the genus Homo. However, as we will see, there is substantial disagreement among paleoanthropologists about the fossils classified as Homo habilis, including whether they come from a single species or multiple, or even whether they should be part of the genus Homo at all.
Homo habilis has a somewhat larger brain size—an average of 650 cubic centimeters (cc)—compared to Australopithecus with less than 500 cc. Additionally, the skull is more rounded and the face less prognathic. However, the postcranial remains show a body size and proportions similar to Australopithecus.
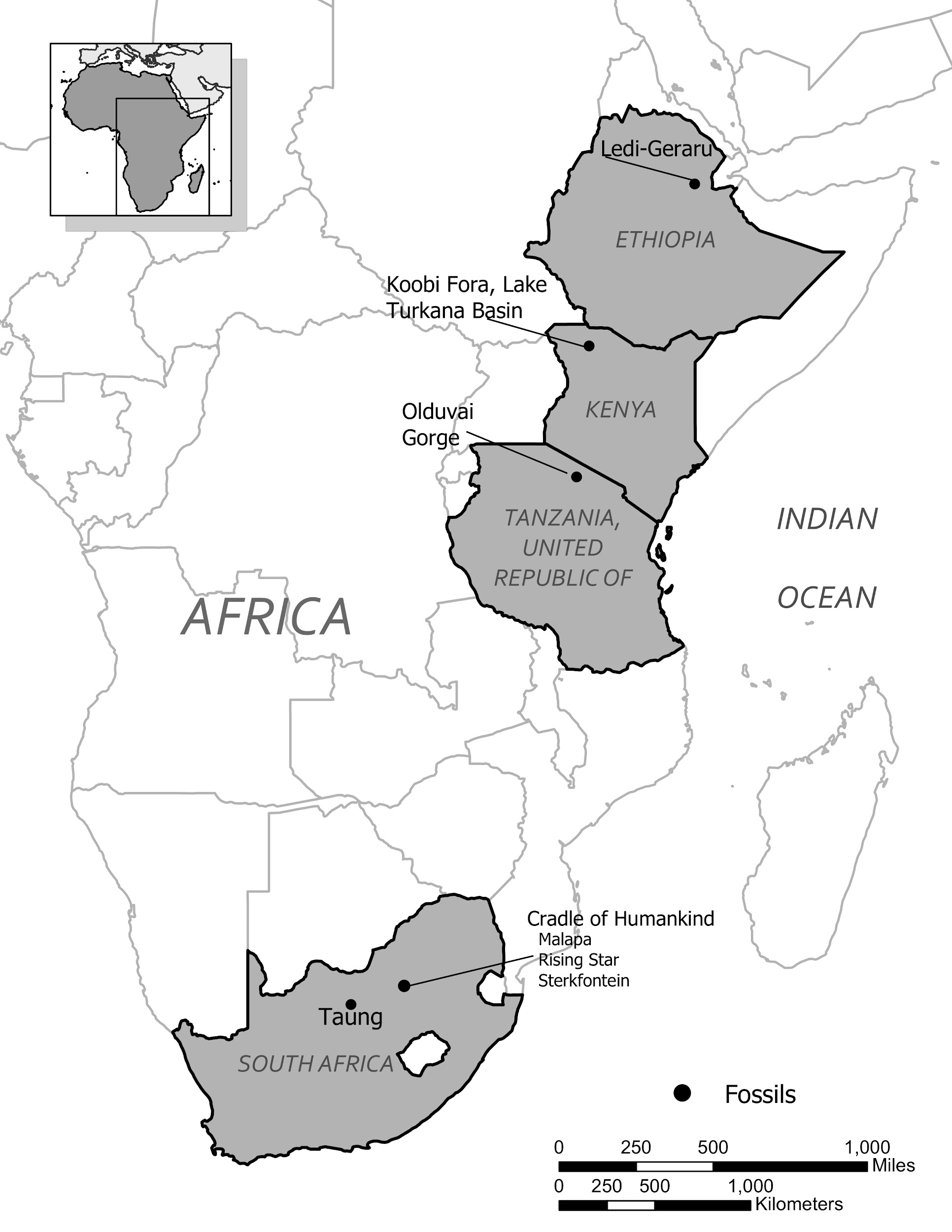
Known dates for fossils identified as Homo habilis range from about 2.5 million years ago to 1.7 million years ago. Recently, a partial lower jaw dated to 2.8 million years from the site of Ledi-Gararu in Ethiopia has been tentatively identified as belonging to the genus Homo (Villmoare et al. 2015). If this classification holds up, it would push the origins of our genus back even further.
Discovery and Naming (just add paragraph not own section)
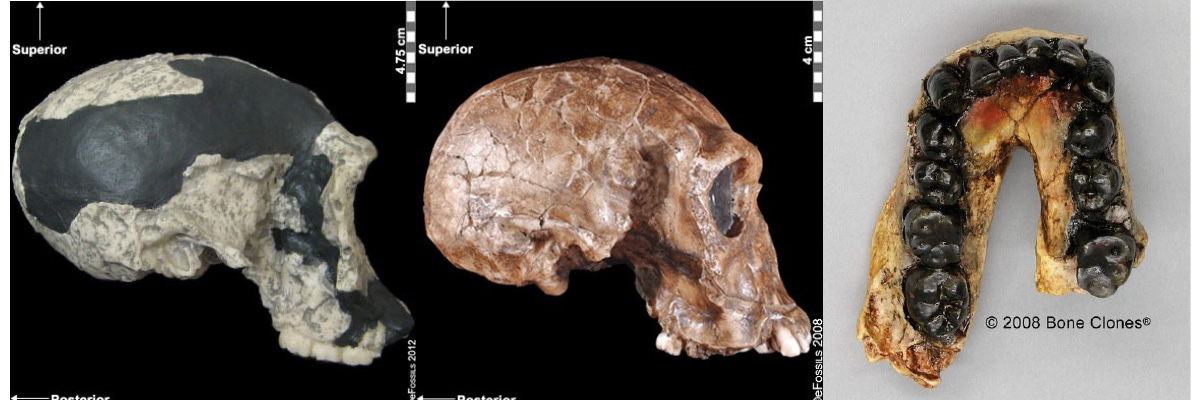
The first fossils to be named Homo habilis were discovered at the site of Olduvai Gorge in Tanzania, East Africa, by members of a team led by Louis and Mary Leakey (Figure 10.4). The Leakey family had been conducting fieldwork in the area since the 1930s and had discovered other hominin fossils at the site, such as the robust Paranthropos boisei. The key specimen, a juvenile individual, was actually found by their 20-year-old son Jonathan Leakey. Louis Leakey invited South African paleoanthropologist Philip Tobias and British anatomist John Napier to reconstruct and analyze the remains. The fossil of the juvenile shown in Figure 10.5 (now known as OH-7) consisted of a lower jaw, parts of the parietal bones of the skull, and some hand and finger bones. The fossil was dated by potassium-argon dating to about 1.75 million years. In 1964, the team published their findings in the scientific journal Nature (Leakey et al. 1964). As described in the publication, the new fossils had smaller molar teeth that were less “bulgy” than australopithecine teeth. Although the primary specimen was not yet fully grown, an estimate of its anticipated adult brain size would make it somewhat larger-brained than australopithecines such as Austalopithecus africanus. The hand bones were capable of a precision grip like a human’s hand. This increased the likelihood that stone tools found earlier at Olduvai Gorge were made by this group of hominins. Based on these findings, the authors inferred that it was a new species that should be classified in the genus Homo. They gave it the name Homo habilis, meaning “handy” or “skilled.”
Controversies over Classification of Homo habilis
Since its initial discovery, many more Homo habilis were discovered in East and South African sites during the 1970s and 1980s (Figure 10.6). As more fossils joined the ranks of Homo habilis, several trends became apparent. First, the fossils were quite variable. While some resembled the fossil specimen first published by Leakey and colleagues, others had larger cranial capacity and tooth size. A well-preserved fossil skull from East Lake Turkana labeled KNM-ER-1470 displayed a larger cranial size along with a strikingly wide face. The diversity of the Homo habilis fossils prompted some scientists to question whether they displayed too much variation to all belong to the same species. They proposed splitting the fossils into at least two groups. The first group resembling the original small-brained specimen would retain the species name Homo habilis; the second group consisting of the larger-brained fossils such as KNM-ER-1470 would be assigned the new name of Homo rudolfensis (see Figure 10.7). Researchers who favored keeping all fossils in Homo habilis argued that sexual dimorphism, adaptation to local environments, or developmental plasticity could be the cause of the differences. For example, modern human body size and body proportions are influenced by variations in climates and nutritional circumstances.
|
Location of Fossils |
Dates |
|
|
Ledi-Gararu, Ethiopia |
2.8 mya |
Partial lower jaw with evidence of both Australopithecus and Homo traits; tentatively considered oldest Early Homo fossil evidence. |
|
Olduvai Gorge, Tanzania |
1.7 mya to 1.8 mya |
Several different specimens classified as Homo habilis, including the type specimen found by Leakey, a relatively complete foot, and a skull with a cranial capacity of about 600 cc. |
|
Koobi Fora, Lake Turkana Basin, Kenya |
1.9 mya |
Several fossils from the Lake Turkana basin show considerable size differences, leading some anthropologists to classify the larger specimen (KNM-ER-1470) as a separate species, Homo rudolfensis. |
|
Sterkfontein and other possible South African cave sites |
about 1.7 mya |
South African caves have yielded fragmentary remains identified as Homo habilis, but secure dates and specifics about the fossils are lacking. |
Given the incomplete and fragmentary fossil record from this time period, it is not surprising that classification has proved contentious. As a scholarly consensus has not yet emerged on the classification status of early Homo, this chapter makes use of the single (inclusive) Homo habilis species designation.
There is also disagreement on whether Homo habilis legitimately belongs in the genus Homo. Most of the fossils first classified as Homo habilis were skulls and teeth. When arm, leg, and foot bones were later found, making it possible to estimate body size, the specimens turned out to be quite small in stature with long arms and short legs. Analysis of the relative strength of limb bones suggested that the species, though bipedal, was much more adapted to arboreal climbing than Homo erectus and Homo sapiens (Ruff 2009). This has prompted some scientists to assert that Homo habilis behaved more like an australopithecine—with a shorter gait and the ability to move around in the trees (Wood and Collard 1999). They were also skeptical of the claim that the brain size of Homo habilis was much larger than that of Australopithecus. They have proposed reclassifying some or all of the Homo habilis fossils into the genus Australopithecus, or even placing them into a newly created genus (Wood 2014).
Other scholars have interpreted the fossil evidence differently. A recent reanalysis of Homo habilis/rudolfensis fossils concluded that they sort into the genus Homo rather than Australopithecus (see Hominin Species Summaries at chapter end). In particular, statistical analysis performed indicates that the Homo habilis fossils differ significantly in average cranial capacity from the australopithecines. They also note that some australopithecine species such as the recently discovered Australopithecus sediba have relatively long legs, so body size may not have been as significant as brain- and tooth-size differences (Anton et al. 2014).
Special Topic: Kamoya Kimeu
Kamoya Kimeu (1938–2022) is arguably the most prolific fossil hunter in the history of paleoanthropology (Figure 10.8). In addition to his many decades of work as a field excavator and project supervisor in East Africa, he also trained field workers and scholars and has served as curator for prehistoric sites for the National Museum of Kenya.

Kamoya Kimeu was born in 1938 in rural southeastern Kenya. Despite a formal education that did not go past the sixth grade, he had an aptitude for languages and familiarity with the plants and animals in the East African bush that led him to a job in Tanzania as a field excavator for Louis and Mary Leakey in 1960. In the years that followed, Kimeu found dozens of major hominin fossils. These included a Paranthropus boisei mandible at Olduvai Gorge, Homo habilis specimen KNM-ER-1813 from the Turkana Basin (shown in Figure 10.5), and a key early modern Homo sapiens fossil from the Omo Valley, Ethiopia. Kimeu’s most famous fossil discovery was the skeleton of a young Homo erectus by the Nariokotome river bed in 1984. This finding was highly significant because it was a nearly complete early hominin skeleton and provided insight into child development within this species. In recognition of his work, Kimeu was awarded the National Geographic Society La Gorce Medal by U.S. President Ronald Reagan in 1985.
Traditionally, there has been a divide between African field workers and foreign research scientists, who would typically conduct seasonal field work in Africa, then travel back to their home institutions to publish their findings. Although Kimeu received widespread acclaim for the Nariokotome discovery, as well as a personal acknowledgement in the publication of the find in the journal Nature, he was not credited as an author. More recently, Kimeu’s intellectual contributions to the field of paleoanthropology have been recognized. In 2021, he received an honorary doctorate degree from Case Western Reserve University in Ohio. Kimeu’s most lasting legacy may be his mentorship of countless field workers and students. Today, there are a small but growing number of Black African paleoanthropologists taking on principal roles in the science of human origins.
Homo habilis Culture and Lifeways
Early Stone Tools
The larger brains and smaller teeth of early Homo are linked to a different adaptive strategy than that of earlier hominins: one dependent on modifying rocks to make stone tools and exploit new food sources. As discussed in Chapter 9, the 3.3-million-year-old stone tools from the Lomekwi 3 site in Kenya were made by earlier hominin species than Homo. However, stone tools become more frequent at sites dating to about 2 million years ago, the time of Homo habilis (Roche et al. 2009). This suggests that these hominins were increasingly reliant on stone tools to make a living.
Stone tools are assigned a good deal of importance in the study of human origins. Examining the form of the tools, the raw materials selected, and how they were made and used can provide insight into the thought processes of early humans and how they modified their environment in order to survive. Paleoanthropologists have traditionally classified collections of stone tools into industries, based on their form and mode of manufacture. There is not an exact correspondence between a tool industry and a hominin species; however, some general associations can be made between tool industries and particular hominins, locations, and time periods.
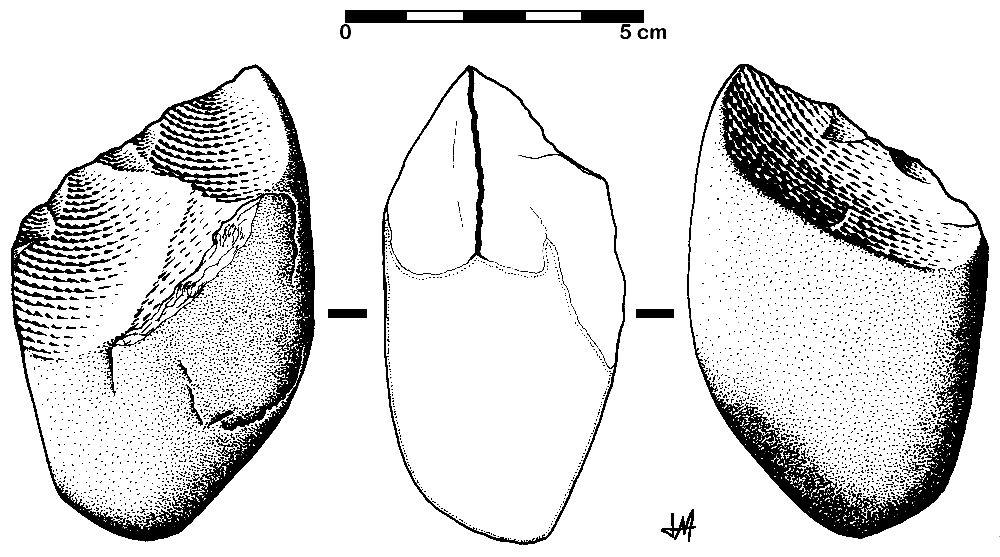
The Oldowan tool industry is named after the site of Olduvai Gorge in Tanzania where the tools were first discovered. The time period of the Oldowan is generally estimated to be 2.5 mya to 1.6 mya. The tools of this industry are described as “flake and chopper” tools—the choppers consisting of stone cobbles with a few flakes struck off them (Figure 10.9). To a casual observer, these tools might not look much different from randomly broken rocks. However, they are harder to make than their crude appearance suggests. The rock selected as the core must be struck by the rock serving as a hammerstone at just the right angle so that one or more flat flakes are removed. This requires selecting rocks that will fracture predictably instead of chunking, as well as the ability to plan ahead and envision the steps needed to create the finished product. The process leaves both the core and the flakes with sharp cutting edges that can be used for a variety of purposes.
Stone Tool Use and the Diet of Early Homo
What were the hominins doing with the tools? One key activity seems to have been butchering animals. Studies of animal bones at the site show cut marks on bones, and leg bones are often cracked open, suggesting that they were extracting the marrow from the bone cavities. It is interesting to consider whether the hominins hunted these animals or acquired them through other means. The butchered bones come from a variety of African mammals, ranging from small antelope to animals as big as wildebeest and elephants! It is difficult to envision slow, small-bodied Homo habilis with their Oldowan tools bringing down such large animals. One possibility is that the hominins were scavenging carcasses from lions and other large cats. Paleoanthropologist Robert Blumenschine has investigated this hypothesis by observing the behavior of present-day animal carnivores and scavengers on the African savanna. When lions abandon a kill after eating their fill, scavenging animals arrive almost immediately to pick apart the carcass. By the time slow-footed hominins arrived on the scene, the carcass would be mostly stripped of meat. However, if hominins could use stone tools to break into the leg bone cavities, they could get to the marrow, a fatty, calorie-dense source of protein (Blumenschine et al. 1987). Reconstructing activities that happened millions of years ago is obviously a difficult undertaking, and paleoanthropologists continue to debate whether scavenging or hunting was more commonly practiced during this time.
Regardless of how they were acquiring the meat, these activities suggest an important dietary shift from the way that the australopithecines were eating. The Oldowan toolmakers were exploiting a new ecological niche that provided them with more protein and calories. And it was not just limited to meat-eating—stone tool use could have made available numerous other subsistence opportunities. A study of microscopic wear patterns on a sample of Oldowan tools indicates that they were used for processing plant materials such as wood, roots or tubers, and grass seeds and stems (Lemorini et al. 2014). In fact, it has been pointed out that the Oldowan toolmakers’ cutting ability (whether for the purposes of consuming meat and plants or for making tools, shelters, or clothing) represents a new and unique innovation, never seen before in the natural world (Roche et al. 2009).
Overall, increasing the use of stone tools allowed hominins to expand their ecological niche and exert more control over their environment. As we’ll see shortly, this pattern continued and became more pronounced with Homo erectus.
Homo erectus: Biological and Cultural Innovations
Two million years ago, a new hominin appeared on the scene. Known as Homo erectus, the prevailing scientific view was that this species was much more like us. These hominins were equipped with bigger brains and large bodies with limb proportions similar to our own. Perhaps most importantly, their way of life is now one that is recognizably human, with more advanced tools, hunting, use of fire, and colonizing new environments outside of Africa.
As will be apparent below, new data suggests that the story is not quite as simple. The fossil record for Homo erectus is much more abundant than that of Homo habilis, but it is also more complex and varied—both with regard to the fossils as well as the geographic context in which they are found. We will first summarize the anatomical characteristics that define Homo erectus, and then discuss the fossil evidence from Africa and the primary geographic regions outside Africa where the species has been located.
Homo erectus Anatomy
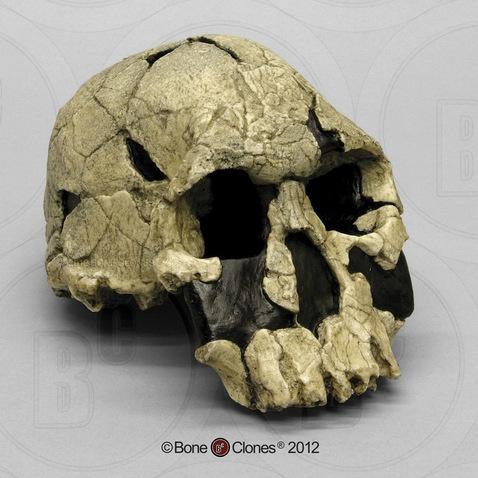
Compared to Homo habilis, Homo erectus showed increased brain size, smaller teeth, and a larger body. However, it also displayed key differences from later hominin species including our own. Although the head of Homo erectus was less ape-like in appearance than the australopithecines, it did not resemble modern humans (Figure 10.10). Compared to Homo habilis, Homo erectus had a larger brain size: an average of about 900 cc compared to 650 cc to 750 cc. Instead of a rounded shape like our skulls, the erectus skull was long and low like a football, with a receding forehead, and a horizontal ridge called an occipital torus that gave the back of the skull a squared-off appearance. The cranial bones are thicker than those of modern humans, and some Homo erectus skulls have a slight thickening along the sagittal suture called a sagittal keel. Large, shelf-like brow ridges hang over the eyes. The face shows less prognathism, and the back teeth are smaller than those of Homo habilis. Instead of a pointed chin, like ours, the mandible of Homo erectus recedes back.
Apart from these features, there is significant variation among Homo erectus fossils from different regions. Scientists have long noted differences between the fossils from Africa and those from Indonesia and China. For example, the Asian fossils tend to have a thicker skull and larger brow ridges than the African specimens, and the sagittal keel described above is more pronounced. Homo erectus fossils from the Republic of Georgia (described in the next section) also display distinctive characteristics. As with Homo habilis, this diversity has prompted a classification debate about whether or not Homo erectus should be split into multiple species. When African Homo erectus is characterized as a separate species, it is called Homo ergaster, while the Asian variant retains the erectus species name because it was discovered first. Here, the species name Homo erectus will be used for both variants.
Homo erectus was thought to have a body size and proportions more similar to modern humans. Unlike Homo habilis and the australopithecines, both of whom were small-statured with long arms and short legs, Homo erectus shows evidence of being fully committed to life on the ground. This meant long, powerfully muscled legs that enabled these hominins to cover more ground efficiently. Indeed, studies of the Homo erectus body form have linked several characteristics of the species to long-distance running in the more open savanna environment (Bramble and Lieberman 2004). Many experts think that hominins around this time had lost much of their body hair, were particularly efficient at sweating, and had darker-pigmented skin—all traits that would support the active lifestyle of such a large-bodied hominin (see Special Topic box, “How We Became Sweaty, Hairless Primates”).
Special Topic: How We Became Hairless, Sweaty Primates (include here)
Much of the information about the body form of Homo erectus comes from the Nariokotome fossil of the Homo erectus youth, described at the beginning of the chapter (see Figure 10.1). However, Homo erectus fossils are turning out to be more varied than previously thought. Homo erectus fossils from sites in Africa, as well as from Dmanisi, Georgia, show smaller body sizes than the Nariokotome boy. Even the Nariokotome skeleton itself has been reassessed: some now predict he would have been about 5 feet and 4 inches when fully grown rather than over 6 feet as initially hypothesized, although there is still disagreement about which measurement is more accurate. One explanation for the range of body sizes could be adaptation to a range of different local environments, just as humans today show reduced body size in poor nutritional environments (Anton and Snodgrass 2012).
Homo erectus in Africa
Although the earliest discoveries of Homo erectus fossils were from Asia, the greatest quantity and best-preserved fossils of the species come from East African sites. The earliest fossils in Africa identified as Homo erectus come from the East African site of Koobi Fora, around Lake Turkana in Kenya, and are dated to about 1.8 million years ago. Other fossil remains have been found in East African sites in Kenya, Tanzania, and Ethiopia. Other notable African Homo erectus finds are a female pelvis from the site of Gona, Ethiopia (Simpson et al. 2008), and a cranium with massive brow ridges from Olduvai Gorge known as Olduvai 9, thought to be about 1.4 million years old.
Until recently, Homo erectus’ presence in southern Africa has not been well documented. However, work at the Drimolen cave site in South Africa has yielded new fossils of Paranthropus robustus, and the cranium of a 2–3 year old child tentatively identified as Homo erectus, dated to about 2 million years (Herries et al. 2020). If substantiated, this would be the oldest discovery to date of Homo erectus anywhere.
Regional Discoveries Outside Africa
It is generally agreed that Homo erectus was the first hominin to migrate out of Africa and colonize Asia and later Europe (although recent discoveries in Asia may challenge this view). Key locations and discoveries of Homo erectus fossils, along with the fossils’ estimated ages, are summarized in Figures 10.11 and 10.12.
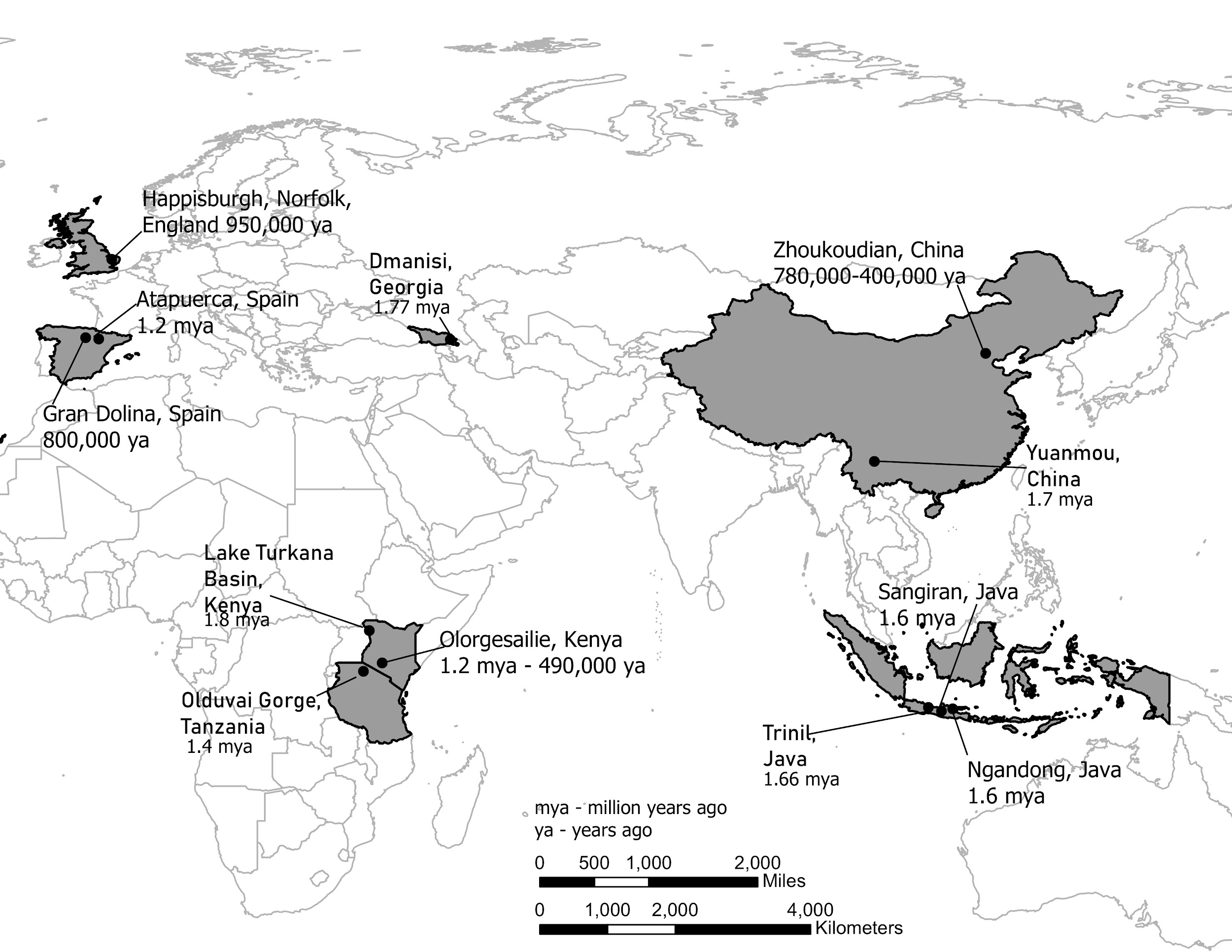
|
Region
|
Sites
|
Dates
|
Significance of Fossils
|
|
East Africa |
East and West Lake Turkana, Kenya; Olduvai Gorge, Tanzania |
1.8 to 1.4 mya |
Earliest evidence of H. erectus; significant variation in skull and facial features. |
|
South Africa |
Drimolen Cave, South Africa |
2 mya |
Recent find of a 2–3 year old child would be oldest H. erectus anywhere to date. |
|
Western Eurasia |
Dmanisi, Republic of Georgia |
1.75 mya |
Smaller brains and bodies than H. erectus from other regions. |
|
Western Europe |
Atapuerca, Spain (Sima del Elefante and Gran Dolina caves) |
1.2 mya– 400,000 ya |
Partial jaw from Atapuerca is oldest evidence of H. erectus in Western Europe. Fossils from Gran Dolina (dated to about 800,000 years) sometimes referred to as H. antecessor. |
|
Indonesia |
Ngandong, Java; Sangiran, Java |
1.6 mya |
Early dispersal of H. erectus to East Asia; Asian H. erectus features. |
|
China |
Zhoukoudian, China; Loess Plateau (Lantian) |
780,000– 400,000 ya; 2.1 mya |
Large sample of H. erectus fossils and artifacts. Recent evidence of stone tools from Loess Plateau suggests great antiquity of Homo in East Asia. |
Indonesia
The first discovery of Homo erectus was in the late 1800s in Java, Indonesia. A Dutch anatomist named Eugene Dubois searched for human fossils with the belief that since orangutans lived there, it might be a good place to look for remains of early humans. He discovered a portion of a skull, a femur, and other bone fragments on a riverbank. While the femur looked human, the top of the skull was smaller and thicker than that of a modern person. Dubois named the fossil Pithecanthropus erectus (“upright ape-man”), popularized in the media at the time as “Java Man.” After later discoveries of similar fossils in China and Africa, they were combined into a single species (retaining the erectus name) under the genus Homo.
Although Homo erectus has a long history in Indonesia, the region’s geology has complicated the dating of fossils and sites. Fossils from the Sangiran Dome, Java, had previously been estimated to be as old as 1.8 million years, but scientists using new dating methods have arrived at a later date of about 1.3 mya (Matsu’ura et al. 2020). On the recent end of the timeline, a cache of H. erectus fossils from the site of Ngandong in Java has yielded a surprisingly young date of 43,000 years, although a newer study with different dating methods concluded that they were between 117,000 to 108,000 years old (Rizal et al. 2020).
China
There is evidence of Homo erectus in China from several regions and time periods. Homo erectus fossils from northern China, collectively known as “Peking Man,” are some of the most famous human fossils in the world. Dated to about 400,000–700,000 years ago, they were excavated from the site of Zhoukoudian, near the outskirts of Beijing. Hundreds of bones and teeth, including six nearly complete skulls, were excavated from a cave in the 1920s and 1930s. Much of the fossils’ fame comes from the fact that they disappeared under mysterious circumstances. As Japan advanced into China during World War II, Chinese authorities, concerned for the security of the fossils, packed up the boxes and arranged for them to be transported to the United States. But in the chaos of the war, they vanished and were never heard about again. Fortunately, an anatomist named Frans Weidenreich had previously studied the bones and made casts and measurements of the skulls, so this valuable information was not lost. More recent excavations at Longgushan “Dragon Bone Cave” at Zhoukoudian—of tools, living sites, and food remains—have revealed much about the lifestyle of Homo erectus during this time.
Despite this long history of research, China, compared to Africa, was perceived as somewhat peripheral to the study of hominin evolution. Although Homo erectus fossils have been found at several sites in China, with dates that make them comparable to those of Indonesian Homo erectus, none seemed to approximate the antiquity of African sites. The notable finds at sites like Nariokotome and Olorgesaille took center stage during the 1970s and 1980s, as scientists focused on elucidating the species’ anatomy and adaptations in its African homeland. In contrast, fewer research projects were focused on East Asian sites (Dennell and Roebroeks 2005; Qiu 2016).
However, isolated claims of very ancient hominin occupation kept cropping up from different locations in Asia. While some were dismissed because of problems with dating methods or stratigraphic context, the 2018 publication of the discovery of 2.1-million-year-old stone tools from China caught everyone’s attention. Based on paleomagnetic techniques that date the associated soils and windblown dust, these tools indicate that hominins in Asia predated those from the Georgian site of Dmanisi by at least 300,000 years (Zhu et al. 2018). In fact, the tools are older than any Homo erectus fossils anywhere. Since no fossils were found with the tools, it isn’t known which species made them, but it opens up the intriguing possibility that hominins could have migrated out of Africa earlier than Homo erectus. These new discoveries are shaking up previously held views of the East Asian human fossil record.
Western Eurasia
An extraordinary collection of fossils from the site of Dmanisi in the Republic of Georgia has revealed the presence of Homo erectus in Western Eurasia between 1.75 million and 1.86 million years ago. Dmanisi is located in the Caucasus mountains in Georgia. When archaeologists began excavating a medieval settlement near the town in the 1980s and came across the bones of extinct animals, they shifted their focus from the historic to the prehistoric era, but they probably did not anticipate going back quite so far in time. The first hominin fossils were discovered in the early 1990s, and since that time, at least five relatively well-preserved crania have been excavated.
There are several surprising things about the Dmanisi fossils. Compared to African Homo erectus, they have smaller brains and bodies. However, despite the small brain size, they show clear signs of Homo erectus traits such as heavy brow ridges and reduced facial prognathism. Paleoanthropologists have pointed to some aspects of their anatomy (such as the shoulders) that appear rather primitive, although their body proportions seem fully committed to terrestrial bipedalism. One explanation for these differences could be that the Dmanisi hominins represent a very early form of Homo erectus that left Africa before increases in brain and body size evolved in the African population.
Second, although the fossils at this location are from the same geological context, they show a great deal of variation in brain size and in facial features. One skull (Skull 5) has a cranial capacity of only 550 cc, smaller than many Homo habilis fossils, along with larger teeth and a protruding face. Scientists disagree on what these differences mean. Some contend that the Dmanisi fossils cannot all belong to a single species because each one is so different. Others assert that the variability of the Dmanisi fossils proves that they, along with all early Homo fossils, including H. habilis and H.rudolfensis, could all be grouped into Homo erectus (Lordkipanidze et al. 2013). Regardless of which point of view ends up dominating, the Dmanisi hominins are clearly central to the question of how to define the early members of the genus Homo.
Europe
Until recently, there was scant evidence of any Homo erectus presence in Europe, and it was assumed that hominins did not colonize Europe until much later than East Asia or Eurasia. One explanation for this was that the harsh climate of Western Europe served as a barrier to settlement. However, recent fossil finds from Spain suggest that Homo erectus could have made it into Europe over a million years ago. In 2008 a mandible from the Atapuerca region in Spain was discovered, dating to about 1.2 million years ago. A more extensive assemblage of fossils from the site of Gran Dolina in Atapuerca have been dated to about 800,000 years ago. In England in 2013 fossilized hominin footprints of adults and children dated to 950,000 years ago were found at the site of Happisburgh, Norfolk, which would make them the oldest human footprints found outside Africa (Ashton et al. 2014).
At this time, researchers aren’t in agreement as to whether the first Europeans belonged to Homo erectus proper or to a later descendent species. Some scientists refer to the early fossils from Spain by the species name Homo antecessor.
Special Topic: How We Became Hairless, Sweaty Primates
As an anthropology instructor teaching human evolution, my students often ask me about human body hair: When did our ancestors lose it and why? It is assumed that our earliest ancestors were as hairy as modern-day apes. Yet, today, we lack thick hair on most parts of our bodies except in the armpits, pubic regions, and tops of our heads. Humans actually have about the same number of hair follicles per unit of skin as chimpanzees, but, the hairs on most of our body are so thin as to be practically invisible. When did we develop this peculiar pattern of hairlessness? Which selective pressures in our ancestral environment were responsible for this unusual characteristic?
Many experts believe that the driving force behind our loss of body hair was the need to effectively cool ourselves. Along with the lack of hair, humans are also distinguished by being exceptionally sweaty: we sweat larger quantities and more efficiently than any other primate. Humans have a larger amount of eccrine sweat glands than other primates and these glands generate an enormous volume of watery sweat. Sweating produces liquid on the skin that cools the body off as it evaporates. It seems likely that hairlessness and sweating evolved together, as a recent DNA analysis has identified a shared genetic pathway between hair follicles and eccrine sweat gland production (Kamberov et al. 2015).
Which particular environmental conditions led to such adaptations? In this chapter, we learned that the climate was a driving force behind many changes seen in the hominin lineage during the Pleistocene. At that time, the climate was increasingly arid and the forest canopy in parts of Africa was being replaced with a more open grassland environment, resulting in increased sun exposure for our ancestors. Compared to the earlier australopithecines, members of the genus Homo were also developing larger bodies and brains, starting to obtain meat by hunting or scavenging carcasses, and crafting sophisticated stone tools.
According to Nina Jablonski, an expert on the evolution of human skin, the loss of body hair and increased sweating capacity are part of the package of traits characterizing the genus Homo. While larger brains and long-legged bodies made it possible for humans to cover long distances while foraging, this new body form had to cool itself effectively to handle a more active lifestyle. Preventing the brain from overheating was especially critical. The ability to keep cool may have also enabled hominins to forage during the hottest part of the day, giving them an advantage over savanna predators, like lions, that typically rest during this time (Jablonski 2010).
When did these changes occur? Although hair and soft tissue do not typically fossilize, several indirect methods have been used to explore this question. One method tracks a human skin color gene. Since chimpanzees have light skin under their hair, it is probable that early hominins also had light skin color. Apes and other mammals with thick fur coats have protection against the sun’s rays. As our ancestors lost their fur, it is likely that increased melanin pigmentation was selected for as a way to shield our ancestors from harmful ultraviolet radiation. A recent genetic analysis determined that one of the genes responsible for melanin production originated about 1.2 million years ago (Rogers et al 2004).
Another line of evidence tracks the coevolution of a rather unpleasant human companion—the louse. A genetic study identified human body louse as the youngest of the three varieties of lice that infest humans, splitting off as a distinct variety around 70,000 years ago (Kittler et al. 2003). Because human body lice can only spread through clothing, this may have been about the time when humans started to regularly wear clothing. However, the split between human head and pubic lice is estimated to have occurred much earlier, about three million years ago (Bower 2003; Reed et al. 2007). When humans lost much of their body hair, lice that used to roam freely around the body were now confined to two areas: the head and pubic region. As a result of this separation, the lice population split into two distinct groups.
Other explanations have been suggested for the loss of human body hair. For example, being hairless makes it more difficult for skin parasites like lice, fleas, and ticks to live on us. Additionally, after bipedality evolved, hairless bodies would also make reproductive organs and female breasts more visible, suggesting that sexual selection may have played a role.
Homo erectus Lifeways
Now, our examination of Homo erectus will turn to its lifeways—how the species utilized its environment in order to survive. This includes making inferences about diet, technology, life history, environments occupied, and perhaps even social organization. As will be apparent, Homo erectus shows significant cultural innovations in these areas, some that you will probably recognize as more “human-like” than any of the hominins previously covered.
Tool Technology: Acheulean Tool Industry
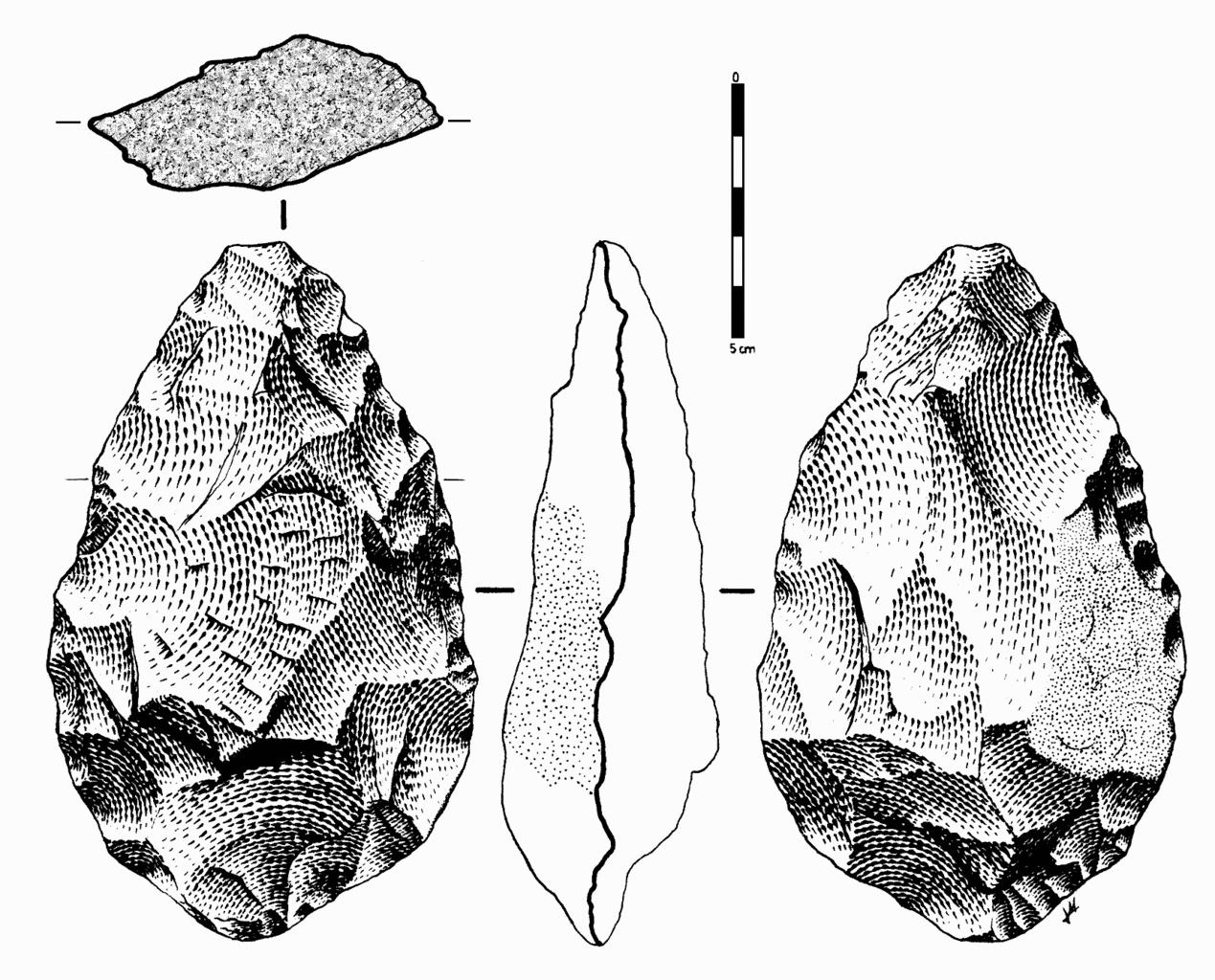
In early African sites associated with Homo erectus, stone tools such as flakes and choppers identified to the Oldowan Industry dominate. Starting at about 1.5 million years ago, some Homo erectus populations began making different forms of tools. These tools—classified together as constituting the Acheulean tool industry—are more complex in form and more consistent in their manufacture. Unlike the Oldowan tools, which were cobbles modified by striking off a few flakes, Acheulean toolmakers carefully shaped both sides of the tool. This type of technique, known as bifacial flaking, requires more planning and skill on the part of the toolmaker; he or she would need to be aware of principles of symmetry when crafting the tool. One of the most common tool forms, the handaxe, is shown in Figure 10.13. As with the tool illustrated below, handaxes tend to be thicker at the base and then come to a rounded point at the tip. Besides handaxes, forms such as scrapers, cleavers, and flake tools are present at Homo erectus sites.
One striking aspect of Acheulean tools is their uniformity. They are more standardized in form and mode of manufacture than the earlier Oldowan tools. For example, the aforementioned handaxes vary in size, but they are remarkably consistent in regard to their shape and proportions. They were also an incredibly stable tool form over time—lasting well over a million years with little change.
Curiously, the Acheulean tools so prominent at African sites are mostly absent in Homo erectus sites in East Asia. Instead, Oldowan-type choppers and scrapers are found at those sites. If this technology seemed to be so important to African Homo erectus, why didn’t East Asian Homo erectus also use the tools? One reason could be environmental differences between the two regions. It has been suggested that Asian Homo erectus populations used perishable material such as bamboo to make tools. Another possibility is that Homo erectus (or even an earlier hominin) migrated to East Asia before the Acheulean technology developed in Africa. The recent discovery of the 2.1-million-year-old tools in China gives credence to this last explanation.
What (if anything) do the Acheulean tools tell us about the mind of Homo erectus? Clearly, they took a fair amount of skill to manufacture. Apart from the actual shaping of the tool, other decisions made by toolmakers can reveal their use of foresight and planning. Did they just pick the most convenient rocks to make their tools, or did they search out a particular raw material that would be ideal for a particular tool? Analysis of Acheulean stone tools suggest that at some sites, the toolmakers selected their raw materials carefully—traveling to particular rock outcrops to quarry stones and perhaps even removing large slabs of rock at the quarries to get at the most desirable material. Such complex activities would require advanced planning and communication with other individuals. However, other Homo erectus sites lack evidence of such selectivity; instead of traveling even a short distance for better raw material, the hominins tended to use what was available in their immediate area (Shipton et al. 2018).
In contrast to Homo erectus tools, the tools of early modern Homo sapiens during the Upper Paleolithic display tremendous diversity across regions and time periods. Additionally, Upper Paleolithic tools and artifacts communicate information such as status and group membership. Such innovation and social signaling seem to have been absent in Homo erectus, suggesting that they had a different relationship with their tools than did Homo sapiens (Coolidge and Wynn 2017). Some scientists assert that these contrasts in tool form and manufacture may signify key cognitive differences between the species, such as the ability to use a complex language.
Subsistence and Diet
In reconstructing the diet of Homo erectus, researchers can draw from multiple lines of evidence. These include stone tools used by Homo erectus, animal bones and occasionally plant remains from Homo erectus sites, and the bones and teeth of the fossils themselves. These data sources suggest that compared to the australopithecines, Homo erectus consumed more animal protein. Coinciding with the appearance of Homo erectus fossils in Africa are archaeological sites with much more abundant stone tools and larger concentrations of butchered animal bones.

It makes sense that a larger body and brain would be correlated with a dietary shift to more calorically dense foods. This is because the brain is a very energetically greedy organ. Indeed, our own human brains require more than 20% of one’s calorie total intake to maintain. When biologists consider the evolution of intelligence in any animal species, it is often framed as a cost/benefit analysis: For large brains to evolve, there has to be a compelling benefit to having them and a way to generate enough energy to fuel them.
One solution that would allow for an increase in human brain size would be a corresponding reduction in the size of the digestive tract (gut). According to the “expensive tissue hypothesis,” initially formulated by Leslie Aiello and Peter Wheeler (1995), a smaller gut would allow for a larger brain without the need for a corresponding increase in the organism’s metabolic rate. More meat in the diet could also fuel the larger brain and body size seen in the genus Homo. Some researchers also believe that body fat percentages increased in hominins (particularly females) around this time, which would have allowed them to be better buffered against environmental disruption such as food shortages (Anton and Snodgrass 2012).
As indicated above, evidence from archaeology and the inferences about Homo erectus body size suggest increased meat eating. How much hunting did Homo erectus engage in compared to the earlier Oldowan toolmakers? Although experts continue to debate the relative importance of hunting versus scavenging, there seems to be stronger evidence of hunting for these hominins. For example, at sites such as Olorgesailie in Kenya (Figure 10.14), there are numerous associations of Acheulean tools with butchered remains of large animals.
However, Homo erectus certainly ate more than just meat. Studies of the tooth surfaces and microscopic wear patterns on hominin teeth indicate that these hominins ate a variety of foods, including some hard, brittle plant foods (Unger and Scott 2009). This would make sense, considering the environment was changing to be more dominated by grasslands in some areas. Roots, bulbs, and tubers (known as underground storage organs) of open savanna plants may have been a primary food source. Indeed, hunter-gatherer groups such as the Hadza of Tanzania rely heavily on such foods, especially during periods when game is scarce. In the unstable environment of the early Pleistocene, dietary versatility would be a definite advantage.
Tool Use, Cooking, and Fire
One key characteristic of the genus Homo is smaller teeth compared to Australopithecus. Why would teeth get smaller? In addition to new types of foods, changes in how food was prepared and consumed likely led to a decrease in tooth size. Think about how you would eat if you didn’t have access to cutting tools. What you couldn’t rip apart with your hands would have to be bitten off with your teeth—actions that would require bigger, more powerful teeth and jaws. As stone tools became increasingly important, hominins began to cut up, tenderize, and process meat and plants, such that they did not have to use their teeth so vigorously.
Cooking food could also have contributed to the reduction in tooth and jaw size. In fact, anthropologist Richard Wrangham (2009) asserts that cooking played a crucial role in human evolution. Cooking provides a head start in the digestive process because of how heat begins to break down food before food even enters the body, and it can help the body extract more nutrients out of meat and plant foods such as starchy tubers.
Obviously cooking requires fire, and the earliest use of fire is a fascinating topic in the study of human evolution. Fire is not only produced by humans; it occurs naturally as a result of lightning strikes. Like other wild animals, early hominins must have been terrified of wildfires, but at some point in time they learned to control fire and put it to good use. Documenting the earliest evidence of fire has been a contentious issue in archaeology because of the difficulty in distinguishing between human-controlled fire and natural burning at hominin sites. Burned areas and ash deposits must have direct associations with human activity to make a case for deliberate fire use. Unfortunately, such evidence is rare at ancient hominin sites, which have been profoundly altered by humans, animals, and geological forces over millions of years. Recently, newer methods—including microscopic analysis of burned rock and bone—have revealed clear evidence of fire use at Koobi Fora, Kenya, dating to 1.5 million years ago (Hlubik et al. 2017).
Migration out of Africa
Homo erectus is generally thought to be the first hominin species to have left Africa. It is hypothesized that they settled in places in Eurasia, such as the Republic of Georgia, Indonesia, and northern China, where fossil evidence of Homo erectus exists. But why would this species have traveled such vast distances to these far-flung regions? To answer this question, we have to consider what we have learned about the biology, culture, and environmental circumstances of Homo erectus. The larger brain and body size of Homo erectus were fueled by a diet consisting of more meat, and their longer, more powerful legs made it possible to walk and run longer distances to acquire food. Since they were eating higher on the food chain, it was necessary for them to extend their home range to find sufficient game. Cultural developments—including better stone tools and new technology such as fire—gave them greater flexibility in adapting to different environments. Finally, the major Pleistocene climate shift discussed earlier in the chapter certainly played a role. Changes in air temperature, precipitation, access to water sources, and other habitat alteration had far-reaching effects on animal and plant communities; this included Homo erectus. If hominins were relying more on hunting, the migration patterns of their prey could have led them to traverse increasingly long distances.
Life History
The life history of a species refers to its overall pattern of growth, development, and reproduction during its lifetime, with the assumption that these characteristics have been shaped by natural selection. The field of human behavioral ecology, explored in more detail in Appendix C, examines the roots of human behavior and life history. Our species, Homo sapiens, is characterized by a unique life history pattern of slow development, an extended period of juvenile dependence, and a long lifespan. Whereas the offspring of great apes achieve self-sufficiency early, human children are dependent on their parents long after weaning. Additionally, human fathers and grandparents (particularly postmenopausal grandmothers) devote substantial time and energy to caring for their children.

Human behavioral ecologists who study modern hunter-gatherer societies have observed that foraging is no easy business (Figure 10.15). Members of these groups engage in complex foraging techniques that take many years to master. An extended juvenile period gives children the time to acquire these skills. It also allows time for large human brains to grow and mature. On the back end, a longer developmental period results in skilled, successful adults, capable of living a long time (Hill and Kaplan 1999). Despite the time and energy demands, females could have offspring at more closely spaced intervals if they could depend on help from fathers and grandmothers (Hawkes et al. 1998).
What can the study of Homo erectus reveal about its life history pattern? Well-preserved fossils such as the Nariokotome boy can provide some insights. We know that apes such as chimpanzees reach maturity more quickly than humans, and there is some evidence that the australopithecines had a growth rate more akin to that of chimpanzees. Scientists have conducted extensive studies of the Nariokotome skeleton’s bones and teeth to assess growth and development. On the one hand, examination of the long bone ends (epiphyses) of the skeleton suggested that he was an early adolescent with a relatively large body mass, though growth had not yet been completed. On the other hand, study of the dentition, including measurement of microscopic layers of tooth enamel called perikymata, revealed a much younger age of 8 or 9. According to Christopher Dean and Holly Smith (2009), the best explanation for this discrepancy between the dental and skeletal age is that Homo erectus had its own distinct growth pattern—reaching maturity more slowly than chimpanzees but faster than Homo sapiens. This suggests that the human life history pattern of slow maturation and lengthy dependency was a more recent development. More work remains on refining this pattern for early Homo, but it is an important topic that sheds light on how and when we developed our unique life history characteristics.
The Big Picture of Early Homo
We are discovering that the evolution of the genus Homo is more complex than what was previously thought. The earlier view of a simple progression from Australopithecus to Homo habilis to Homo erectus as clearly delineated stages in human evolution just doesn’t hold up anymore.
As is apparent from the information presented here, there is tremendous variability during this time. While fossils classified as Homo habilis show many of the characteristics of the genus Homo, such as brain expansion and smaller tooth size, the small body size and long arms are more akin to australopithecines. There is also tremendous variability within the fossils assigned to Homo habilis, so there is little consensus on whether it is one or multiple species of Homo, a member of the genus Australopithecus, or even a yet-to-be-defined new genus. Similarly, there are considerable differences in skull morphology and body size and form of Homo erectus, of which some specimens show more similarity to Homo habilis than previously thought.
What does this diversity mean for how we should view early Homo? First, there isn’t an abrupt break between Australopithecus and Homo habilis or even between Homo habilis and Homo erectus. Characteristics we define as Homo don’t appear as a unified package; they appear in the fossil record at different times. This is known as mosaic evolution. Indeed, fossil species such as Australopithecus sediba, as well as Homo naledi and Homo floresiensis (who will be introduced in Chapter 11), have displayed unexpected combinations of primitive and derived traits.
We can consider several explanations for the diversity we see within early Homo from about 2.5 million to 1.5 million years ago. One possibility is the existence of multiple contemporaneous species of early Homo during this period. In light of the pattern of environmental instability discussed earlier, it shouldn’t be surprising to see fossils from different parts of Africa and Eurasia display tremendous variability. Multiple hominin forms could also evolve in the same region, as they diversified in order to occupy different ecological niches. However, even the presence of multiple species of hominin does not preclude their interacting and interbreeding with one another. As you’ll see in Appendix D, sequencing of ancient hominin genomes has led to deeper understanding of genetic relationships between extinct species such as the Neanderthals and Denisovans.
Diversity of brain and body sizes could also reflect developmental plasticity—short-term adaptations within a lifetime (Anton et al. 2014). These have the advantage of being more flexible than genetic natural selection, which could only occur over many generations. For example, among human populations today, different body sizes are thought to be adaptations to different climate or nutritional environments. Under Pleistocene conditions of intense variability, a more flexible strategy of adaptation would be valuable.
New discoveries are also questioning old assumptions about the behavior of Homo habilis and Homo erectus. Just as the fossil evidence doesn’t neatly separate Australopithecus and Homo, evidence of the lifeways of early Homo show similar diversity. For example, one of the traditional dividing lines between Homo and Australopithecus was thought to be stone tools: Homo made them; Australopithecus didn’t. However, the recent discovery of stone tools from Kenya dating to 3.3 million years ago challenges this point of view. Similarly, the belief that Homo erectus was the first species to settle outside Africa may now come into question with the report of 2.1-million-year-old stone tools from China. If this find is supported by additional evidence, it may cause a reevaluation of Homo erectus being the first to leave Africa. Instead, there could have been multiple earlier migrations of hominins such as Homo habilis or even Australopithecus species.
These various lines of evidence about the genus Homo point out the need for a more nuanced view of this period of human evolution. Rather than obvious demarcations between species and their corresponding behavioral advancements, it now looks like many behaviors were shared among species. Earlier hominins that we previously didn’t think had the capability could have been doing things like expanding out of Africa or using stone tools. Meanwhile, some other hominins that we had considered more advanced didn’t actually have the full suite of “human” characteristics previously expected.
From a student’s perspective, all this complexity probably seems frustrating. It would be ideal if the human story were a straightforward, sequential narrative. Unfortunately, it seems that human evolution was not a nice, neat trajectory of increasingly humanlike traits and behaviors; rather, it is emblematic of the untidy but exciting nature of the study of human evolution.
Despite some haziness dominating the early Homo narrative, we can identify some overall trends for the million-year period associated with early Homo. These trends include brain expansion, a reduction in facial prognathism, smaller jaw and tooth size, larger body size, and evidence of full terrestrial bipedalism. These traits are associated with a key behavioral shift that emphasizes culture as a flexible strategy to adapt to unpredictable environmental circumstances. Included in this repertoire are the creation and use of stone tools to process meat obtained by scavenging and later hunting, a utilization of fire and cooking, and the roots of the human life history pattern of prolonged childhood, cooperation in child raising, and the practice of skilled foraging techniques. In fact, it’s apparent that the cultural innovations are driving the biological changes, and vice versa, fueling a feedback loop that continues during the later stages of human evolution.
Hominin Species Summaries
|
Hominin |
Homo habilis |
|
Dates |
2.5 million years ago to 1.7 million years ago |
|
Region(s) |
East and South Africa |
|
Famous discoveries |
Olduvai Gorge, Tanzania; Koobi Fora, Kenya; Sterkfontein, South Africa |
|
Brain size |
650 cc average (range from 510 cc to 775 cc) |
|
Dentition |
Smaller teeth with thinner enamel compared to Australopithecus; parabolic dental arcade shape |
|
Cranial features |
Rounder cranium and less facial prognathism than Australopithecus |
|
Postcranial features |
Small stature; similar body plan to Australopithecus |
|
Culture |
Oldowan tools |
|
Other |
N/A |
|
Hominin |
Homo erectus |
|
Dates |
1.8 million years ago to about 110,000 years ago |
|
Region(s) |
East and South Africa; West Eurasia; China and Southeast Asia |
|
Famous discoveries |
Lake Turkana, Olorgesailie, Kenya; Java, Indonesia; Zhoukoudian, China; Dmanisi, Republic of Georgia |
|
Brain size |
Average 900 cc; range between 650 cc and 1,100 cc |
|
Dentition |
Smaller teeth than Homo habilis |
|
Cranial features |
Long, low skull with robust features including thick cranial vault bones and large brow ridge, sagittal keel, and occipital torus |
|
Postcranial features |
Larger body size compared to Homo habilis; body proportions (longer legs and shorter arms) similar to Homo sapiens |
|
Culture |
Acheulean tools (in Africa); evidence of increased hunting and meat-eating; use of fire; migration out of Africa |
|
Other |
N/A |
Review Questions
- Describe the climate during the early Pleistocene. Explain why climate is important for understanding the evolution of early Homo.
- List the key anatomical characteristics that are generally agreed to define the genus Homo.
- Why has classification of early Homo fossils proved difficult? What are some explanations for the variability seen in these fossils?
- Compare and contrast the Oldowan and Acheulean tool industries.
- Name some specific behaviors associated with Homo erectus in the areas of tool use, subsistence practices, migration patterns, and other cultural innovations.
Key Terms
Acheulean: Tool industry characterized by teardrop-shaped stone handaxes flaked on both sides.
Developmental plasticity: The capability of an organism to modify its phenotype during development in response to environmental cues.
Human behavioral ecology: The study of human behavior from an evolutionary and ecological perspective.
Life history: The broad pattern of a species’ life cycle, including development, reproduction, and longevity.
Mosaic evolution: Different characteristics evolve at different rates and appear at different stages.
Occipital torus: A ridge on the occipital bone in the back of the skull.
Oldowan: Earliest stone-tool industry consisting of simple flakes and choppers.
Perikymata: Microscopic ridges on the surface of tooth enamel that serve as markers of tooth development.
Pleistocene: Geological epoch dating from 2.6 million years ago to about 11,000 years ago.
Pliocene: Geological epoch dating from 5.3 to 2.6 million years ago.
Prognathism: Condition where the lower face and jaw protrude forward from a vertical plane.
Sagittal keel: A thickened area along the top of the skull.
About the Author

Bonnie Yoshida-Levine, Ph.D.
Grossmont College, bonnie.yoshida@gcccd.edu
Bonnie Yoshida-Levine is an instructor of anthropology at Grossmont College, where she teaches biological anthropology and archaeology. She received her bachelor’s degree in history from the University of California, Los Angeles, and her M.A. and Ph.D. degrees in anthropology from the University of California, Santa Barbara. Her dissertation research focused on the bioarchaeology of early civilizations in north coastal Peru. Bonnie has also collaborated on archaeological field projects in Bolivia and coastal California.
FOR FURTHER EXPLORATION
Boaz, Noel Thomas, and Russell L. Ciochon. 2004. Dragon Bone Hill: An Ice-Age Saga of Homo erectus. New York: Oxford University Press.
Human Evolution by the Smithsonian Institution. Produced by the Smithsonian National Museum of Natural History, this website covers many aspects of human evolution including 3-D models of hominin fossils.
Lewin, Roger, and Robert A. Foley. 2004. Principles of Human Evolution. Oxford, UK: Blackwell Publishing.
Mutu, Kari. “Honour Finds Kenya’s Oldest Fossil Hunter Kamoya Kimeu.” The East African, July 19, 2021.
Nordling, Linda. “Raising Up African Paleoanthropologists.” SAPIENS, September 28, 2021. Accessed February 24, 2023. https://www.sapiens.org/biology/african-paleoanthropologists/.
Risen, Clay. “Kamoya Kimeu, Fossil-Hunting ‘Legend’ in East Africa Is Dead.” New York Times, August 11, 2022. Accessed February 24, 2023. https://www.nytimes.com/2022/08/11/science/kamoya-kimeu-dead.html/.
Stoneking, Mark. 2015. “Of Lice and Men: The Molecular Evolution of Human Lice.” Lecture, Center for Academic Research & Training in Anthropogeny, San Diego, California, October 16, 2015. Accessed February 24, 2023. https://carta.anthropogeny.org/events/unique-features-human-skin.
Tarlach, Gemma. 2015. “The First Humans to Know Winter.” Discover, February 26. https://www.discovermagazine.com/planet-earth/the-first-humans-to-know-winter
Ungar, Peter S. 2017. Evolution’s Bite: A Story of Teeth, Diet, and Human Origins. Princeton, NJ: Princeton University Press.
References
Aiello, Leslie C., and Peter Wheeler. 1995. “The Expensive-Tissue Hypothesis.” Current Anthropology 36 (2): 199–221.
Anton, Susan C., Richard Potts, and Leslie C. Aiello. 2014. “Evolution of Early Homo: An Integrated Biological Perspective.” Science 345 (6192) doi: 10.1126/science.1236828.
Anton, Susan C., and J. Josh Snodgrass. 2012. “Origins and Evolution of Genus Homo: New Perspectives.” Current Anthropology 53 (S6): S479–S496.
Ashton, Nick, Simon G. Lewis, Isabelle De Groote, Sarah M. Duffy, Martin Bates, Richard Bates, Peter Hoare, et al. 2014. “Hominin Footprints from Early Pleistocene Deposits at Happisburgh, UK.” PLOS ONE 9 (2): e88329.
Belmaker, Miriam. 2010. “Early Pleistocene Faunal Connections between Africa and Eurasia: An Ecological Perspective.” In Out of Africa I: The First Hominin Colonization of Eurasia, edited by John G. Fleagle, John J. Shea, Frederick E. Grine, Andrea L. Baden, and Richard E. Leakey, 183–205. Dordrecht: Springer Netherlands.
Blumenschine, Robert, Henry T. Bunn, Valerius Geist, Fumiko Ikawa-Smith, Curtis W. Marean, Anthony G. Payne, John Tooby, J. Nikolaas, and Van Der Merwe. 1987. “Characteristics of an Early Hominid Scavenging Niche [and Comments and Reply].” Current Anthropology 28 (4): 383–407.
Bower, Bruce. 2004. “Evolution’s Buggy Ride.” Science News 166 (15): 230–230.
Bramble, Dennis M., and Daniel E. Lieberman. 2004. “Endurance Running and the Evolution of Homo.” Nature 432 (7015): 345–352.
Coolidge, Frederick L., and Thomas Grant Wynn. 2017. The Rise of Homo Sapiens: The Evolution of Modern Thinking. New York: Oxford University Press.
Dean, M. Christopher, and B. Holly Smith. 2009. “Growth and Development of the Nariokotome Youth, KNM-WT 15000.” In The First Humans–Origin and Early Evolution of the Genus Homo: Contributions from the Third Stony Brook Human Evolution Symposium and Workshop October 3–7, 2006, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 101–120. Dordrecht: Springer Netherlands.
deMenocal, Peter B. 2014. “Climate Shocks.” Scientific American 311 (3): 48–53.
Dennell, Robin, and Wil Roebroeks. 2005. “An Asian Perspective on Early Human Dispersal from Africa.” Nature 438 (7071): 1099–1104.
Hawkes, Kristen, James F. O’Connell, Nicholas G. Blurton Jones, Helen Alvarez, and Eric L. Charnov. 1998. “Grandmothering, Menopause, and the Evolution of Human Life Histories.” Proceedings of the National Academy of Sciences 95 (3): 1336–1339.
Herries, A. I. R., J. M. Martin, A. B. Leece, J. W. Adams, G. Boschian, R. Joannes-Boyau, T. R. Edwards, et al. 2020. “Contemporaneity of Australopithecus, Paranthropus, and early Homo erectus in South Africa.” Science 368 (6486). https://doi.org/10.1126/science.aaw7293
Hill, Kim, and Hillard Kaplan. 1999. “Life History Traits in Humans: Theory and Empirical Studies.” Annual Review of Anthropology 28: 397–430.
Hlubik, Sarah, Francesco Berna, Craig Feibel, David Braun, and John W. K. Harris. 2017. “Researching the Nature of Fire at 1.5 Mya on the Site of FxJj20 AB, Koobi Fora, Kenya, Using High-Resolution Spatial Analysis and FTIR Spectrometry.” Current Anthropology 58 (S16): S243–S257.
Jablonski, Nina G. 2010. “The Naked Truth.” Scientific American 302 (2): 42–49.
Kamberov, Yana G., Elinor K. Karlsson, Gerda L. Kamberova, Daniel E. Lieberman, Pardis C. Sabeti, Bruce A. Morgan, and Clifford J. Tabin. 2015. “A Genetic Basis of Variation in Eccrine Sweat Gland and Hair Follicle Density.” Proceedings of the National Academy of Sciences 112 (32): 9932–9937.
Kittler, R., M. Kayser, and M. Stoneking. 2003. “Molecular Evolution of Pediculus Humanus and the Origin of Clothing.” Current Biology 13 (16): 1414–1417.
Leakey, Louis S. B., Phillip V. Tobias, and John R. Napier. 1964. “A New Species of Genus Homo from Olduvai Gorge.” Nature 202: 308–312.
Lemorini, Cristina, Thomas W. Plummer, David R. Braun, Alyssa N. Crittenden, Peter W. Ditchfield, Laura C. Bishop, Fritz Hertel, et al. 2014. “Old Stones’ Song: Use-Wear Experiments and Analysis of the Oldowan Quartz and Quartzite Assemblage from Kanjera South (Kenya).” Journal of Human Evolution 72: 10–25.
Lisiecki, Lorraine E., and Maureen E. Raymo. 2005. “A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records.” Paleoceanography 20 (1)
Lordkipanidze, David, Marcia S. Ponce de León, Ann Margvelashvili, Yoel Rak, G. Philip Rightmire, Abesalom Vekua, and Christoph P. E. Zollikofer. 2013. “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo.” Science 342 (6156): 326–333.
Matsu’ura, S., M. Kondo, T. Danhara, S. Sakata, H. Iwano, T. Hirata, I. Kurniawan, et al. 2020. “Age Control of the First Appearance Datum for Javanese Homo erectus in the Sangiran Area.” Science 367 (6474): 210–214.
Qiu, Jane. 2016. “How China Is Rewriting the Book on Human Origins.” Nature 535: 22–25.
Reed, David L., Jessica E. Light, Julie M. Allen, and Jeremy J. Kirchman. 2007. “Pair of Lice Lost or Parasites Regained: The Evolutionary History of Anthropoid Primate Lice.” BMC Biology 5 (1): 7. doi: 10.1186/1741-7007-5-7.
Rizal, Y., K. E. Westaway, Y. Zaim, G. D. van den Bergh, E. A. Bettis, 3rd, M. J. Morwood, O. F. Huffman, R. Grün, et al. 2020. “Last Appearance of Homo erectus at Ngandong, Java, 117,000–108,000 Years Ago.” Nature 577 (7790): 381–385.
Roche, Helene, Robert J. Blumenschine, and John J. Shea. 2009. “Origins and Adaptations of Early Homo: What Archeology Tells Us.” In The First Humans: Origin and Early Evolution of the Genus Homo, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 135–147. New York: Springer.
Rogers, Alan R., David Iltis, and Stephen Wooding. 2004. “Genetic Variation at the MC1R l Locus and the Time since Loss of Human Body Hair.” Current Anthropology 45 (1): 105–108.
Ruff, Christopher. 2009. “Relative Limb Strength and Locomotion in Homohabilis.” American Journal of Physical Anthropology 138 (1): 90–100.
Shipton, Ceri, James Blinkhorn, Paul S. Breeze, Patrick Cuthbertson, Nick Drake, Huw S. Groucutt, Richard P. Jennings, et al. 2018. “Acheulean Technology and Landscape Use at Dawadmi, Central Arabia.” PloS one 13 (7): e0200497–e0200497.
Simpson, Scott W., Jay Quade, Naomi E. Levin, Robert Butler, Guillaume Dupont-Nivet, Melanie Everett, and Sileshi Semaw. 2008. “A Female Homoerectus Pelvis from Gona, Ethiopia.” Science 322 (5904): 1089–1092.
Ungar, Peter S., and Robert S. Scott. 2009. “Dental Evidence for Diets of Early Homo.” In The First Humans: Origin and Early Evolution of the Genus Homo, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 121–134. New York: Springer.
Villmoare, Brian, William H. Kimbel, Chalachew Seyoum, Christopher J. Campisano, Erin N. DiMaggio, John Rowan, David R. Braun, J. Ramón Arrowsmith, and Kaye E. Reed. 2015. “Early Homo at 2.8 Ma From Ledi-Geraru, Afar, Ethiopia.” Science 347 (6228): 1352–1355.
Wood, Bernard. 2014. “Human Evolution: Fifty Years after Homohabilis.” Nature 508 (7494): 31–33.
Wood, Bernard, and Mark Collard. 1999. “The Changing Face of Genus Homo.” Evolutionary Anthropology 8 (6): 195–207.
Wrangham, Richard. 2009. Catching Fire: How Cooking Made Us Human. New York: Basic Books.
Zhu, Zhaoyu, Robin Dennell, Weiwen Huang, Yi Wu, Shifan Qiu, Shixia Yang, and Zhiguo Rao. 2018. “Hominin Occupation of the Chinese Loess Plateau Since about 2.1 Million Years Ago.” Nature 559: 608–612.
Acknowledgments
The author gratefully acknowledges funding from the California Community Colleges Chancellor’s Office Zero Textbook Cost Degree Grant Program—Implementation Phase 2.
A geological epoch between the Miocene and Pleistocene.
Jonathan M. G. Perry, Ph.D., Western University of Health Sciences
Stephanie L. Canington, Ph.D., University of Pennsylvania
This chapter is a revision from "Chapter 8: Primate Evolution” by Jonathan M. G. Perry and Stephanie L. Canington. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Understand the major trends in primate evolution from the origin of primates to the origin of our own species.
- Learn about primate adaptations and how they characterize major primate groups.
- Discuss the kinds of evidence that anthropologists use to find out how extinct primates are related to each other and to living primates.
- Recognize how the changing geography and climate of Earth have influenced where and when primates have thrived or gone extinct.
The first fifty million years of primate evolution was a series of adaptive radiations leading to the diversification of the earliest lemurs, monkeys, and apes. The primate story begins in the canopy and understory of conifer-dominated forests, with our small, furtive ancestors subsisting at night, beneath the notice of day-active dinosaurs.
From the ancient plesiadapiforms (archaic primates) to the earliest groups of true primates (euprimates) (Bloch and Boyer 2002), the origin of our own order is characterized by the struggle for new food sources and microhabitats in the arboreal setting. Climate change forced major extinctions as the northern continents became increasingly dry, cold, and seasonal and as tropical rainforests gave way to deciduous forests, woodlands, and eventually grasslands. Lemurs, lorises, and tarsiers—once diverse groups containing many species—became rare, except for lemurs in Madagascar, where there were no anthropoid competitors and perhaps few predators. Meanwhile, anthropoids (monkeys and apes) likely emerged in Asia and then dispersed across parts of the northern hemisphere, Africa, and ultimately South America. The movement of continents, shifting sea levels, and changing patterns of rainfall and vegetation contributed to the developing landscape of primate biogeography, morphology, and behavior. Today’s primates provide modest reminders of the past diversity and remarkable adaptations of their extinct relatives. This chapter explores the major trends in primate evolution from the origin of the Order Primates to the beginnings of our own lineage, providing a window into these stories from our ancient past.
Major Hypotheses About Primate Origins
For many groups of mammals, there is a key feature that led to their success. A good example is powered flight in bats. Primates lack a feature like this (see Chapter 5). Instead, if there is something unique about primates, it is probably a group of features rather than one single thing. Because of this, anthropologists and paleontologists struggle to describe an ecological scenario that could explain the rise and success of our own order. Three major hypotheses have been advanced to consider the origin of primates and to explain what makes our order distinct among mammals (Figure 8.1); these are described below.

Arboreal Hypothesis
In the 1800s, many anthropologists viewed all animals in relation to humans. That is, animals that were more like humans were considered to be more “advanced” and those lacking humanlike features were considered more “primitive.” This way of thinking was particularly obvious in studies of primates. A more modern way of referring to members of a group that lack certain evolutionary innovations seen in other members is to call them plesiomorphic (literally “anciently shaped”). The state of their morphological features is sometimes referred to as ancestral traits.
Thus, when anthropologists sought features that separate primates from other mammals, they focused on features that were least developed in lemurs and lorises, more developed in monkeys, and most developed in apes (Figure 8.2). Frederic Wood Jones, one of the leading anatomist-anthropologists of the early 1900s, is usually credited with the Arboreal Hypothesis of primate origins (Jones 1916). This hypothesis holds that many of the features of primates evolved to improve locomotion in the trees; this way of getting around is referred to as arboreal. For example, the grasping hands and feet of primates are well suited to gripping tree branches of various sizes and our flexible joints are good for reorienting the extremities in many different ways. A mentor of Jones, Grafton Elliot Smith, had suggested that the reduced olfactory system, acute vision, and forward-facing eyes of primates are adaptations for making accurate leaps and bounds through a complex, three-dimensional canopy (Smith 1912). The forward orientation of the eyes in primates causes the visual fields to overlap, enhancing depth perception, especially at close range. Evidence to support this hypothesis includes the facts that many extant primates are arboreal, and the plesiomorphic members of most primate groups are dedicated arborealists. The Arboreal Hypothesis was well accepted by most anthropologists at the time and for decades afterward.

Visual Predation Hypothesis
In the late 1960s and early 1970s, Matt Cartmill studied and tested the idea that the characteristic features of primates evolved in the context of arboreal locomotion. Cartmill noted that squirrels climb trees (and even vertical walls) very effectively, even though they lack some of the key adaptations of primates. As members of the Order Rodentia, squirrels also lack the hand and foot anatomy of primates. They have claws instead of flattened nails and their eyes face more laterally than those of primates. Cartmill reasoned that there must be some other explanation for the unique traits of primates. He noted that some nonarboreal animals share at least some of these traits with primates; for example, cats and predatory birds have forward-facing eyes that enable visual field overlap. Cartmill suggested that the unique suite of features in primates is an adaptation to detecting insect prey and guiding the hands (or feet) to catch insects (Cartmill 1972). His hypothesis emphasizes the primary role of vision in prey detection and capture; it is explicitly comparative, relying on form-function relationships in other mammals and nonmammalian vertebrates. According to Cartmill, many of the key features of primates evolved for preying on insects in this special manner (Cartmill 1974).
Angiosperm-Primate Coevolution Hypothesis
The visual predation hypothesis was unpopular with some anthropologists. One reason for this is that many primates today are not especially predatory. Another is that, whereas primates do seem well adapted to moving around in the smallest, terminal branches of trees, insects are not necessarily easier to find there. A counterargument to the visual predation hypothesis is the angiosperm-primate coevolution hypothesis. Primate ecologist Robert Sussman (Sussman 1991) argued that the few primates that eat mostly insects often catch their prey on the ground rather than in tree branches. Furthermore, predatory primates often use their ears more than their eyes to detect prey. Finally, most early primate fossils show signs of having been omnivorous rather than insectivorous. Instead, he argued, the earliest primates were probably seeking fruit. Fruit (and flowers) of angiosperms (flowering plants) often develop in the terminal branches. Therefore, any mammal trying to access those fruits must possess anatomical traits that allow them to maintain their hold on thin branches and avoid falling while reaching for the fruits. Primates likely evolved their distinctive visual traits and extremities in the Paleocene (approximately 65 million to 54 million years ago) and Eocene (approximately 54 million to 34 million years ago) epochs, just when angiosperms were going through a revolution of their own—the evolution of large, fleshy fruit that would have been attractive to a small arboreal mammal. Sussman argued that, just as primates were evolving anatomical traits that made them more efficient fruit foragers, angiosperms were also evolving fruit that would be more attractive to primates to promote better seed dispersal. This mutually beneficial relationship between the angiosperms and the primates was termed coevolution or more specifically diffuse coevolution.
At about the same time, D. Tab Rasmussen noted several parallel traits in primates and the South American woolly opossum, Caluromys. He argued that early primates were probably foraging on both fruits and insects (Rasmussen 1990). As is true of Caluromys today, early primates probably foraged for fruits in the terminal branches of angiosperms, and they probably used their visual sense to aid in catching insects. Insects are also attracted to fruit (and flowers), so these insects represent a convenient opportunity for a primarily fruit-eating primate to gather protein. This solution is a compromise between the visual predation hypothesis and the angiosperm-primate coevolution hypothesis. It is worth noting that other models of primate origins have been proposed, and these include the possibility that no single ecological scenario can account for the origin of primates.
THE ORIGIN OF PRIMATES
Paleocene: Mammals in the Wake of Dinosaur Extinctions
Placental mammals, including primates, originated in the Mesozoic Era (approximately 251 million to 65.5 million years ago), the Age of Dinosaurs. During this time, most placental mammals were small, probably nocturnal, and probably avoided predators via camouflage and slow, quiet movement. It has been suggested that the success and diversity of the dinosaurs constituted a kind of ecological barrier to Mesozoic mammals. The extinction of the dinosaurs (and many other organisms) at the end of the Cretaceous Period (approximately 145.5–65.5 million years ago) might have opened up these ecological niches, leading to the increased diversity and disparity in mammals of the Tertiary Period (approximately 65.5–2.5 million years ago).
The Paleocene was the first epoch in the Age of Mammals. Soon after the Cretaceous-Tertiary (K-T) extinction event, new groups of placental mammals appear in the fossil record. Many of these groups achieved a broad range of sizes and lifestyles as well as a great number of species before declining sometime in the Eocene (or soon thereafter). These groups were ultimately replaced by the modern orders of placental mammals (Figure 8.3). It is unknown whether these replacements occurred gradually, for example by competitive exclusion, or rapidly, perhaps by sudden geographic dispersals with replacement. In some senses, the Paleocene might have been a time of recovery from the extinction event; it was cooler and more seasonal globally than the subsequent Eocene.

Plesiadapiforms, the Archaic Primates
The Paleocene epoch saw the emergence of several families of mammals that have been implicated in the origin of primates. These are the plesiadapiforms, which are archaic primates, meaning they possessed some primate features and lacked others. The word plesiadapiform means “almost adapiform,” a reference to some similarities between some plesiadapiforms and some adapiforms (or adapoids; later-appearing true primates)—mainly in the molar teeth. Because enamel fossilizes better than other parts of the body, the molar teeth are the parts most often found and first discovered for any new species. Thus, dental similarities were often the first to be noticed by early mammalian paleontologists, partly explaining why plesiadapiforms were thought to be primates. Major morphological differences between plesidapiforms and euprimates (true primates) were observed later when more parts of plesiadapiform skeletons were discovered. Many plesiadapiforms have unusual anterior teeth and most have digits possessing claws rather than nails. So far, no plesiadapiform ever discovered has a postorbital bar (seen in extant strepsirrhines) or septum (as seen in haplorhines), and whether or not the auditory bulla was formed by the petrosal bone remains unclear for many plesiadapiform specimens. Nevertheless, there are compelling reasons (partly from new skeletal material) for including plesiadapiforms within the Order Primates.
Geographic and Temporal Distribution
Purgatorius is generally considered to be the earliest primate. This Paleocene mammal is known from teeth that are very plesiomorphic for a primate. It has some characteristics that suggest it is a basal plesiadapiform, but there is very little to link it specifically with euprimates (see Clemens 2004). Its ankle bones suggest a high degree of mobility, signaling an arboreal lifestyle (Chester et al. 2015). Purgatorius is plesiomorphic enough to have given rise to all primates, including the plesiadapiforms. However, new finds suggest that this genus was more diverse and had more differing tooth morphologies than previously appreciated (Wilson Mantilla et al. 2021). Plesiadapiform families were numerous and diverse during parts of the Paleocene in western North America and western Europe, with some genera (e.g., Plesiadapis; see Figure 8.4) living on both continents (Figure 8.5). Thus, there were probably corridors for plesiadapiform dispersal between the two continents, and it stands to reason that these mammals were living all across North America, including in the eastern half of the continent and at high latitudes. A few plesiadapiforms have been described from Asia (e.g., Carpocristes), but the affinities of these remain uncertain.
|
Family
|
Genera
|
Morphology
|
Location
|
Age1
|
|
Paromomyidae |
Ignacius |
Long, dagger-like, lower incisor. |
North America and Europe |
Early Paleocene to Late Eocene |
|
Carpolestidae |
Carpolestes |
Plagiaulacoid dentition. Limb adaptations to terminal branch feeding. Grasping big toe. |
North America, Europe, and Asia |
Middle Paleocene to Early Eocene |
|
Plesiadapidae |
Plesiadapis |
Mitten-like upper incisor. Diastema. Large body size for group. |
North America and Europe |
Middle Paleocene to Early Eocene |
|
1 Derived from Fleagle 2013. |
|
|||

General Morphological Features
Although there is much morphological variation among the families of plesiadapiforms, some common features unite the group. Most plesiadapiforms were small, the largest being about three kilograms (approximately 7 lbs.; Plesiadapis cookei). They had small brains and fairly large snouts, with eyes that faced more laterally than in euprimates. Many species show reduction and/or loss of the canine and anterior premolars, with the resulting formation of a rodent-like diastema (a pronounced gap between the premolars and the incisors, with loss of at least the canine); this probably implies a herbivorous diet. Some families appear to have had very specialized diets, as suggested by unusual tooth and jaw shapes.
Arguably the most interesting and unusual family of plesiadapiforms is the Carpolestidae. They are almost exclusively from North America (with a couple of possible members from Asia), and mainly from the Middle and Late Paleocene. Their molars are not very remarkable, being quite similar to those of some other plesiadapiforms (e.g., Plesiadapidae). However, their lower posterior premolars (p4) are laterally compressed and blade-like with vertical serrations topped by tiny cuspules. This unusual dental morphology is termed plagiaulacoid (Simpson 1933). The upper premolar occlusal surfaces are broad and are covered with many small cuspules; the blade-like lower premolar might have cut across these cuspules, between them, or both.

Many plesiadapiforms have robust limb bones with hallmarks of arboreality. Instead of having nails, most taxa had sharp claws on most or all of the digits. The extremities show grasping abilities comparable to those of primates and some arboreal marsupials. Nearly complete skeletons have yielded a tremendous wealth of information on locomotor and foraging habits. Many plesiadapiforms appear to have been able to cling to vertical substrates (like a broad tree trunk) using their sharp claws, propelling themselves upward using powerful hindlimbs, bounding along horizontal supports, grasping smaller branches, and moving head-first down tree trunks. In carpolestids in particular, the skeleton appears to have been especially well adapted to moving slowly and carefully in small terminal branches (Figure 8.6).
Debate: Relationship of Plesiadapiforms to True Primates
In the middle of the twentieth century, treeshrews (Order Scandentia) were often considered part of the Order Primates, based on anatomical similarities between some treeshrews and primates. For many people, plesiadapiforms represented intermediates between primates and treeshrews, so plesiadapiforms were included in Primates as well.
Studies of reproduction and brain anatomy in treeshrews and lemurs suggested that treeshrews are not primates (e.g., Martin 1968). This was soon followed by the suggestion to also expel plesiadapiforms (Martin 1972) from the Order Primates. Like treeshrews, plesiadapiforms lack a postorbital bar, nails, and details of the ear region that characterize true primates. Many paleoanthropologists were reluctant to accept this move to banish plesiadapiforms (e.g., F. S. Szalay, P. D. Gingerich).
Later, K. Christopher Beard (1990) found that in some ways, the digits of paromomyid plesiadapiforms are actually more similar to those of dermopterans (Order Dermoptera), the closest living relatives of primates, than they are to those of primates themselves (but see Krause 1991). At the same time, Richard Kay and colleagues (1990) found that cranial circulation patterns and auditory bulla morphology in the paromomyid, Ignacius (see Figure 8.4), are more like those of dermopterans than of primates.
For many anthropologists, this one-two punch effectively removed plesiadapiforms from the Order Primates. In the last two decades, the tide of opinion has turned again, with many researchers reinstating plesiadapiforms as members of the Order Primates. New and more complete specimens demonstrate that the postcranial skeletons of plesiadapiforms, including the hands and feet, were primate-like, not dermorpteran-like (Bloch and Boyer 2002, 2007). New fine-grained CT scans of relatively complete plesiadapiform skulls revealed that they share some key traits with primates to the exclusion of other placental mammals (Bloch and Silcox 2006). Most significant was the suggestion that Carpolestes simpsoni possessed an auditory bulla formed by the petrosal bone, like in all living primates.
The debate about the status of plesiadapiforms continues, owing to a persistent lack of key bones in some species and owing to genuine complexity of the anatomical traits involved. Maybe plesiadapiforms were the ancestral stock from which all primates arose, with some plesiadapiforms (e.g., carpolestids) nearer to the primate stem than others.
Adapoids and Omomyoids, the First True Primates
Geographic and Temporal Distribution
The first universally accepted fossil primates are the adapoids (Superfamily Adapoidea) and the omomyoids (Superfamily Omomyoidea). These groups become quite distinct over evolutionary time, filling mutually exclusive niches for the most part. However, the earliest adapoids are very similar to the earliest omomyoids.
The adapoids were mainly diurnal and herbivorous, with some achieving larger sizes than any plesiadapiforms (10 kg; 22 lbs.). By contrast, the omomyoids were mainly nocturnal, insectivorous and frugivorous, and small.
Both groups appear suddenly at the start of the Eocene, where they are present in western North America, western Europe, and India (Figure 8.7). This wide dispersal of early primates was probably due to the presence of rainforest corridors extending far into northern latitudes.

In North America and Europe, both groups achieved considerable diversity in the Middle Eocene, then mostly died out at the end of that epoch (Figure 8.8). In some Eocene rock formations in the western United States, adapoids and omomyoids make up a major part of the mammalian fauna. The Eocene of India has yielded a modest diversity of euprimates, some of which are so plesiomorphic that it is difficult to know whether they are adapoids or omomyoids (or even early anthropoids).
|
Family
|
Genera
|
Morphology
|
Location
|
Age1
|
|
Cercamoniidae |
Donrussellia |
Variable in tooth number and jaw shape. |
Europe and Asia |
Early to Late Eocene |
|
Asiadapidae2 |
Asiadapis |
Plesiomorphic teeth and jaw resemble early Omomyids. |
Asia |
Early Eocene |
|
Caenopithecidae3 |
Darwinius |
Robust jaws with crested molars. Fewer premolars. |
Europe, Africa, North America, and Asia |
Middle to Late Eocene |
|
Adapidae |
Adapis |
Fused mandible. Long molar crests. Large size and large chewing muscles. |
Europe |
Late Eocene to Early Oligocene |
|
Sivaladapidae |
Sivaladapis |
Some large with robust jaws. |
Asia |
Middle Eocene to Late Miocene |
|
Notharctidae4 |
Notharctus |
Canine sexual dimorphism. Lemur-like skull. Clinging and leaping adaptations. |
North America and Europe |
Early to Middle Eocene |
|
Omomyidae5 |
Teilhardina |
Small, nocturnal, frugivorous or insectivorous. Tarsier-like skull in some. |
North America, Europe, and Asia |
Early Eocene to Early Miocene |
|
Microchoeridae6 |
Necrolemur |
Long bony ear tubes. Tarsier-like lower limb adaptations for leaping. |
Europe and Asia |
Early Eocene to Early Oligocene |
|
1 Derived from Fleagle 2013. 2 See Dunn et al. 2016 and Rose et al. 2018. 3 See Kirk and Williams 2011 and Seiffert et al. 2009. 4 See Gregory 1920. 5 See Beard and MacPhee 1994 and Strait 2001. 6 See Schmid 1979. |
|
|||
Adapoids and omomyoids barely survived the Eocene-Oligocene extinctions, when colder temperatures, increased seasonality, and the retreat of rainforests to lower latitudes led to changes in mammalian biogeography. In North America, one genus (originally considered an omomyoid but recently reclassified as Adapoidea) persisted until the Miocene: Ekgmowechashala (Rose and Rensberger 1983). This taxon has highly unusual teeth and might have been a late immigrant to North America from Asia. In Asia, one family of adapoids, the Sivaladapidae, retained considerable diversity as late as the Late Miocene.
Adapoid Diversity
Adapoids were very diverse, particularly in the Eocene of North America and Europe. They can be divided into six families, with a few species of uncertain familial relationship. As a group, adapoids have some features in common, although much of what they share is plesiomorphic. Important features include the hallmarks of euprimates: postorbital bar, flattened nails, grasping extremities, and a petrosal bulla (Figures 8.9 and 8.10). In addition, some adapoids retain the ancestral dental formula of 2.1.4.3; that is, in each quadrant of the mouth, there are two incisors, one canine, four premolars, and three molars. In general, the incisors are small compared to the molars, but the canines are relatively large, with sexual dimorphism in some species. Cutting crests on the molars are well developed in some species, and the two halves of the mandible were fused at the midline in some species. Some adapoids were quite small (Anchomomys at a little over 100 g), and some were quite large (Magnadapis at 10 kg; 22 lbs.). Furthermore, the spaces and attachment features for the chewing muscles were truly enormous in some species, suggesting that these muscles were very large and powerful. Taken together, this suggests an overall adaptive profile of diurnal herbivory. The canine sexual dimorphism in some species suggests a possible mating pattern of polygyny, as males in polygynous primate species often compete with each other for mates and have especially large canine teeth.


Omomyoid Diversity
Like adapoids, omomyoids appeared suddenly at the start of the Eocene and then became very diverse with most species dying out before the Oligocene. Omomyoids are known from thousands of jaws with teeth, relatively complete skulls for about a half-dozen species, and very little postcranial material. Omomyoids were relatively small primates, with the largest being less than three kilograms (approximately 7 lbs.; Macrotarsius montanus). All known crania possess a postorbital bar, which in some has been described as “incipient closure.” Some—but not all—known crania have an elongated bony ear tube extending lateral to the location of the eardrum, a feature seen in living tarsiers and catarrhines. The anterior teeth tend to be large, with canines that are usually not much larger than the incisors. Often it is difficult to distinguish closely related species using molar morphology, but the premolars tend to be distinct from one species to another. The postcranial skeleton of most omomyoids shows hallmarks of leaping behavior reminiscent of that of tarsiers. In North America, omomyoids became very diverse and abundant. In fact, omomyoids from Wyoming are sufficiently abundant and from such stratigraphically controlled conditions that they have served as strong evidence for the gradual evolution of anatomical traits over time (Rose and Bown 1984).
Teilhardina (Figure 8.11; see Figure 8.2) is one of the earliest and arguably the most plesiomorphic of omomyoids. Teilhardina has several species, most of which are from North America, with one from Europe (T. belgica) and one from Asia (T. asiatica). The species of this genus are anatomically similar and the deposits from which they are derived are roughly contemporaneous. Thus, this small primate likely dispersed across the northern continents very rapidly (Smith et al. 2006).

The Emergence of Modern Primate Groups
Origins of Crown Strepsirrhines
Until the turn of this century, very little was known about the origins of the crown (living) strepsirrhines. The Quaternary record of Madagascar contains many amazing forms of lemurs, including giant sloth-like lemurs, lemurs with perhaps monkey-like habits, lemurs with koala-like habits, and even a giant aye-aye (Godfrey and Jungers 2002). However, in Madagascar, early Tertiary continental sediments are lacking, and there is no record of lemur fossils before the Pleistocene.
The fossil record of galagos is slightly more informative. Namely, there are Miocene African fossils that are very likely progenitors of lorisids (Simpson 1967). However, these are much like modern galagos and do not reveal anything about the relationship between crown strepsirrhines and Eocene fossil primates (but see below regarding Propotto). A similar situation exists for lorises in Asia: there are Miocene representatives, but these are substantially like modern lorises. The discovery of the first definite toothcomb canine (a hallmark of stresirrhines) in 2003 provided the “smoking gun” for the origin of crown strepsirrhines (Seiffert et al. 2003). Recently, several other African primates have been recognized as having strepsirrhine affinities (Marivaux et al. 2013; Seiffert 2012). The enigmatic Fayum primate Plesiopithecus is known from a skull that has been compared to aye-ayes and to lorises (Godinot 2006; Simons and Rasmussen 1994a).
The now-recognized diversity of stem strepsirrhines from the Eocene and Oligocene of Afro-Arabia is strong evidence to suggest that strepsirrhines originated in that geographic area. This implies that lorises dispersed to Asia subsequent to an African origin. It is unknown what the first strepsirrhines in Madagascar were like. However, it seems likely that the lemuriform-lorisiform split occurred in continental Africa, followed by dispersal of lemuriform stock to Madagascar. Recent evidence suggests that Propotto, a Miocene primate from Kenya originally described as a potto antecedent, actually forms a clade with Plesiopithecus and the aye-aye; this might suggest that strepsirrhines dispersed to Madagascar from continental Africa more than once (Gunnell et al. 2018).
The Fossil Record of Tarsiers
Tarsiers are so unusual that they fuel major debates about primate taxonomy. Tarsiers today are moderately diverse but geographically limited and not very different in their ecological habits—especially considering that the split between them and their nearest living relative probably occurred over 50 million years ago. If omomyoids are excluded, then the fossil record of tarsiers is very limited. Two fossil species from the Miocene of Thailand have been placed in the genus Tarsius, as has an Eocene fossil from China (Beard et al. 1994). These, and Xanthorhysis from the Eocene of China, are all very tarsier-like. In fact, it is striking that Tarsius eocaenus from China was already so tarsier-like as early as the Eocene. This suggests that tarsiers achieved their current morphology very early in their evolution and have remained more or less the same while other primates changed dramatically. Two additional genera, Afrotarsius from the Oligocene of Egypt and Libya and Afrasia from the Eocene of Myanmar, have also been implicated in tarsier origins, though the relationship between them and tarsiers is unclear (Chaimanee et al. 2012). More recently, a partial skeleton of a small Eocene primate from China, Archicebus achilles (dated to approximately 55.8 million to 54.8 million years ago), was described as the most basal tarsiiform (Ni et al. 2013). This primate is reconstructed as a diurnal insectivore and an arboreal quadruped that did some leaping—but not to the specialized degree seen in living tarsiers. The anatomy of the eye in living tarsiers suggests that their lineage passed through a diurnal stage, so Archicebus (and diurnal omomyoids) might represent such a stage.
Climate Change and the Paleogeography of Modern Primate Origins
Changing global climate has had profound effects on primate dispersal patterns and ecological habits over evolutionary time. Primates today are strongly tied to patches of trees and particular plant parts such as fruits, seeds, and immature leaves. It is no surprise, then, that the distribution of primates mirrors the distribution of forests. Today, primates are most diverse in the tropics, especially in tropical rainforests. Global temperature trends across the Tertiary have affected primate ranges. Following the Cretaceous-Tertiary extinction event, cooler temperatures and greater seasonality characterized the Paleocene. In the Eocene, temperatures (and probably rainfall) increased globally and rainforests likely extended to very high latitudes. During this time, euprimates became diverse. With cooling and increased aridity at the end of the Eocene, many primate extinctions occurred in the northern continents and the surviving primates were confined to lower latitudes in South America, Afro-Arabia, Asia, and southern Europe. Among these survivors are the progenitors of the living groups of primates: lemurs and lorises, tarsiers, platyrrhines (monkeys of the Americas), and catarrhines (monkeys and apes of Africa and Asia) (Figure 8.12).

Competing Hypotheses for the Origin of Anthropoids
There is considerable debate among paleoanthropologists as to the geographic origins of anthropoids. In addition, there is debate regarding the source group for anthropoids. Three different hypotheses have been articulated in the literature. These are the adapoid origin hypothesis, the omomyoid origin hypothesis, and the tarsier origin hypothesis (Figure 8.13).

Adapoid Origin Hypothesis
Resemblances between some adapoids and some extant anthropoids include fusion of the mandibular symphysis, overall robusticity of the chewing system, overall large body size, features that signal a diurnal lifestyle (like relatively small eye sockets), and ankle bone morphology. Another feature in common is canine sexual dimorphism, which is present in some species of adapoids (probably) and in several species of anthropoids.
These features led some paleoanthropologists in the last half of the 20th century to suggest that anthropoids came from adapoid stock (Gingerich 1980; Simons and Rasmussen 1994b). One of the earliest supporters of the link between adapoids and anthropoids was Hans Georg Stehlin, who described much of the best material of adapoids and compared these Eocene primates to South American monkeys (Stehlin 1912). In more recent times, the adapoid origin hypothesis was reinforced by resemblances between these European adapoids (especially Adapis and Leptadapis) and some early anthropoids from the Fayum Basin (e.g., Aegyptopithecus, see below; Figure 8.14).
|
Family
|
Genera
|
Morphology
|
Location
|
Age1
|
|
Propliopithecidae2 |
Aegyptopithecus |
Large size. Cranial sexual dimorphism, large canines. Robust jaws and rounded molars. Partially ossified ear tube (in some). Robust skeleton; quadruped. |
Africa |
Late Eocene to Early Oligocene |
|
Parapithecidae3 |
Apidium |
Medium size. Retention of three premolars per quadrant. Rounded molars and premolars with large central cusps. Adaptations for leaping in the lower limb. |
Africa |
Late Eocene to Late Oligocene |
|
Proteopithecidae4 |
Proteopithecus |
Small size. Retention of three premolars per quadrant. Arboreal quadrupeds that ate fruit. |
Africa |
Late Eocene |
|
Oligopithecidae5 |
Catopithecus |
Small size. Skull has postorbital septum and unfused mandible. Deep jaws. Diet of fruits. Generalized quadruped. |
Africa |
Late Eocene |
|
Eosimiidae |
Eosimias |
Deep jaw with vertical unfused symphysis. Pointed incisors and canines. Crowded premolars. |
Asia |
Middle Eocene |
|
Amphipithecidae6 |
Pondaungia |
Deep jaws. Molars generally rounded with wide basins. |
Asia |
Middle Eocene to Early Oligocene |
|
1 Derived from Fleagle 2013. 2 See Gebo and Simons 1987 and Simons et al. 2007. 3 See Feagle and Simons 1995 and Simons 2001. 4 See Simons and Seiffert 1999. 5 See Simons and Rasmussen 1996. 6 See Kay et al. 2004. |
|
|||
Unfortunately for the adapoid hypothesis, most of the shared features listed above probably emerged independently in the two groups as adaptations to a diet of hard and/or tough foods. For example, fusion of the mandibular symphysis likely evolved as a means to strengthen the jaw against forces that would pull the two halves away from each other, in the context of active chewing muscles on both sides of the head generating great bite forces. This context would also favor the development of robust jaws, large chewing muscles, shorter faces, and some other features shared by some adapoids and some anthropoids.
As older and more plesiomorphic anthropoids were found in the Fayum Basin, it became clear that the earliest anthropoids from Africa did not possess these features of jaw robusticity (Seiffert et al. 2009). Furthermore, many adapoids never evolved these features. Fusion of the mandibular symphysis in adapoids is actually quite different from that in anthropoids and probably occurred during juvenile development in the former (Beecher 1983; Ravosa 1996). Eventually, the adapoid origin hypothesis fell out of favor among most paleoanthropologists, although the description of Darwinius is a recent revival of that idea (Franzen et al. 2009; but see Seiffert et al. 2009, Williams et al. 2010b).
Omomyoid Origin Hypothesis
Similarities in cranial and hindlimb morphology between some omomyoids and extant tarsiers have led to the suggestion that tarsiers arose from some kind of omomyoid. In particular, Necrolemur has many features in common with tarsiers, as does the North American Shoshonius, which is known from a few beautifully preserved (although distorted) crania. Tarsiers and Shoshonius share exclusively some features of the base of the cranium; however, Shoshonius does not have any sign of postorbital closure, and it lacks the bony ear tube of tarsiers. Nevertheless, some of the resemblances between some omomyoids and tarsiers suggest that tarsiers might have originated from within the Omomyoidea (Beard 2002; Beard and MacPhee 1994). In this scenario, although living tarsiers and living anthropoids might be sister taxa, they might have evolved from different omomyoids, possibly separated from each other by more than 50 million years of evolution, or from anthropoids evolved from some non-omomyoid fossil group. The arguments against the omomyoid origin hypothesis are essentially the arguments for the tarsier origin hypothesis (see below). Namely, tarsiers and anthropoids share many features (especially of the soft tissues) that must have been retained for many millions of years or must have evolved convergently in the two groups. Furthermore, a key hard-tissue feature shared between the two extant groups, the postorbital septum, was not present in any omomyoid. Therefore, that feature must have arisen convergently in the two extant groups or must have been lost in omomyoids. Neither scenario is very appealing, although recent arguments for convergent evolution of the postorbital septum in tarsiers and anthropoids have arisen from embryology and histology of the structure (DeLeon et al. 2016).
Tarsier Origin Hypothesis
Several paleoanthropologists have suggested that there is a relationship between tarsiers and anthropoids to the exclusion of omomyoids and adapoids (e.g., Cartmill and Kay 1978; Ross 2000; Williams and Kay 1995). Tarsiers and anthropoids today share several traits, including many soft-tissue features related to the olfactory system (e.g., the loss of a hairless external nose and loss of the median cleft running from the nose to the mouth, as possessed by strepsirrhines), and aspects of the visual system (e.g., the loss of a reflective layer at the back of the eye, similarities in carotid circulation to the brain, and mode of placentation). Unfortunately, none of these can be assessed directly in fossils. Some bony similarities between tarsiers and anthropoids include an extra air-filled chamber below the middle ear cavity, reduced bones within the nasal cavity, and substantial postorbital closure; these can be assessed in fossils, but the distribution of these traits in omomyoids does not yield clear answers. Furthermore, several similarities between tarsiers and anthropoids are probably due to similarities in sensory systems, which might have evolved in parallel for ecological reasons. Although early attempts to resolve the crown primates with molecular data were sometimes equivocal or in disagreement with one another, more recent analyses (including those of short interspersed elements) suggest that tarsiers and anthropoids are sister groups to the exclusion of lemurs and lorises (Williams et al. 2010a). However, this does not address omomyoids, all of which are far too ancient for DNA extraction.
The above three hypotheses are not the only possibilities for anthropoid origins. It may be that anthropoids are neither the closest sister group of tarsiers, nor evolved from adapoids or omomyoids. In recent years, two new groups of Eocene Asian primates have been implicated in the origin of anthropoids: the eosimiids and the amphipithecids. It is possible that one or the other of these two groups gave rise to anthropoids. Regardless of the true configuration of the tree for crown primates, the three major extant groups probably diverged from each other quite long ago (Seiffert et al. 2004).
Early Anthropoid Fossils in Africa


The classic localities yielding the greatest wealth of early anthropoid fossils are those from the Fayum Basin in Egypt (Simons 2008; Figure 8.15). The Fayum is a veritable oasis of fossil primates in an otherwise spotty early Tertiary African record. Since the 1960s, teams led by E. L. Simons have discovered several new species of early anthropoids, some of which are known from many parts of the skeleton and several individuals (Figure 8.16).
The Fayum Jebel Qatrani Formation and Birket Qarun Formation between them have yielded a remarkable array of terrestrial, arboreal, and aquatic mammals. These include ungulates, bats, sea cows, elephants, hyraces, rodents, whales, and primates. Also, many other vertebrates, like water birds, were present. The area at the time of deposition (Late Eocene through Early Oligocene) was probably very wet, with slow-moving rivers, standing water, swampy conditions, and lots of trees (see Bown and Kraus 1988). In short, it was an excellent place for primates.
General Morphology of Anthropoids
The anthropoids known from the Fayum (and their close relatives from elsewhere in East Africa and Afro-Arabia) bear many of the anatomical hallmarks of extant anthropoids; however, there are plesiomorphic forms in several families that lack one or more anthropoid traits. All Fayum anthropoids known from skulls possess postorbital closure, most had fused mandibular symphyses, and most had ring-like ectotympanic bones. Tooth formulae were generally either 2.1.3.3 or 2.1.2.3. Fayum anthropoids ranged in size from the very small Qatrania and Biretia (less than 500 g) to the much-larger Aegyptopithecus (approximately 7 kg; 15 lbs.). Fruit was probably the main component of the diet for most or all of the anthropoids, with some of them supplementing with leaves (Kay and Simons 1980; Kirk and Simons 2001; Teaford et al. 1996). Most Fayum anthropoids were probably diurnal above-branch quadrupeds. Some of them (e.g., Apidium; see Figure 8.14) were probably very good leapers (Gebo and Simons 1987), but none show specializations for gibbon-style suspensory locomotion. Some of the Fayum anthropoids are known from hundreds of individuals, permitting the assessment of individual variation, sexual dimorphism, and in some cases growth and development. The description that follows provides greater detail for the two best known Fayum anthropoid families, the Propliopithecidae and the Parapithecidae; the additional families are summarized briefly.
Fayum Anthropoid Families
The Propliopithecidae (see Figure 8.14) include the largest anthropoids from the fauna, and they are known from several crania and some postcranial elements. They have been suggested to be stem catarrhines, although perhaps near the split between catarrhines and platyrrhines. The best known propliopithecid is Aegyptopithecus, known from many teeth, crania, and postcranial elements (Figure 8.17) .

Parapithecidae are an extremely abundant and unusual family of anthropoids from the Fayum. The parapithecid Apidium is known from many jaws with teeth, crushed and distorted crania, and several skeletal elements. Parapithecus is known from cranial material including a beautiful, undistorted cranium. This genus shows extreme reduction of the incisors, including complete absence of the lower incisors in P. grangeri (Simons 2001). This trait is unique among primates. Parapithecids were once thought to be the ancestral stock of platyrrhines; however, their platyrrhine-like features are probably ancestral retentions, so the most conservative approach is to consider them stem anthropoids.
The Proteopithecidae were small frugivores that probably mainly walked along horizontal branches on all fours. TThey are considered stem anthropoids. The best known genus, Proteopithecus, is represented by dentitions, crania, and postcranial elements.
The Oligopithecidae share a mixture of traits that makes them difficult to classify more specifically within anthropoids. The best known member, Catopithecus, is known from crania that demonstrate a postorbital septum and from mandibles that lack symphyseal fusion. They share the catarrhine tooth formula of 2.1.2.3 and have a canine honing complex that involves the anterior lower premolar. The postcranial elements known for the group suggest generalized arboreal quadrupedalism. The best known member, Catopithecus, is known from crania that demonstrate a postorbital septum and from mandibles that lack symphyseal fusion (Simons and Rasmussen 1996). The jaws are deep, with broad muscle attachment areas and crested teeth. Catopithecus was probably a little less than a kilogram in weight.
Other genera of putative anthropoids from the Fayum include the very poorly known Arsinoea, the contentious Afrotarsius, and the enigmatic Nosmips. The last of these possesses traits of several major primate clades and defies classification (Seiffert et al. 2010).
Early Anthropoid Fossils in Asia
For the last half of the 1900s, researchers believed that Africa was the unquestioned homeland of early anthropoids (see Fleagle and Kay 1994). However, two very different groups of primates from Asia soon began to change that. One was an entirely new discovery (Eosimiidae), and the other was a poorly known group discovered decades prior (Amphipithecidae). Soon, attention on anthropoid origins began to shift eastward (see Ross and Kay 2004; Simons 2004). If anthropoids arose in Asia instead of Africa, then this implies that the African early anthropoids either emigrated from Asia or evolved their anthropoid traits in parallel with living anthropoids.
Eosimiids
First described in the 1990s, the eosimiids are best represented by Eosimias (see Figure 8.14; Figure 8.18). This tiny “dawn monkey” is known from relatively complete jaws with teeth, a few small fragments of the face, and some postcranial elements (Beard et al. 1994; Beard et al. 1996; Gebo et al. 2000). Eosimias (along with the other less-well-known genera in its family) bears some resemblance to tarsiers as well as anthropoids. Unfortunately, no good crania are known for this family, and the anatomy of, for example, the posterior orbital margin could be very revealing as to higher-level relationships.

Amphipithecids
Amphipithecids are small- to medium-size primates (up to 10 kg; 22 lbs.). Most are from the Eocene Pondaung Formation in Myanmar (Early–Middle Eocene), but one genus is known from Thailand. Some dental similarities with anthropoids were noted early on, such as deep jaws and wide basins that separate low molar cusps. The best known genera were Pondaungia and Amphipithecus (Ciochon and Gunnell 2002; see Figure 8.14). Another amphipithecid, Siamopithecus from Thailand, has very rounded molars and was probably a seed-eater (Figure 8.19). In addition to teeth and jaws, some cranial fragments, ankle material, and ends of postcranial bones have been found for Pondaungia. There are important resemblances between the postcranial bones of Pondaungia and those of adapoids, suggesting adapoid affinities for the amphipithecidae. This would imply that the resemblances with anthropoids in the teeth are convergent, based on similarities in diet (see Ciochon and Gunnell 2002). Unfortunately, the association between postcranial bones and teeth is not definite. With other primates in these faunas (including eosimiids), one cannot be certain that the postcranial bones belong with the teeth. Some researchers suggest that some bones belong to a sivaladapid (or asiadapid) and others to an early anthropoid (Beard et al. 2007; Marivaux et al. 2003). Additional well-associated material of amphipithecids would help to clear up this uncertainty.

Platyrrhine Dispersal to South America
Today there is an impressive diversity of primates in South and Central America. These are considered to be part of a single clade, the Platyrrhini. Primates colonized South America sometime in the Eocene from an African source. In the first half of the 20th century, the source of platyrrhines was a matter of major debate among paleontologists, with some favoring a North American origin (e.g., Simpson 1940).
Part of the reason for this debate is that South America was an island in the Eocene. Primates needed to cross open ocean to get there from either North America or Africa, although the distance from the former was shorter. Morphology yields clues to platyrrhine origins. The first known primates in South America have more in common morphologically with African primates than with North American ones. At the time, anthropoids were popping up in North Africa, whereas the only euprimates in North America were adapoids and omomyoids. Despite lacking a bony ear tube, early platyrrhines shared a great deal with other anthropoids, including full postorbital closure and fusion of the mandibular symphysis.
The means by which a population of small North African primates managed to disperse across the Atlantic and survive to colonize South America remains a mystery. The most plausible scenario is one of rafting. That is, primates must have been trapped on vegetation that was blown out to sea by a storm. The vegetation then became a sort of life raft, which eventually landed ashore, dumping its passengers in South America. Rodents probably arrived in South America in the same way (Antoine et al. 2012).
Once ashore, platyrrhines must have crossed South America fairly rapidly because the earliest-known primates from that continent are from Peru (Bond et al. 2015). Soon after that, platyrrhines were in Bolivia, namely Branisella. By the Miocene, platyrrhines were living in extreme southern Argentina and were exploiting a variety of feeding niches. The Early Miocene platyrrhines were all somewhat plesiomorphic in their morphology, but some features that likely arose by ecological convergence suggest (to some) relationships with extant platyrrhine families. This has led to a lively debate about the pattern of primate evolution in South America (Kay 2015; Kay and Fleagle 2010; Rosenberger 2010). By the Middle Miocene, clear representatives of modern families were present in a diverse fauna from La Venta, Colombia (Wheeler 2010). The Plio-Pleistocene saw the emergence of giant platyrrhines as well as several taxa of platyrrhines living on Caribbean islands (Cooke et al. 2016).
The story of platyrrhines seems to be one of amazing sweepstakes dispersal, followed by rapid diversification and widespread geographic colonization of much of South America. After that, dramatic extinctions resulted in the current, much-smaller geographic distribution of platyrrhines. These extinctions were probably caused by changing climates, leading to the contraction of forests. Platyrrhines dispersed to the Caribbean and to Central America, with subsequent extinctions in those regions that might have been related to interactions with humans. Unlike anthropoids of Africa and Asia, platyrrhines do not seem to have evolved any primarily terrestrial forms and so have always been highly dependent on forests.
Special Topic: Jonathan Perry and Primates of the Extreme South
Many primates are very vulnerable to ecological disturbance because they are heavily dependent on fruit to eat and trees to live in. This is one reason why so many primates are endangered today and why many of them went extinct due to climatic and vegetational changes in the past. I (Jonathan Perry) have conducted paleontological research focusing on primates that lived on the edge of their geographic distribution. This research has taken me to extreme environments in the Americas: southern Patagonia, the Canadian prairies, western Wyoming, and the badlands of eastern Oregon.
Santa Cruz Province in Argentina is as far south as primates have ever lived. The Santa Cruz fauna of the Miocene has yielded a moderate diversity of platyrrhines, each with slightly different dietary adaptations. These include Homunculus, first described by Florentino Ameghino in 1891 (Figure 8.20). Recent fieldwork by my colleagues and I in Argentina has revealed several skulls of Homunculus as well as many parts of the skeleton (Kay et al. 2012). The emerging profile of this extinct primate is one of a dedicated arboreal quadruped that fed on fruits and leaves. Many of the foods eaten by Homunculus must have been very tough and were probably covered and impregnated with grit; we suspect this because the cheek teeth are very worn down, even in young individuals, and because the molar tooth roots were very large, presumably to resist strong bite forces (Perry et al. 2010, 2014).

I began working in Argentina while a graduate student at Duke University. I participated as a field assistant in a team led by my Ph.D. advisor, Richard F. Kay, and Argentine colleagues Sergio F. Vizcaíno and M. Susana Bargo. Most of the localities examined belong to a suite of beach sites known since the 1800s and visited by many field parties from various museums in the early 1900s. Since 2003, our international team of paleontologists from the U.S. and Argentina has visited these localities every single year (Figure 8.21). Over time, new fossils and new students have led to new projects and new approaches, including the use of microcomputed tomography (microCT) to visualize and analyze internal structures of the skeleton.

Planet of Apes
Geologic Activity and Climate Change in the Miocene
The Miocene Epoch was a time of mammalian diversification and extinction, global climate change, and ecological turnover. In the Miocene, there was an initial warming trend across the globe with the expansion of subtropical forests, followed by widespread cooling and drying with the retreat of tropical forests and replacement with more open woodlands and eventually grasslands. It was also a time of major geologic activity. On one side of the globe, South America experienced the rise of the Andes Mountains. On the other side, the Indian subcontinent collided with mainland Asia, resulting in the rise of the Himalayan Mountains. In Africa, volcanic activity promoted the development of the East African Rift System. Critical to the story of ape evolution was the exposure of an intercontinental landbridge between East Africa and Eurasia, permitting a true planet of apes (Figure 8.22).

Geographic Distribution: Africa, Asia, Europe
The world of the Miocene had tremendous ape diversity compared to today. The earliest records of fossil apes are from Early Miocene deposits in Africa. However, something dramatic happened around 16 million years ago. With the closure of the ancient Tethys Sea, the subsequent exposure of the Gomphotherium Landbridge, and a period of global warming, the Middle–Late Miocene saw waves of emigration of mammals (including primates) out of Africa and into Eurasia, with evidence of later African re-entry for some (Harrison 2010). Some of the mammals that dispersed from Africa to Eurasia and back were apes. Though most of these early apes left no modern descendants, some of them gave rise to the ancestors of modern apes—including hominins (Figure 8.23).

Where Are the Monkeys? Diversity in the Miocene
Whereas the Oligocene deposits in the Fayum of Egypt have yielded the earliest-known catarrhine fossils, the Miocene demonstrates some diversification of Cercopithecoidea. However, compared to the numerous and diverse Miocene apes (see below), monkeys of the Miocene are very rare and restricted to a single extinct family, the Victoriapithecidae (Figure 8.24). This family contains the earliest definite cercopithecoids. These monkeys are found from northern and eastern Africa between 20 million and 12.5 million years ago (Miller et al. 2009). The best known early African monkey is Victoriapithecus (Figure 8.25), a small-bodied (approximately 7 kg; 15 lbs.), small-brained monkey. Bilophodonty, known to be a hallmark of molar teeth of modern cercopithecoid, was present to some extent in Victoriapithecids. Victoriapithecus has been reconstructed as being more frugivorous and perhaps spent more time on the ground (terrestrial locomotion) than in the trees (arboreal locomotion; Blue et al. 2006). The two major groups of cercopithecoids today are cercopithecines and colobines. The earliest records demonstrating clear members of each of these two groups are at the end of the Miocene. Examples include the early colobine Microcolobus from Kenya and the early cercopithecine Pliopapio from Ethiopia.
|
Family
|
Genera
|
Morphology
|
Location
|
Age1
|
|
Victoriapithecidae2 |
Victoriapithecus |
Long, sloping face. Round, narrowly spaced orbits. Deep cheek bones. Well-developed sagittal crest. |
Africa |
Early to Middle Miocene |
|
Proconsulidae3 |
Proconsul |
Short face. Generalized dentition. Arboreal quadruped. Probably tailless. |
Africa and Arabia |
Early to Middle Miocene |
|
Pongidae |
Gigantopithecus |
Largest primate ever. Deep jaws and low rounded molars. |
Asia |
Miocene to Present |
|
1 Derived from Fleagle 2013. 2 See Benefit and McCrossin 1997 and Fleagle 2013. 3 See Begun 2007. |
|
|||

The Story of Us, the Apes
African Ape Diversity
The Early Miocene of Africa has yielded around 14 genera of early apes (Begun 2003). Many of these taxa have been reconstructed as frugivorous arboreal quadrupeds (Kay 1977). One of the best studied of these genera is the East African Proconsul (Family Proconsulidae; see Figure 8.24). Several species have been described, with body mass reconstructions ranging from 17 to 50 kg (approximately 37–110 lbs.). A paleoenvironmental study reconstructed the habitat of Proconsul to be a dense, closed-canopy tropical forest (Michel et al. 2014). No caudal vertebrae (tail bones) have been found in direct association with Proconsul postcrania, and the morphology of the sacrum is consistent with Proconsul lacking a tail (Russo 2016; Ward et al. 1991).
Overall, the African ape fossil record in the Late Miocene is sparse, with seven fossil localities dating between eleven and five million years ago (Pickford et al. 2009). Nevertheless, most species of great apes live in Africa today. Where did the progenitors of modern African apes arise? Did they evolve in Africa or somewhere else? The paucity of apes in the Late Miocene of Africa stands in contrast to the situation in Eurasia. There, ape diversity was high. Furthermore, several Eurasian ape fossils show morphological affinities with modern hominoids (apes). Because of this, some paleoanthropologists suggest that the ancestors of modern African great apes recolonized Africa from Eurasia toward the end of the Miocene (Begun 2002). However, discoveries of Late Miocene hominoids like the Kenyan Nakalipithecus (9.9 million to 9.8 million years ago), the Ethiopian Chororapithecus (10.7 million to 10.1 million years ago), and the late-Middle Miocene Namibian Otavipithecus (13 million to 12 million years ago) fuel an alternative hypothesis—namely that African hominoid diversity was maintained throughout the Miocene and that one of these taxa might, in fact, be the last common ancestor of extant African apes (Kunimatsu et al. 2007; Mocke et al. 2002). The previously underappreciated diversity of Late Miocene apes in Africa might be due to poor sampling of the fossil record in Africa.
Eurasian Ape Diversity
With the establishment of the Gomphotherium Landbridge (a result of the closure of the Eastern Mediterranean seaway; Rögl 1999), the Middle Miocene was an exciting time for hominoid radiations outside of Africa (see Figure 8.23). Eurasian hominoid species exploited their environments in many different ways in the Miocene. Food exploitation ranged from soft-fruit feeding in some taxa to hard-object feeding in others, in part owing to seasonal fluctuations and the necessary adoptions of fallback foods (DeMiguel et al. 2014). For example, the molars of Oreopithecus bambolii (Family Hominidae) have relatively long lower-molar shearing crests, suggesting that this hominoid was very folivorous (Ungar and Kay 1995). Associated with variation in diet, there is great variation in the degree to which cranial features (e.g., zygomatic bone or supraorbital tori) are developed across the many taxa (Cameron 1997); however, Middle Miocene fossils tend to exhibit relatively thick molar enamel and relatively robust jaws (Andrews and Martin 1991).
In Spain, the cranium with upper dentition, part of a mandible, and partial skeleton of Pliobates (Family Pliobatidae), a small-bodied ape (4–5 kg; 9–11 lbs.), was discovered in deposits dating to 11.6 million years ago (Alba et al. 2015). It is believed to be a frugivore with a relative brain size that overlaps with modern cercopithecoids. The fossilized postcrania of Pliobates suggest that this ape might have had a unique style of locomotion, including the tendency to walk across the branches of trees with its palms facing downward and flexible wrists that permitted rotation of the forearm during climbing. However, the anatomy of the distal humerus differs from those of living apes in ways that suggest that Pliobates was less efficient at stabilizing its elbow while suspended (Benefit and McCrossin 2015). Two other recently described apes from Spain, Pierolapithecus and Anoiapithecus, are known from relatively complete skeletons. Pierolapithecus had a very projecting face and thick molar enamel as well as some skeletal features that suggest (albeit controversially) a less suspensory locomotor style than in extant apes (Moyà-Solà et al. 2004). In contrast to Pierolapithecus, the slightly younger Anoiapithecus has a very flat face (Moyà-Solà et al. 2009).
Postcranial evidence for suspensory or well-developed orthograde behaviors in apes does not appear until the Late Miocene of Europe. Primary evidence supporting these specialized locomotor modes includes the relatively short lumbar vertebrae of Oreopithecus (Figure 8.26) and Dryopithecus (Maclatchy 2004). Further, fossil material of the lower torso of O. bambolii (which dates to the Pan-hominin divergence) conveys a higher degree of flexion-extension abilities in the lumbar region (lower back) than what is possible in extant apes. Additionally, the hindlimb of O. bambolii is suggested to have supported powerful hip adduction during climbing (Hammond et al. 2020). The Late Miocene saw the extinction of most of the Eurasian hominoids in an event referred to as the Vallesian Crisis (Agustí et al. 2003). Among the latest surviving hominoid taxa in Eurasia were Oreopithecus and Gigantopithecus, the latter of which held out until the Pleistocene in Asia and was probably even sympatric with Homo erectus (Cachel 2015).

The Origins of Extant Apes
The fossil record of the extant apes is somewhat underwhelming: it ranges from being practically nonexistent for some taxa (e.g., chimpanzees) to being a little better for others (e.g., humans). There are many possible reasons for these differences in fossil abundance, and many are associated with the environmental conditions necessary for the fossilization of bones. One way to understand the evolution of extant apes that is not so dependent on the fossil record is via molecular evolutionary analyses. This can include counting up the differences in the genetic sequence between two closely related species to estimate the amount of time since these species shared a common ancestor. This is called a molecular clock, and it is often calibrated using fossils of known absolute age that stand in for the last common ancestor of a particular clade. Molecular clock estimates have placed the Hylobatidae and Hominidae split between 19.7 million and 24.1 million years ago, the African ape and Asian ape split between 15.7 million and 19.3 million years ago, and the split of Hylobatidae into its current genera between 6.4 million and 8 million years ago (Israfil et al. 2011).
Small Ape Origins and Fossils
Unfortunately, the fossil record for the small (formerly “lesser”) apes is meager, particularly in Miocene deposits. One possible early hylobatid is Laccopithecus robustus, a Late Miocene catarrhine from China (Harrison 2016). Although it does share some characteristics with modern gibbons and siamangs (including an overall small body size and a short face), Laccopithecus most likely represents a plesiomorphic stem catarrhine and is therefore distantly related to extant apes (Jablonski and Chaplin 2009). A more likely candidate for the hylobatid stem is another Late Miocene taxon from China, Yuanmoupithecus xiaoyuan. Interpretation of its phylogenetic standing, however, is complicated by contradicting dental features—some of them quite plesiomorphic—which some believe best place Yuanmoupithecus as a stem hylobatid (Harrison 2016). Recently, a Middle Miocene Indian fossil ape, Kapi ramnagarensis, has extended the fossil record of small apes by approximately five million years. Its teeth are suggestive of a shift to a more frugivorous diet and it is likely a stem hylobatid (Gilbert et al. 2020). The history of Hylobatidae becomes clearer in the Pleistocene, with fossils representing extant genera.
Great Ape Origins and Fossils
The most extensive fossil record of a modern great ape is that of our own genus, Homo. The evolution of our own species will be covered in Chapter 9. The evolutionary history of the Asian great ape, the orangutan (Pongo), is becoming clearer. Today, orangutans are found only on the islands of Borneo and Sumatra. However, Pleistocene-aged teeth, attributed to Pongo, have been found in Cambodia, China, Laos, Peninsular Malaysia, and Vietnam—demonstrating the vastness of the orangutan’s previous range (Ibrahim et al. 2013; Wang et al. 2014). Sivapithecus from the Miocene of India and Pakistan is represented by many specimens, including parts of the face. Sivapithecus is very similar to Pongo, especially in the face, and it probably is closely related to ancestral orangutans (Pilbeam 1982). Originally, jaws and teeth belonging to the former genus Ramapithecus were thought to be important in the origin of humans (Simons 1961), but now these are recognized as specimens of Sivapithecus (Kelley 2002). Postcranial bones of Sivapithecus, however, suggest a more generalized locomotor mode—including terrestrial locomotion—than seen in Pongo (Pilbeam et al. 1990). Stable carbon and oxygen isotope data from dental enamel have reconstructed the paleoecological space of Sivapithecus (as well as the contemporaneous Late Miocene pongine Khoratpithecus) within the canopies of forested habitats (Habinger et al. 2022).
A probable close relative of Sivapithecus is the amazing Gigantopithecus (see Figure 8.24). Known only from teeth and jaws from China and India (e.g., Figure 8.27), this ape probably weighed as much as 270 kg (595 lbs.) and was likely the largest primate ever (Bocherens et al. 2017). Because of unique features of its teeth (including molarized premolars and patterns of wear) and its massive size, it has been reconstructed as a bamboo specialist, somewhat like the modern panda. Small silica particles (phytoliths) from grasses have been found stuck to the molars of Gigantopithecus (Ciochon et al. 1990). Recent studies evaluating the carbon isotope composition of the enamel sampled from Gigantopithecus teeth suggest that this ape exploited a wide range of vegetation, including fruits, leaves, roots, and bamboo (Bocherens et al. 2017). Its face is reminiscent of that of modern orangutans and it might belong in the same family, Pongidae (Kelley 2002).

In Africa, the first fossil to be confidently attributed to Pan, and known to be the earliest evidence of a chimpanzee, was described based on teeth found in Middle Pleistocene deposits in the Eastern Rift Valley of Kenya (McBrearty and Jablonski 2005). Paleoenvironmental reconstructions of this locality suggest that this early chimpanzee was living in close proximity to early Homo in a closed-canopy wooded habitat. Similarly, fossil teeth and mandibular remains attributed to two species of Middle-Late Miocene apes—Chororapithecus abyssinicus (from Ethiopia; Suwa et al. 2007) and Nakalipithecus nakayamai (from Kenya; Kunimatsu et al. 2007)—have been suggested as basal members of the gorilla clade.
While the deposits of Eastern Africa have yielded a profound record of our fossil hominin ancestors, the continent’s rainforests remain a “palaeontological desert” (Rosas et al. 2022). Clearly, more work is needed to fill in the large gaps in the fossil record of the nonhuman great apes. The twentieth century witnessed the discovery of many hominin fossils in East Africa, which have been critical for improving our understanding of human evolution. While twenty-first-century conservationists fight to prevent the extinction of the living great apes, perhaps efforts by twenty-first-century paleoanthropologists will yield the evolutionary story of these, our closest relatives.
Review Questions
- Compare three major hypotheses about primate origins, making reference to each one’s key ecological reason for primate uniqueness.
- Explain how changes in temperature, rainfall, and vegetation led to major changes in primate biogeography over the Early Tertiary.
- List some euprimate features that plesiadapiforms have and some that they lack.
- Contrast adapoids and omomyoids in terms of life habits.
- Describe one piece of evidence for each of the adapoid, omomyoid, and tarsier origin hypotheses for anthropoids.
- Discuss the biogeography of the origins of African great apes and orangutans using examples from the Miocene ape fossil record.
Key Terms
Adapoidea: Order: Primates. One of the earliest groups of euprimates (true primates; earliest records from the early Eocene).
Adaptive radiations: Rapid diversifications of single lineages into many species which may present unique morphological features in response to different ecological settings.
Ancestral traits: Features that were inherited from a common ancestor and which remain (largely) unchanged.
Anthropoids:Group containing monkeys and apes, including humans.
Auditory bulla: The rounded bony floor of the middle ear cavity.
Bilophodonty: Dental condition in which the cusps of molar teeth form ridges (or lophs) separated from each other by valleys (seen, e.g., in modern catarrhine monkeys).
Catarrhines: Order: Primates; Suborder: Anthropoidea; Infraorder: Catarrhini. Group, with origins in Africa and Asia, that contains monkeys and apes, including humans.
Clade:Group containing all of the descendants of a single ancestor. A portion of a phylogenetic tree represented as a bifurcation (node) in a lineage and all of the branches leading forward in time from that bifurcation.
Convergent evolution: The independent evolution of a morphological feature in animals not closely related (e.g., wings in birds and bats).
Crown: Smallest monophyletic group (clade) containing a specified set of extant taxa and all descendants of their last common ancestor.
Diastema: Space between adjacent teeth.
Diffuse coevolution: The ecological interaction between whole groups of species (e.g., primates) with whole groups of other species (e.g., fruiting trees).
Ectotympanic: Bony ring or tube that holds the tympanic membrane (eardrum).
Euprimates: Order: Primates. True primates or primates of modern aspect.
Haplorhines: Group containing catarrhines, platyrrhines, and tarsiers.
Hominins: Modern humans and any extinct relatives more closely related to us than to chimpanzees.
Mandibular symphysis: Fibrocartilaginous joint between the left and right mandibular segments, located in the midline of the body.
Omomyoidea: Order: Primates; Superfamily: Omomyoidea. One of the earliest groups of euprimates (true primates; earliest record in the early Eocene).
Petrosal bone: The portion of the temporal bone that houses the inner ear apparatus.
Plagiaulacoid: Dental condition where at least one of the lower cheek-teeth (molars or premolars) is a laterally compressed blade.
Platyrrhines: Order: Primates; Suborder: Anthropoidea; Infraorder: Platyrrhini. Group containing monkeys found in the Americas.
Plesiadapiforms: Order: Plesiadapiformes. Archaic primates or primate-like placental mammals (Early Paleocene–Late Eocene).
Plesiomorphic: Having features that are shared by different groups which arose from a common ancestor.
Stem: Taxa that are basal to a given crown group but are more closely related to the crown group than to the closest living sister taxon of the crown group.
Strepsirrhines: Order: Primates; Suborder: Stresirrhini. Group containing lemurs, lorises, and galagos (does not include tarsiers).
Toothcomb: Dental condition found in modern strepsirrhines in which the lower incisors and canines are laterally compressed and protrude forward at a nearly horizontal inclination. This structure is used in grooming.
About the Authors

Jonathan M. G. Perry, Ph.D.
Western University of Health Sciences, Oregon, jperry@westernu.edu
Jonathan Perry was trained as a paleontologist and primatologist at the University of Alberta, Duke University, and Stony Brook University. His research focuses on the relationship between food, feeding, and craniodental anatomy in primates both living and extinct. This work includes primate feeding behavior, comparative anatomy, biomechanics, and field paleontology. He has taught courses on primate evolution at the undergraduate and graduate level.

Stephanie L. Canington, B.A., Ph.D.
University of Pennsylvania, scaning@upenn.edu
Stephanie Canington is a postdoctoral researcher at the University of Pennsylvania. Her current research is on the links between food properties, feeding behavior, and jaw morphology in lemurs that live in varying forms of captivity.
For Further Exploration
Beard, Chris. 2004. The Hunt for the Dawn Monkey: Unearthing the Origins of Monkeys, Apes, and Humans. Berkeley: University of California Press.
Begun, David R. 2010. “Miocene Hominids and the Origins of the African Apes and Humans.” Annual Review of Anthropology 39: 67–84.
Fleagle, John G. 2013. Primate Adaptation and Evolution. Third edition. San Diego, CA: Academic Press.
Gebo, Daniel L., ed. 1993. Postcranial Adaptations in Nonhuman Primates. Dekalb: Northern Illinois University Press.
Godfrey, Laurie R., and William L. Jungers. 2002. “Quaternary Fossil Lemurs.” In The Primate Fossil Record, edited by Walter C. Hartwig, 97–121. Cambridge: Cambridge University Press.
Godinot, Marc. 2006. “Lemuriform Origins as Viewed from the Fossil Record.” Folia Primatologica 77 (6): 446–464.
Kay, Richard F. 2018. “100 Years of Primate Paleontology.” American Journal of Physical Anthropology 165 (4): 652–676.
Marivaux, Laurent. 2006. “The Eosimiid and Amphipithecid Primates (Anthropoidea) from the Oligocene of the Bugti Hills (Balochistan, Pakistan): New Insight into Early Higher Primate Evolution in South Asia.” Palaeovertebrata, Montpellier 34 (1–2): 29–109.
Martin, R. D. 1990. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton University Press.
Rose, Kenneth D., Marc Godinot, and Thomas M. Bown. 1994. “The Early Radiation of Euprimates and the Initial Diversification of Omomyidae.” In Anthropoid Origins: The Fossil Evidence, edited by John G. Fleagle and Richard F. Kay, 1–28. New York: Plenum Press.
Ross, Callum F. 1999. “How to Carry Out Functional Morphology.” Evolutionary Anthropology 7 (6): 217–222.
Seiffert, Erik R. 2012. “Early Primate Evolution in Afro-Arabia.” Evolutionary Anthropology: Issues, News, and Reviews 21(6): 239–253.
Szalay, Frederic S., and Eric Delson. 1979. Evolutionary History of the Primates. New York: Academic Press.
Ungar, Peter S. 2002. “Reconstructing the Diets of Fossil Primates.” In Reconstructing Behavior in the Primate Fossil Record, edited by Joseph Plavcan, Richard F. Kay, William Jungers, and Carel P. van Schaik, 261–296. New York: Kluwer Academic/Plenum Publishers.
References
Agustí, J., A. Sanz de Siria, and M. Garcés M. 2003. “Explaining the End of the Hominoid Experiment in Europe.” Journal of Human Evolution 45 (2): 145–153.
Alba, David M., Sergio Almécija, Daniel DeMiguel, Josep Fortuny, Miriam Pérez de los Ríos, Marta Pina, Josep M. Robles, and Salvador Moyà-Solà. 2015. “Miocene Small-Bodied Ape from Eurasia Sheds Light on Hominoid Evolution.” Science 350 (6260): aab2625.
Andrews, Peter, and Lawrence Martin. 1991. “Hominoid Dietary Evolution.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 334 (1270): 199–209.
Antoine, Pierre-Oliver, Laurent Marivaux, Darren A. Croft, Guillaume Billet, Morgan Ganerød, Carlos Jaramillo, Thomas Martin, et al. 2012. “Middle Eocene Rodents from Peruvian Amazonia Reveal the Pattern and Timing of Caviomorph Origins and Biogeography.” Proceedings of the Royal Society B: Biological Sciences 279 (1732): 1319–1326.
Beard, K. Christopher. 1990. “Gliding Behaviour and Palaeoecology of the Alleged Primate Family Paromomyidae (Mammalia, Dermoptera).” Nature 345 (6273): 340–341.
Beard, K. Christopher. 2002. “Basal Anthropoids.” In The Primate Fossil Record, edited by William C. Hartwig, 133–150. Cambridge: Cambridge University Press.
Beard, K. Christopher, and R. D. E. MacPhee. 1994. “Cranial Anatomy of Shoshonius and the Antiquity of Anthropoidea.” In Anthropoid Origins: The Fossil Evidence, edited by John G. Fleagle and Richard F. Kay, 55–98. New York: Plenum Press.
Beard, K. Christopher, Laurent Marivaux, Soe Thura Tun, Aung Naing Soe, Yaowalak Chaimanee, Wanna Htoon, Bernard Marandat, Htun Htun Aung, and Jean-Jacques Jaeger. 2007. “New Sivaladapid Primates from the Eocene Pondaung Formation of Myanmar and the Anthropoid Status of Amphipithecidae.” Bulletin of Carnegie Museum of Natural History 39: 67–76.
Beard, K. Christopher, Tao Qi, Mary R. Dawson, Banyue Wang, and Chuankuei Li. 1994. “A Diverse New Primate Fauna from Middle Eocene Fissure-Fillings in Southeastern China.” Nature 368 (6472): 604–609.
Beard, K. Christopher, Yongsheng Tong, Mary R. Dawson, Jingwen Wang, and Xueshi Huang. 1996. “Earliest Complete Dentition of an Anthropoid Primate from the Late Middle Eocene of Shanxi Province, China.” Science 272 (5258): 82–85.
Beecher, Robert M. 1983. “Evolution of the Mandibular Symphysis in Notharctinae (Adapidae, Primates).” International Journal of Primatology 4 (1): 99–112.
Begun, David R. 2002. “European Hominoids.” In The Primate Fossil Record, edited by William C. Hartwig, 339–368. Cambridge: Cambridge University Press.
Begun, David R. 2003. “Planet of the Apes.” Scientific American 289 (2): 74–83.
Begun, David R. 2007. “Fossil Record of Miocene Hominoids.” In Handbook of Paleoanthropology, edited by Winfried Henke and Ian Tattersall, 921–977. New York: Springer.
Benefit, Brenda R., and Monte L. McCrossin. 1997. “Earliest Known Old World Monkey Skull.” Nature 388 (6640): 368–371.
Benefit, Brenda R., and Monte L. McCrossin. 2015. “A Window into Ape Evolution.” Science 350 (6260): 515–516.
Bloch, Jonathan I., and David M. Boyer. 2002. “Grasping Primate Origins.” Science 298 (5598): 1606–1610.
Bloch, Jonathan I., and David M. Boyer. 2007. “New Skeletons of Paleocene-Eocene Plesiadapiformes: A Diversity of Arboreal Positional Behaviors in Early Primates.” In Primate Origins: Adaptations and Evolution, edited by Matthew J. Ravosa and Marian Dagosto, 535–581. New York: Springer.
Bloch, Jonathan I., and Mary T. Silcox. 2006. “Cranial Anatomy of the Paleocene Plesiadapiform Carpolestes simpsoni (Mammalia, Primates) Using Ultra High-Resolution X-ray Computed Tomography, and the Relationships of Plesiadapiforms to Euprimates.” Journal of Human Evolution: 50 (1): 1–35.
Blue, Kathleen T., Monte L. McCrossin, and Brenda R. Benefit. 2006. “Terrestriality in a Middle Miocene Context: Victoriapithecus from Maboko, Kenya.” In Human Origins and Environmental Backgrounds, edited by Hidemi Ishida, Russell Tuttle, Martin Pickford, Naomichi Ogihara, and Masato Nakatsukasa, 45–58. New York: Springer.
Bocherens, Hervé, Friedemann Schrenk, Yaowalak Chaimanee, Ottmar Kullmer, Doris Mörike, Diana Pushkina, and Jean-Jacques Jaeger. 2017. “Flexibility of Diet and Habitat in Pleistocene South Asian Mammals: Implications for the Fate of the Giant Fossil Ape Gigantopithecus.” Quaternary International 434 (A): 148–155.
Bond, Mariano, Marcelo F. Tejedor, Kenneth E. Campbell Jr., Laura Chornogubsky, Nelson Novo, and Francisco Goin. 2015. “Eocene Primates of South America and the African Origins of New World Monkeys.” Nature 520 (7548): 539–541.
Bown, T. M., and M. J. Kraus. 1988. “Geology and Paleoenvironment of the Oligocene Jebel Qatrani Formation and Adjacent Rocks, Fayum Depression, Egypt.” Professional Paper, 1452. Washington, DC: U.S. Geological Survey Professional Papers.
Cachel, Susan. 2015. Fossil Primates. Vol. 69. Cambridge: Cambridge University Press.
Cameron, David W. 1997. “A Revised Systematic Scheme for the Eurasian Miocene Fossil Hominidae.” Journal of Human Evolution 33 (4): 449–477.
Cartmill, Matt. 1972. “Arboreal Adaptations and the Origin of the Order Primates.” In The Functional and Evolutionary Biology of Primates, edited by Russell Tuttle, 97–122. Chicago: Aldine-Atherton.
Cartmill, Matt. 1974. “Rethinking Primate Origins.” Science 184 (4135): 436–443.
Cartmill, Matt, and Richard F. Kay. 1978. “Craniodental Morphology, Tarsier Affinities, and Primate Suborders.” In Recent Advances in Primatology: Evolution, edited by D. J. Chivers and K. A. Joysey, 205–214. London: Academic Press.
Casanovas-Vilar, Isaac, David M. Alba, Miguel Garcés, Josep M. Robles, and Salvador Moyà-Solà. 2011. “Updated Chronology for the Miocene Hominoid Radiation in Western Eurasia.” Proceedings of the National Academy of Sciences 108 (14): 5554-5559. https://doi:10.1073/pnas.1018562108.
Chaimanee, Yaowalak, Olivier Chavasseau, K. Christopher Beard, Aung Aung Kyaw, Aung Naing Soe, Chit Sein, Vincent Lazzari, et al. 2012. “Late Middle Eocene Primate from Myanmar and the Initial Anthropoid Colonization of Africa.” Proceedings of the National Academy of Sciences of the United States of America 109 (26): 10293–10297.
Chester, Stephen G. B., Jonathan I. Bloch, Doug M. Boyer, and William A. Clemens. 2015. “Oldest Known Euarchontan Tarsals and Affinities of Paleocene Purgatorius to Primates.” Proceedings of the National Academy of Sciences of the United States of America 112 (5): 1487–1492.
Ciochon, Russell L., and Gregg F. Gunnell. 2002. “Chronology of Primate Discoveries in Myanmar: Influences on the Anthropoid Origins Debate.” Yearbook of Physical Anthropology 45(S35): 2–35.
Ciochon, R. L., D. R. Piperno, and R. G. Thompson. 1990. “Opal Phytoliths Found on the Teeth of the Extinct Ape Gigantopithecus blacki: Implications for Paleodietary Studies.” Proceedings of the National Academy of Sciences of the United States of America 87 (20): 8120–8124.
Clemens, William A. 2004. “Purgatorius (Plesiadapiformes, Primates?, Mammalia), a Paleocene Immigrant into Northeastern Montana: Stratigraphic Occurrences and Incisor Proportions.” Bulletin of Carnegie Museum of Natural History 36: 3–13.
Cooke, Siobhán B., Justin T. Gladman, Lauren B. Halenar, Zachary S. Klukkert, and Alfred L. Rosenberber. 2016. “The Paleobiology of the Recently Extinct Platyrrhines of Brazil and the Caribbean.” In Molecular Population Genetics, Evolutionary Biology and Biological Conservation of Neotropical Primates, edited by Manuel Ruiz-Garcia and Joseph Mark Shostell, 41–89. New York: Nova Publishers.
DeLeon, Valerie B., Timothy D. Smith, and Alfred L. Rosenberger. 2016. “Ontogeny of the Postorbital Region in Tarsiers and Other Primates.” Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 299 (12): 1631–1645.
DeMiguel, Daniel, David M. Alba, and Salvador Moyà-Solà. 2014. “Dietary Specialization during the Evolution of Western Eurasian Hominoids and the Extinction of European Great Apes.” PLoS ONE 9 (5): e97442. https://doi.org/10.1371/journal.pone.0097442.
Dunn, Rachel H., Kenneth D. Rose, Rajendra Rana, Kishore Kumar, Ashok Sahni, and Thierry Smith. 2016. “New Euprimate Postcrania from the Early Eocene of Gujarat, India, and the Strepsirrhine–Haplorhine Divergence.” Journal of Human Evolution 99: 25–51.
Fleagle, John G. 2013. Primate Adaptation and Evolution, Third Edition. San Diego, CA: Academic Press.
Fleagle, John G., and Richard F. Kay. 1994. Anthropoid Origins. New York: Plenum Press.
Franzen, Jens Lorenz, Phillip D. Gingerich, Jörg Habersetzer, Jørn Hurum, von Wighart Koenigswald, and B. Holly Smith. 2009. “Complete Primate Skeleton from the Middle Eocene of Messel in Germany: Morphology and Paleobiology.” PLoS ONE 4 (5): e5723. doi:10.1371/journal.pone.0005723.
Gebo, Daniel L., Marian Dagosto, K. Christopher Beard, Tao Qi, and Jingwen Wang. 2000. “The Oldest Known Anthropoid Postcranial Fossils and the Early Evolution of Higher Primates.” Nature 404 (6775): 276–278.
Gebo, Daniel L., and Elwyn L. Simons. 1987. “Morphology and Locomotor Adaptations of the Foot in Early Oligocene Anthropoids.” American Journal of Physical Anthropology 74 (1): 83–101.
Gilbert, Christopher C., Alejandra Ortiz, Kelsey D. Pugh, Christopher J. Campisano, Biren A. Patel, Ningthoujam Premjit Singh, John G. Fleagle, and Rajeev Patnaik. 2020. “New Middle Miocene Ape (Primates: Hylobatidae) from Ramnagar, India, Fills Major Gaps in the Hominoid Fossil Record.” Proceedings of the Royal Society B 287(1934): 20201655.
Gingerich, P. D. 1980. “Eocene Adapidae, Paleobiogeography, and the Origin of South American Platyrrhini.” In Evolutionary Biology of the New World Monkeys and Continental Drift, edited by Russell L. Ciochon and A. Brunetto Chiarelli, 123–138. New York: Plenum Press.
Godfrey, Laurie R., and William L. Jungers. 2002. “Quaternary Fossil Lemurs.” In The Primate Fossil Record, edited by Walter C. Hartwig, 97–121. Cambridge: Cambridge University Press.
Godinot, Marc. 2006. “Lemuriform Origins as Viewed from the Fossil Record.” Folia Primatologica 77 (6): 446–464.
Gregory, William K. 1920. “On the Structure and Relations of Notharctus, an American Eocene Primate.” Memoirs of the American Museum of Natural History (N.S.) 3 (2).
Gunnell, Gregg F., Doug M. Boyer, Anthony R. Friscia, Steven Heritage, Frederik Kyalo Manthi, Ellen R. Miller, Hesham M. Sallam, Nancy B. Simmons, Nancy J. Stevens, and Erik R. Seiffert. 2018. “Fossil Lemurs from Egypt and Kenya Suggest an African Origin for Madagascar’s Aye-aye.” Nature Communications 9 (3193): 1–12.
Habinger, S. G., O. Chavasseau, J. J. Jaeger, Y. Chaimanee, A. N. Soe, C. Sein, and H. Bocherens. 2022. “Evolutionary Ecology of Miocene Hominoid Primates in Southeast Asia.” Scientific Reports 12 (1): 1–12.
Hammond, Ashley, Lorenzo Rook, Alisha D.Anaya, Elisabetta Cioppi, Loïc Costeur, Salvadore Moyà-Solà, and Sergio Almécija. 2020. “Insights into the Lower Torso in Late Miocene Hominoid Oreopithecus bambolii.” Proceedings of the National Academy of Sciences 117 (1): 278–284.
Harrison, Terry. 2010. “Apes among the Tangled Branches of Human Origins.” Science 327 (5965): 532–534.
Harrison, Terry. 2016. “The Fossil Record and Evolutionary History of Hylobatids.” In Evolution of Gibbons and Siamang, edited by Ullrich H. Reichard, Hirohisa Hirai, and Claudia Barelli, 91–110. New York: Springer.
Ibrahim, Yasamin Kh., Lim Tze Tshen, Kira E. Westaway, Earl of Cranbrook, Louise Humphrey, Ross Fatihah Muhammad, Jian-xin Zhao, and Lee Chai Peng. 2013. “First Discovery of Pleistocene Orangutan (Pongo sp.) Fossils in Peninsular Malaysia: Biogeographic and Paleoenvironmental Implications.” Journal of Human Evolution 65 (6): 770–797.
Israfil, Hulya, Sarah M. Zehr, Alan R. Mootnick, Maryellen Ruvolo, and Michael E. Steiper. 2011. “Unresolved Molecular Phylogenies of Gibbons and Siamangs (Family: Hylobatidae) Based on Mitochondrial, Y-linked, and X-linked Loci Indicate a Rapid Miocene Radiation or Sudden Vicariance Event.” Molecular Phylogenetics and Evolution 58 (3): 447–455.
Jablonski, Nina G., and George Chaplin. 2009. “The Fossil Record of Gibbons.” In The Gibbons, edited by Danielle Whittaker and Susan Lappan, 111–130. New York: Springer.
Jones, F. Wood. 1916. Arboreal Man. London: Edward Arnold.
Kay, Richard F. 1977. “Diets of Early Miocene African Hominoids.” Nature 268 (5621): 628–630.
Kay, Richard F. 2015. “Biogeography in Deep Time: What Do Phylogenetics, Geology, and Paleoclimate Tell Us about Early Platyrrhine Evolution?” Molecular Phylogenetics and Evolution 82 (B): 358–374.
Kay, Richard F., and John G. Fleagle. 2010. “Stem Taxa, Homoplasy, Long Lineages, and the Phylogenetic Position of Dolichocebus.” Journal of Human Evolution 59 (2): 218–222.
Kay, Richard F., Jonathan M. G. Perry, Michael Malinzak, Kari L. Allen, E. Christopher Kirk, J. Michael Plavcan, and John G. Fleagle. 2012. “Paleobiology of Santacrucian Primates.” In Early Miocene Paleobiology in Patagonia: High-Latitude Paleocommunities of the Santa Cruz Formation, edited by Sergio F. Vizcaíno, Richard F. Kay, and M. Susana Bargo, 306–330. Cambridge: Cambridge University Press.
Kay, Richard F., Daniel O Schmitt, Christopher J. Vinyard, Jonathan M. G. Perry, Nobuo Shigehara, Masanaru Takai, and Naoko Egi. 2004. “The Paleobiology of Amphipithecidae, South Asian Late Eocene Primates.” Journal of Human Evolution 46 (1): 3–25.
Kay, Richard F., and Elwyn L. Simons. 1980. “The Ecology of Oligocene African Anthropoidea.” International Journal of Primatology 1 (1): 21–37.
Kay, Richard F., Richard W. Thorington, and Peter Houde. 1990. “Eocene Plesiadapiform Shows Affinities with Flying Lemurs Not Primates.” Nature 345 (6273): 342–344.
Kelley, Jay. 2002. “The Hominoid Radiation in Asia.” In The Primate Fossil Record, edited by Walter C. Hartwig, 369–384. Cambridge: Cambridge University Press.
Kirk, E. Christopher, and Elwyn L. Simons. 2001. “Diets of Fossil Primates from the Fayum Depression of Egypt: A Quantitative Analysis of Molar Shearing.” Journal of Human Evolution 40 (3): 203–229.
Kirk, E. Christopher, and Blythe A. Williams. 2011. “New Adapiform Primate of Old World Affinities from the Devil’s Graveyard Formation of Texas.” Journal of Human Evolution 61 (2): 156–168.
Krause, David W. 1991. “Were Paromomyids Gliders? Maybe, Maybe Not.” Journal of Human Evolution 21 (3): 177–188.
Kunimatsu, Yutaka, Masato Nakatsukasa, Yoshihiro Sawada, Tetsuya Sakai, Masayuki Hyodo, Hironobu Hyodo, Tetsumaru Itaya, et al. 2007. “A New Late Miocene Great Ape from Kenya and Its Implications for the Origins of African Great Apes and Humans.” Proceedings of the National Academy of Sciences of the United States of America 104 (49): 19220–19225.
Maclatchy, Laura. 2004. “The Oldest Ape.” Evolutionary Anthropology: Issues, News, and Reviews 13 (3): 90–103.
Marivaux, Laurent, Yaowalak Chaimanee, Stéphane Ducrocq, Bernard Marandat, Jean Sudre, Aung Naing Soe, Soe Thura Tun, Wanna Htoon, and Jean-Jacques Jaeger. 2003. “The Anthropoid Status of a Primate from the Late Middle Eocene Pondaung Formation (Central Myanmar): Tarsal Evidence.” Proceedings of the National Academy of Sciences of the United States of America 100 (23): 13173–13178.
Marivaux, Laurent, Anusha Ramdarshan, El Mabrouk Essid, Wissem Marzougui, Hayet Khayati Ammar, Renaud Lebrun, Bernard Marandat, Gilles Merzeraud, Rodolphe Tabuce, and Monique Vianey-Liaud. 2013. “Djebelemur, a Tiny Pre-ToothCombed Primate from the Eocene of Tunisia: A Glimpse into the Origin of Crown Strepsirrhines.” PLoS ONE 8 (12): e80778. doi.org/10.1371/journal.pone.0080778.
Martin, R. D. 1968. “Towards a New Definition of Primates.” Man (N.S.) 3 (3): 377–401.
Martin, R. D. 1972. “Adaptive Radiation and Behaviour of the Malagasy Primates.” Philosophical Transactions of the Royal Society B: Biological Sciences 264 (862): 295–352.
Martin, R. D. 1990. Primate Origins and Evolution, a Phylogenetic Reconstruction. Princeton: Princeton University Press.
McBrearty, Sally, and Nina G. Jablonski. 2005. “First Fossil Chimpanzee.” Nature 437 (7055): 105–108.
Michel, Lauren A., Daniel J. Peppe, James A. Lutz, Stephen G. Driese, Holly M. Dunsworth, William E. H. Harcourt-Smith, William H. Horner, Thomas Lehmann, Sheila Nightingale, and Kieran P. McNulty. 2014. “Remnants of an Ancient Forest Provide Ecological Context for Early Miocene Fossil Apes.” Nature Communications 5: 1-9.
Miller, E. R., B. R. Benefit, M. L. McCrossin, J. M. Plavcan, M. G. Leakey, A. N. El-Barkooky, M. A. Hamdan, M. K. A. Gawad, S. M. Hassan, and E. L. Simons. 2009. “Systematics of Early and Middle Miocene Old World Monkeys.” Journal of Human Evolution 57 (3): 195–211.
Mocke, H., M. Pickford, B. Senut, and D. Gommery. 2022. “New Information about African Late Middle Miocene to Latest Miocene (13–5.5 Ma) Hominoidea. Communications of the Geological Survey of Namibia 24: 33–66.
Moyà-Solà, Salvadore, David M. Alba, Sergio Almécija, Isaac Casanovas-Vilar, Meike Köhler, Soledad De Esteban-Trivigno, Josep M. Robles, Jordi Galindo, and Josep Fortuny. 2009. “A Unique Middle Miocene European Hominoid and the Origins of the Great Ape and Human Clade.” Proceedings of the National Academy of Sciences of the United States of America 106 (24): 9601–9606.
Moyà-Solà, Salvador, Meike Köhler, David M. Alba, Isaac Casanovas-Vilar, and Jordi Galindo. 2004. “Pierolapithecus catalaunicus, a New Middle Miocene Great Ape from Spain.” Science 306 (5700): 1339–1344.
Ni, Xijun, Daniel L. Gebo, Marian Dagosto, Jin Meng, Paul Tafforeau, John J. Flynn, and K. Christopher Beard. 2013. “The Oldest Known Primate Skeleton and Early Haplorhine Evolution.” Nature 498 (7452): 60–64.
Perry, Jonathan M. G., Richard F. Kay, Sergio F. Vizcaíno, and M. Susana Bargo. 2010. “Tooth Root Size, Chewing Muscle Leverage, and the Biology of Homunculus patagonicus (Primates) from the Late Early Miocene of Patagonia.” Ameghiniana 47 (3): 355–371.
Perry, Jonathan M. G., Richard F. Kay, Sergio F. Vizcaíno, and M. Susana Bargo. 2014. “Oldest Known Cranium of a Juvenile New World Monkey (Early Miocene, Patagonia, Argentina): Implications for the Taxonomy and the Molar Eruption Pattern of Early Platyrrhines.” Journal of Human Evolution 74: 67–81.
Pickford, Martin, Yves Coppens, Brigitte Senut, Jorge Morales, and José Braga. 2009. “Late Miocene Hominoid from Niger.” Comptes Rendus Palevol 8 (4): 413–425.
Pilbeam, David. 1982. “New Hominoid Skull Material from the Miocene of Pakistan.” Nature 295 (5846): 232–234.
Pilbeam, David, Michael D. Rose, John C. Barry, and S. M. Ibrahim Shah. 1990. “New Sivapithecus Humeri from Pakistan and the Relationship of Sivapithecus and Pongo.” Nature 348 (6298): 237–239.
Rasmussen, D. Tab. 1990. “Primate Origins: Lessons from a Neotropical Marsupial.” American Journal of Primatology 22 (4): 263–277.
Ravosa, Matthew J. 1996. “Mandibular Form and Function in North American and European Adapidae and Omomyidae.” Journal of Morphology 229 (2): 171–190.
Rögl, Fred. 1999. “Mediterranean and Paratethys Palaeogeography during the Oligocene and Miocene.” In Hominoid Evolution and Climatic Change in Europe, edited by Jorge Agustí, Lorenzo Rook, and Peter Andrews, 8–22. Cambridge: Cambridge University Press.
Rosas, A., A. García-Tabernero, D. Fidalgo, M. Fero Meñe, C. Ebana Ebana, F. Esono Mba, and P. Saladie. 2022. “The Scarcity of Fossils in the African Rainforest: Archaeo-Paleontological Surveys and Actualistic Taphonomy in Equatorial Guinea.” Historical Biology 34 (8): 1–9.
Rose, Kenneth D., and Thomas M. Bown. 1984. “Gradual Phyletic Evolution at the Generic Level in Early Eocene Omomyoid Primates.” Nature 309 (5965): 250–252.
Rose, Kenneth D., Rachel H. Dunn, Kishor Kumar, Jonathan M. G. Perry, Kristen A. Prufrock, Rajendra S. Rana, and Thierry Smith. 2018. “New Fossils from Tadkeshwar Mine (Gujarat, India) Increase Primate Diversity from the Early Eocene Cambay Shale.” Journal of Human Evolution 122: 93–107.
Rose, Kenneth D., and John M. Rensberger. 1983. “Upper Dentition of Ekgmowechashala (Omomyoid Primate) from the John Day Formation, Oligo-Miocene of Oregon.” Folia Primatologica 41(1-2): 102–111.
Rosenberger, Alfred L. 2010. “Platyrrhines, PAUP, Parallelism, and the Long Lineage Hypothesis: A Reply to Kay et al. (2008).” Journal of Human Evolution 59 (2): 214–217.
Ross, Callum F. 2000. “Into the Light: The Origins of Anthropoidea.” Annual Review of Anthropology 29: 147–194.
Ross, Callum F., and Richard F. Kay, eds. 2004. Anthropoid Origins: New Visions. New York: Kluwer Academic/Plenum Publishers.
Russo, Gabrielle A. 2016. “Comparative Sacral Morphology and the Reconstructed Tail Lengths of Five Extinct Primates: Proconsul heseloni, Epipliopithecus vindobonensis, Archaeolemur edwardsi, Megaladapis grandidieri, and Palaeopropithecus kelyus.” Journal of Human Evolution 90: 135–162.
Schmid, Peter. 1979. “Evidence of Microchoerine Evolution from Dielsdorf (Zürich Region, Switzerland): A Preliminary Report.” Folia Primatologica 31 (4): 301–311.
Seiffert, Erik R. 2012. “Early Primate Evolution in Afro-Arabia.” Evolutionary Anthropology: Issues, News, and Reviews 21 (6): 239–253.
Seiffert, Erik R., Jonathan M. G. Perry, Elwyn L. Simons, and Doug M. Boyer. 2009. “Convergent Evolution of Anthropoid-like Adaptations in Eocene Adapiform Primates.” Nature 461 (7267): 1118–1121.
Seiffert, Erik R., Elwyn L. Simons, and Yousry Attia. 2003. “Fossil Evidence for an Ancient Divergence of Lorises and Galagos.” Nature 422 (6930): 421–424.
Seiffert, Erik R., Elwyn L. Simons, Doug M. Boyer, Jonathan M. G. Perry, Timothy M. Ryan, and Hesham M. Sallam. 2010. “A Fossil Primate of Uncertain Affinities from the Earliest Late Eocene of Egypt.” Proceedings of the National Academy of Sciences of the United States of America 107 (21): 9712–9717.
Seiffert, Erik R., Elwyn L. Simons, and Cornelia V. M. Simons. 2004. “Phylogenetic, Biogeographic, and Adaptive Implications of New Fossil Evidence Bearing on Crown Anthropoid Origins and Early Stem Catarrhine Evolution.” In Anthropoid Origins: New Visions, edited by Callum F. Ross and Richard F. Kay, 157–182. New York: Kluwer/Plenum Publishing.
Simons, Elwyn L. 1961. “The Phyletic Position of Ramapithecus.” Postilla 57: 1–9.
Simons, Elwyn L. 2001. “The Cranium of Parapithecus grangeri, an Egyptian Oligocene Anthropoidean Primate.” Proceedings of the National Academy of Sciences of the United States of America 98 (4): 7892–7897.
Simons, Elwyn L. 2004. “The Cranium and Adaptations of Parapithecus grangeri, a Stem Anthropoid From the Fayum Oligocene of Egypt.” In Anthropoid Origins: New Visions, edited by Callum F. Ross and Richard F. Kay, 183–204. New York: Kluwer/Plenum Publishing.
Simons, Elwyn L. 2008. “Eocene and Oligocene Mammals of the Fayum, Egypt.” In Elwyn Simons: A Search for Origins, edited by John G. Fleagle and Christopher C. Gilbert, 87–105. New York: Springer.
Simons, Elwyn L., and D. Tab Rasmussen. 1994a. “A Remarkable Cranium of Plesiopithecus teras (Primates, Prosimii) from the Eocene of Egypt.” Proceedings of the National Academy of Sciences of the United States of America 91(21): 9946–9950.
Simons, Elwyn L., and D. Tab Rasmussen. 1994b. “A Whole New World of Ancestors: Eocene Anthropoideans from Africa.” Evolutionary Anthropology 3 (4): 128–139.
Simons, Elwyn L., and D. Tab Rasmussen. 1996. “Skull of Catopithecus browni, an Early Tertiary Catarrhine.” American Journal of Physical Anthropology 100 (2): 261–292.
Simons, Elwyn L., and Erik R. Seiffert. 1999. “A Partial Skeleton of Proteopithecus sylviae (Primates Anthropoidea): First Associated Dental and Postcranial Remains of an Eocene Anthropoidean.” Comptes Rendus de l'Académie des Sciences, Paris 329 (12): 921–927.
Simons, Elwyn L., Erik R. Seiffert, Timothy M. Ryan, and Yousry Attia. 2007. “A Remarkable Female Cranium of the Early Oligocene Anthropoid Aegyptopithecus zeuxis (Catarrhini, Propliopithecidae).” Proceedings of the National Academy of Sciences of the United States of America 104 (21): 8731–8736.
Simpson, George Gaylord. 1933. “The ‘Plagiaulacoid’ Type of Mammalian Dentition: A Study of Convergence.” Journal of Mammalogy 14 (2): 97–107.
Simpson, George Gaylord. 1940. “Review of the Mammal-Bearing Tertiary of South America.” Proceedings of the American Philosophical Society 83 (5): 649–709.
Simpson, George Gaylord. 1967. “The Tertiary Lorisiform Primates of Africa.” Bulletin of the Museum of Comparative Zoology at Harvard University 136: 39–62.
Smith, G. Elliot. 1912. “The Evolution of Man.” Smithsonian Institute Annual Report 2012: 553–572.
Smith, Thierry, Kenneth D. Rose, and Philip D. Gingerich. 2006. “Rapid Asia–Europe–North America Geographic Dispersal of Earliest Eocene Primate Teilhardina during the Paleocene–Eocene Thermal Maximum.” Proceedings of the National Academy of Sciences of the United States of America 103 (30): 11223–11227.
Stehlin, Hans G. 1912. “Die säugetiere des schweizerischen Eocaens. Siebenter teil, erst hälfte: Adapis” [“The Mammals of the Swiss Eocene. Part Seven, First Half: Adapis”]. Abhandlungen der Schweizerischen Paläontologischen Gesellschaft 38: 1165–1298.
Strait, Suzanne G. 2001. “Dietary Reconstruction of Small-Bodied Omomyoid Primates.” Journal of Vertebrate Paleontology 21 (2): 322–334.
Sussman, Robert W. 1991. “Primate Origins and the Evolution of Angiosperms.” American Journal of Primatology 23 (4): 209–223.
Suwa, Gen, Reiko T. Kono, Shigehiro Katoh, Berhane Asfaw, and Yonas Beyene. 2007. “A New Species of Great Ape from the Late Miocene Epoch in Ethiopia.” Nature 448 (7156): 921–924.
Teaford, Mark F., Mary C. Maas, and Elwyn L. Simons. 1996. “Dental Microwear and Microstructure in Early Oligocene Primates from the Fayum, Egypt: Implications for Diet.” American Journal of Physical Anthropology 101 (4): 527–543.
Ungar, Peter S., and Richard F. Kay. 1995. “The Dietary Adaptations of European Miocene Catarrhines.” Proceedings of the National Academy of Sciences of the United States of America 92 (12): 5479–5481.
Wang, Cui-Bin, Ling-Xia Zhao, Chang-Zhu Jin, Yuan Wang, Da-Gong Qin, and Wen-Shi Pan. 2014. “New Discovery of Early Pleistocene Orangutan Fossils from Sanhe Cave in Chongzuo, Guangxi, Southern China.” Quaternary International 354: 68–74.
Ward, C. V., A. Walker, and M. F. Teaford. 1991. “Proconsul Did Not Have a Tail.” Journal of Human Evolution 21 (3): 215–220.
Wheeler, Brandon C. 2010. “Community Ecology of the Middle Miocene Primates of La Venta, Colombia: The Relationship between Ecological Diversity, Divergence Time, and Phylogenetic Richness.” Primates 51 (2): 131–138.
Williams, Blythe A., and Richard F. Kay. 1995. “The Taxon Anthropoidea and the Crown Clade Concept.” Evolutionary Anthropology 3 (6): 188–190.
Williams, Blythe A., Richard F. Kay, and E. Christopher Kirk. 2010a. “New Perspectives on Anthropoid Origins.” Proceedings of the National Academy of the United States of America 107 (11): 4797–4804.
Williams, Blythe A., Richard F. Kay, E. Christopher Kirk, and Callum F. Ross. 2010b. “Darwinius masillae Is a European Middle Eocene Stem Strepsirrhine—A Reply to Franzen et al.” Journal of Human Evolution 59(5): 567–573.
Wilson Mantilla, G. P., S. G. B. Chester, W. A. Clemens, J. R. Moore, C. J. Sprain, B. T. Hovatter, W. S. Mitchell, W. W. Mans, R. Mundil, and P. R. Renne. 2021. “Earliest Palaeocene Purgatoriids and the Initial Radiation of Stem Primates.” Royal Society Open Science 8(2):210050. doi:10.1098/rsos.210050.
Acknowledgments
We are immensely grateful to the editors of this book, Drs. Beth Shook, Lara Braff, Katie Nelson, and Kelsie Aguilera, for their time and commitment to making this knowledge freely accessible to all, and for giving us the opportunity to participate in this important project.
Lower Paleolithic, the earliest stone tool culture.
Amanda Wolcott Paskey, M.A., Cosumnes River College
AnnMarie Beasley Cisneros, M.A., American River College
This chapter is a revision from "Chapter 11: Archaic Homo” by Amanda Wolcott Paskey and AnnMarie Beasley Cisneros. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Identify the main groupings of Archaic Homo sapiens, such as Neanderthals.
- Explain how shifting environmental conditions required flexibility of adaptations, both anatomically and behaviorally.
- Describe the unique anatomical and cultural characteristics of Archaic Homo sapiens, including Neanderthals, in contrast to other hominins.
- Articulate how Middle Pleistocene hominin fossils fit into evolutionary trends including cranial capacity (brain size) development, cultural innovations, and migration patterns.
- Identify the shared traits, regional variations, and local adaptations among Archaic Homo sapiens.
- Detail the increased complexity and debates surrounding the classification of hominins in light of transitional species, species admixture, etc.
Breaking the Stigma of the "Caveman"
What do you think of when you hear the word “caveman”? Perhaps you imagine a character from a film such as The Croods, Tarzan, and Encino Man or from the cartoon The Flintstones. Maybe you picture the tennis-playing, therapy-going hairy Neanderthals from Geico Insurance commercials. Or perhaps you imagine characters from The Far Side or B.C. comics. Whichever you picture, the character in your mind is likely stooped over with a heavy brow, tangled long locks and other body hair, and clothed in animal skins, if anything. They might be holding a club with a confused look on their face, standing at the entrance to a cave or dragging an animal carcass to a fire for their next meal (see Figure 11.1). You might have even signed up to take this course because of what you knew—or expected to learn—about “cavemen.”

These images have long been the stigma and expectation about our ancestors at the transition to modern Homo sapiens. Tracing back to works as early as Carl Linnaeus, scientists once propagated and advanced this imagery, creating a clear picture in the minds of early scholars that informed the general public, even through today, that Archaic Homo sapiens, “cavemen,” were somehow fundamentally different and much less intelligent than we are now. Unfortunately, this view is overly simplistic, misleading, and incorrect. Understanding what Archaic Homo sapiens were actually like requires a much more complex and nuanced picture, one that comes into sharper focus as continuing research uncovers more about the lives of our not-too-distant (and not-too-different) ancestors.
The first characterizations of Archaic Homo sapiens were formed from limited fossil evidence in a time when ethnocentric and species-centric perspectives (anthropocentrism) were more widely accepted and entrenched in both society and science. Today, scientists are working from a more complete fossil record from three continents (Africa, Asia, and Europe), and genetic evidence informs their analyses and conclusions. The existence of Archaic Homo sapiens marks an exciting point in our lineage—a point at which many modern traits had emerged and key refinements were on the horizon. Anatomically, humans today are not that much different from Archaic Homo sapiens.
This chapter will examine how the environment with which Archaic Homo sapiens had to contend shaped their biological and cultural evolution. It will also examine the key anatomical traits that define this group of fossils, focusing on the most well-known of them, including Neanderthals. The chapter will describe cultural innovations that aided their adaptation to the changing environment, as well as their geographic distribution and regional variations. Additionally, exciting new research is examined that suggests even greater nuance and complexity during this time period.
The Changing Environment
While modern climate change is of critical concern today due to its cause (human activity) and pace (unprecedentedly rapid), the existence of global climate change itself is not a recent phenomenon. The focus of this chapter, the Middle Pleistocene (roughly between 780 kya and 125 kya), is the time period in which Archaic Homo sapiens appears in the fossil record—a time that witnessed some of the most drastic climatic changes in human existence. During this time period, there were 15 major and 50 minor glacial events in Europe, alone.
What exactly is glaciation? When scientists talk about glacial events, they are referring to the climate being in an ice age. This means that the ocean levels were much lower than today, because much of the earth’s water was tied up in large glaciers or ice sheets. Additionally, the average temperature would have been much cooler, which would have better supported an Arctic or tundra-adapted plant-and-animal ecosystem in northern latitudes. The most interesting and relevant features of Middle Pleistocene glacial events are the sheer number of them and their repeated bouts: this era alternated between glacial periods and warmer periods, known as interglacials. In other words, the planet wasn’t in an ice age the whole time.
You can see the dramatic and increasing fluctuations in temperature, recorded through foraminifera, in Figure 11.2. The distance between highs and lows demonstrates the severity of temperature shifts. Much as the Richter scale represents more intense earthquakes with more dramatic peaks, so too does this chart, which uses dramatic peaks to demonstrate intense temperature swings.
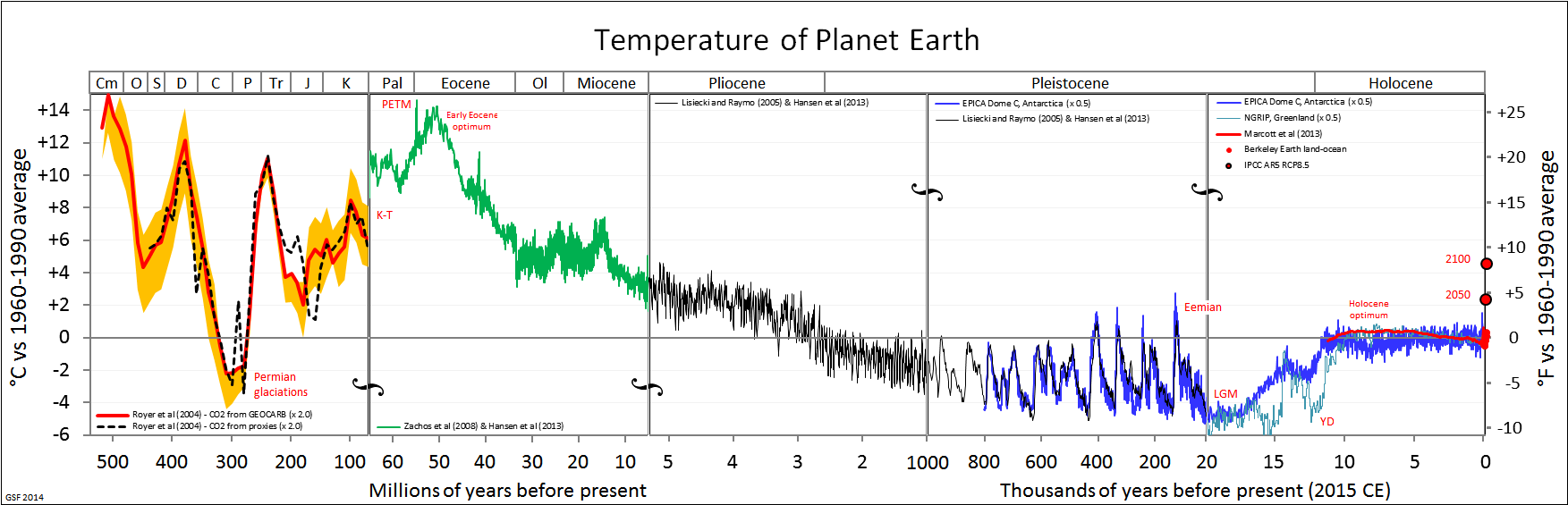
Glacial periods are defined by Earth’s average temperature being lower. Worldwide, temperatures are reduced, with cold areas becoming even colder. Huge portions of the landscape may have become inaccessible during glacial events due to the formation of glaciers and massive ice sheets. In Europe, the Scandinavian continental glacier covered what is today Ireland, England, Sweden, Norway, Denmark, and some of continental Europe. Plant and animal communities shifted to lower latitudes along the periphery of ice sheets. Additionally, some new land was opened during glacials. Evaporation with little runoff reduced sea levels by as much as almost 150 meters, shifting coastlines outward by in some instances as much as almost 100 kilometers. Additionally, land became exposed that connected what were previously unconnected continents such as Africa into Yemen at the Gulf of Aden.
Glacial periods also affected equatorial regions and other regions that are today thought of as warmer or at least more temperate parts of the globe, including Africa. While these areas were not covered with glaciers, increased global glaciation resulted in lower sea levels and expanded coastlines. Cooler temperatures were accompanied by the drying of the climate, which caused significantly reduced rainfall, increased aridity, and the expansion of deserts. It is an interesting question to consider whether the same plants and animals that lived in these regions prior to the ice ages would be able to survive and thrive in this new climate. Plant and animal communities shifted in response to the changing climate, whenever possible.
Surviving During the Middle Pleistocene
Rather than a single selective force, the Middle Pleistocene was marked by periods of fluctuation, not just cold periods. Interglacials interrupted glaciations, reversing trends in sea level, coastline, temperature, precipitation, and aridity, as well as glacier size and location. Interglacials are marked by increased rainfall and a higher temperature, which causes built-up ice in glaciers to melt. This leads to glacial retreat, which is the shrinking of glaciers and the movement of the glaciers back toward the poles, as we’ve seen in our lifetime. During interglacials, sea levels increase, flooding some previously exposed coastlines and continental connections. In addition, plant and animal communities shift accordingly, often finding more temperate climates to the north and less arid and more humid climates in the tropics (Van Andel and Tzedakis 1996).
Scientists have found that the Olorgesailie region in southern Kenya was at various times in the Middle Pleistocene a deep lake, a drought-dried lakebed with an area criss-crossed by small streams, and a grassland. While various animal species would have moved in and out of the area as the climate shifted, some animal species went extinct, and new, often related, species took up residence. The trend, scientists noted, was that animals with more specialized features went extinct and animals with more generalized features, such as animals we see today, survived in this changing climatic time period. For example, a zebra with specialized teeth for eating grass was ultimately replaced by a zebra that could eat both grass and other types of vegetation. If this small, localized example shows such a dramatic change in terms of the environment and the plant and animal biocommunities, what would have been the impact on humans?
There is no way humans could have escaped the effects of Middle Pleistocene climate change, no matter what region of the world they were living in. As noted earlier, and as evidenced by what was seen in the other biotic communities, humans would have faced changing food sources as previous sources of food may have gone extinct or moved to a different latitude. Depending on where they were living, fresh water may have been limited. Durial glacials, lower sea levels would have given humans more land to live on, while the interglacials would have reduced the available land through the increase in rainfall and associated sea level rise. Dry land connections between the continents would have made movement from one continent to another by foot easier at times than today, although these passageways were not consistently available through the Middle Pleistocene due to the glacial/interglacial cycle. Finally, as evidenced by the Olorgesailie region in Kenya, during the Middle Pleistocene animal species that were overly specialized to one particular type of environment were less likely to survive when compared to their more generalized counterparts. Evidence suggests that this same pattern may have held true for Archaic Homo sapiens, in terms of their ability to survive this dramatic period of climate change.
Defining Characteristics of Archaic Homo sapiens
Archaic Homo sapiens share our species name but are distinguished by the term “Archaic” as a way of recognizing both the long period of time between their appearance and ours, as well as the way in which human traits have continued to evolve over time—making Archaic Homo sapiens look slightly different from us today, despite being considered the same species. Living throughout Africa, and the Middle East during the Middle Pleistocene, Archaic Homo sapiens are considered, in many ways, transitional between Homo erectus and modern Homo sapiens (see Figure 11.3). Archaic Homo sapiens share the defining trait of an increased brain size of at least 1,100 cc and averaging 1,200 cc, although there are significant regional and temporal (time) variations. Because of these variations, scientists disagree on whether these fossils represent a single, variable species or multiple, closely related species (sometimes called Homo antecessor, Homo bodoensis, Homo heidelbergensis, Homo georgicus, Homo neanderthalensis, and Homo rhodesiensis).
An active area of scholarship in the discipline involves reconciling the diversity of species from this time period and establishing the phylogenetic relationships between them. The term “Archaic Homo sapiens” can mean different things to different scholars within the discipline. The intent of this chapter is to provide an understanding of the diversity of this time period and provide data used to make interpretations from among the most likely possibilities. Although we recognize that some anthropologists split many of these fossils into separate species, until the issue is resolved at the discipline level, this chapter will rely on the widely used naming conventions that refer to many fossils from this time period as Archaic Homo sapiens. We will discuss these contemporaneous fossils as a unit, with the exception of a particularly unique population living in Europe and West Asia known as the Neanderthals, which we will examine separately.
|
Trait |
Homo erectus |
Archaic Homo sapiens (including Neaderthals) |
Anatomically Modern Homo sapiens |
|
Time |
1.8 mya–200,000 ya |
600,000–40,000 ya |
315,000 ya–today |
|
Brain size |
900 cc |
1,200 cc (1,500 cc when including Neanderthals) |
1,400 cc |
|
Skull Shape |
Long and low, angular |
Intermediate |
Short and high, globular |
|
Forehead |
Absent |
Emerging |
Present |
|
Nasal Region |
Projecting nasal bones (bridge of the nose), no midfacial prognathism |
Wider nasal aperture and some midfacial prognathism, particularly pronounced among Neanderthals |
Narrower nasal aperture, no midfacial prognathism |
|
Chin |
Absent |
Absent |
Present |
|
Other Facial Features |
Large brow ridge and large projecting face |
Intermediate |
Small brow ridge and retracted face |
|
Other Skull Features |
Nuchal torus, sagittal keel, thick cranial bone |
Projecting occipital bone, often called occipital bun in Neanderthals; intermediate thickness of cranial bone |
Small bump on rear of skull, if anything; thin cranial bone |
|
Dentition |
Large teeth, especially front teeth |
Slightly smaller teeth; front teeth still large; retromolar gap in Neanderthals |
Smaller teeth |
|
Postcranial Features |
Robust bones of skeleton |
Robust bones of skeleton |
More gracile bones of skeleton |
When comparing Homo erectus, Archaic Homo sapiens, and anatomically modern Homo sapiens, one can see that Archaic Homo sapiens are intermediate in their physical form. For some features, this follows the trends first seen in Homo erectus with other features having early, less developed forms of traits seen in modern Homo sapiens. For example, Archaic Homo sapiens trended toward less angular and higher skulls than Homo erectus. However, the archaic skulls were not as short and globular and had less developed foreheads compared to anatomically modern Homo sapiens. Archaic Homo sapiens had smaller brow ridges and a less-projecting face than Homo erectus and slightly smaller teeth, although incisors and canines were often about as large as those of Homo erectus. Archaic Homo sapiens also had a wider nasal aperture, or opening for the nose, and a forward-projecting midfacial region, which is later seen more fully developed among Neanderthals and is known as midfacial prognathism. The occipital bone often projected and the cranial bone was of intermediate thickness, somewhat reduced from Homo erectus but not nearly as thin as that of anatomically modern Homo sapiens. The postcrania remained fairly robust. Identifying a set of features that is unique to Archaic Homo sapiens is a challenging task, due to both individual and geographic variation—these developments were not all present to the same degree in all individuals. Neanderthals are the exception, as they had several unique traits that made them notably different from modern Homo sapiens as well as their closely related Archaic cousins.
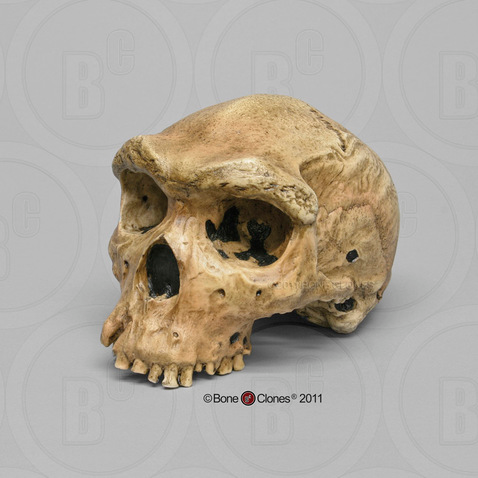
The one thing that is clear about Archaic Homo sapiens is that, despite general features, there is a lot of regional variation, which is first seen in the different Homo erectus specimens across Asia and Africa. While the general features of Archaic Homo sapiens, identified earlier, are present in the fossils of this time period, there are significant regional differences. The majority of this regional variation lies in the degree to which fossils have features more closely aligned with Homo erectus or with anatomically modern Homo sapiens.
To illustrate this point, we will examine three exemplary specimens, one from each of the three continents on which Archaic Homo sapiens lived. First, in Africa, a specimen from Broken Hill is one of several individuals found in the Kabwe lead mine in Zambia. It had a large brain (1,300 cc) and taller cranium as well as many Homo erectus-like skull features, including massive brow ridges, a large face, and thick cranial bones (Figure 11.4). Second, one partial crania from Dali, China, is representative of Archaic Homo sapiens in Asia, with large and robust features with heavy brow ridges, akin to what is seen in Homo erectus, and a large cranial capacity intermediate between Homo erectus and anatomically modern Homo sapiens (Figure 11.5). Third, an almost-complete skeleton was found in northern Spain at Atapuerca. Atapuerca 5 (Figure 11.6) has thick cranial bone, an enlarged cranial capacity, intermediate cranial height, and a more rounded cranium than seen previously. Additionally, Atapuerca 5 demonstrates features that foreshadow Neanderthals, including increased midfacial prognathism. After examining some of the fossils such as those from Kabwe, Dali, and Atapuerca, the transitional nature of Archaic Homo sapiens is clear: their features place them squarely between Homo erectus and modern Homo sapiens.
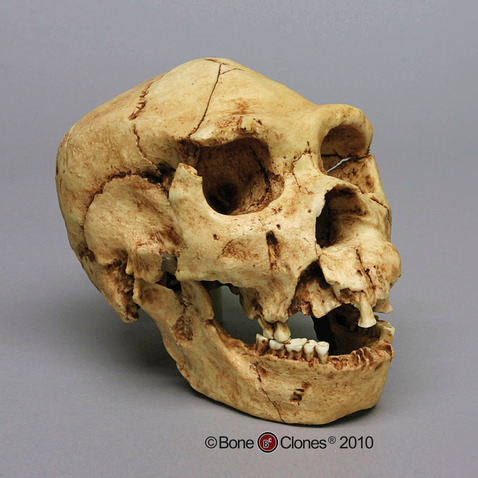
Due to the transitional nature of Archaic Homo sapiens, identifying the time period with which they are associated is problematic and complex. Generally, it is agreed that Archaic Homo sapiens lived between 600,000 and 200,000 years ago, with regional variation and overlap between Homo erectus on the early end of the spectrum and modern Homo sapiens and Neanderthals on the latter end. The earliest-known Archaic Homo sapiens fossils tentatively date to about 600,000 years ago in Africa, to around 300,000 years ago in Asia, and to about 350,000 years ago in Europe (and potentially as early as 600,000 years ago). Determining the end point of Archaic Homo sapiens is also problematic since it largely depends upon when the next subspecies of Homo sapiens appears and the classification of highly intermediate specimens. For example, in Africa, the end of Archaic Homo sapiens is met with the appearance of modern Homo sapiens, while in Europe it is the appearance of Neanderthals that is traditionally seen as marking the transition from other Archaic Homo sapiens.
It is important to remember that this time period is represented by many branching relationships and assuming an evolutionary trajectory that follows a single linear path would not be correct. Even still, Archaic Homo sapiens mark an important chapter in the human lineage, connecting more ancestral forms, such as Homo erectus, to modern Homo sapiens. During this period of climatic transition and fluctuation, Archaic Homo sapiens mirror the challenges of their environments. Showing increasing regional variation due to the need for local adaptation, there is no single archetype for this group; the defining characteristic seems to be variability.
Neanderthals
One well-known population of Archaic Homo sapiens are the Neanderthals, named after the site where they were first discovered in the Neander Valley, or “thal” in German, located near Dusseldorf, Germany. Popularly known as the stereotypical “cavemen” examined at the outset of this chapter, recent research is upending long-held beliefs about this group of Archaics. Neanderthal behavior was increasingly complex, far beyond what was exhibited by even other Archaic Homo sapiens discussed throughout this chapter. We implore you to forget the image of the iconic caveman and have an open mind when exploring the fossil evidence of the Neanderthals.
It is important to understand why Neanderthals are separated from other Archaic Homo sapiens. Unlike the rest of Archaic Homo sapiens, Neanderthals are easily defined and identified in many ways. Evidence suggests the time period when Neanderthals lived was between 150,000 and 40,000 years ago. There is a clear geographic boundary of where Neanderthals lived: western Europe, the Middle East, and western Asia. No Neanderthal fossils have ever been discovered outside of this area, including Africa. This is a bit curious, as other Archaics seem to have adapted in Africa and then migrated elsewhere, but Neanderthals’ regional association makes sense in light of the environment to which they were best adapted: namely, extreme cold weather. Additionally, Neanderthals have a unique and distinct cluster of physical characteristics. While a few aspects of Neanderthals are shared among some Archaic Homo sapiens, such as the types of tools, most Neanderthal anatomical and behavioral attributes are unique to them.
Neanderthals lived during some of the coldest times during the last Ice Age and at far northern latitudes. This means Neanderthals were living very close to the glacial edge, rather than in a more temperate region of the globe like some of their Archaic Homo sapiens relatives. While able to survive in arctic conditions, most Neanderthal sites dating to the glacial periods were found farther away from the severe cold, in a steppe tundra-like environment, which would have been more hospitable to Neanderthals, and their food sources, both flora and fauna (Ashton 2002; Nicholson 2017; Richter 2006).Their range likely expanded and contracted along with European glacial events, moving into the Middle East during glacial events when Europe became even cooler, and when the animals they hunted would have moved for the same reason. During interglacials, when Europe warmed a bit, Neanderthals and their prey would have been able to move back into Western Europe. Clearly, the true hallmark of Neanderthals is their adaptation to an unstable environment, shifting between warm and cold, as the climate was in constant flux throughout their existence (Adler et al. 2003; Boettger et al. 2009).
Many of the Neanderthals’ defining physical features are more extreme and robust versions of traits seen in other Archaic Homo sapiens, clustered in this single population. Brain size, namely an enlargement of the cranial capacity, is one such trait. The average Neanderthal brain size is around 1,500 cc, and the range for Neanderthal brains can extend to upwards of 1,700 cc. The majority of the increase in the brain occurs in the occipital region, or the back part of the brain, resulting in a skull that has a large cranial capacity with a distinctly long and low shape that is slightly wider than previous forms at the far back of the skull. Modern humans have a brain size comparable to that of Neanderthals; however, our brain expansion occurred in the frontal region of the brain, not the back, as in Neanderthal brains. This difference is also the main reason why Neanderthals lack the vertical forehead that modern humans possess. They simply did not need an enlarged forehead, because their brain expansion occurred in the rear of their brain. Due to cranial expansion, the back of the Neanderthal skull is less angular (as compared to Homo erectus), but not as rounded as Homo sapiens, producing an elongated shape, akin to a football.
Another feature that continues the trend noted in previous hominins is the enlargement of the nasal region, or the nose. Neanderthal noses are large and have a wide nasal aperture, which is the opening for the nose. While the nose is only made up of two bones, the nasals, the true size of the nose can be determined by looking at other facial features, including the nasal aperture, and the angle of the nasal and maxillary, or facial bones. In Neanderthals, these indicate a large, forward-projecting nose that appears to be pulled forward away from the rest of the face. This feature is further emphasized by the backward-sloping nature of the cheekbones, or the zygomatic arches. The unique shape and size of the Neanderthal nose is often characterized by the term midfacial prognathism—a jutting out of the middle portion of the face, or nose. This is in sharp contrast to the prognathism exhibited by other hominins, who exhibited prognathism, or the jutting out, of their jaws.
The teeth of the Neanderthals follow a similar pattern seen in the Archaic Homo sapiens, which is an overall reduction in size, especially as compared to the extremely large teeth seen in the genus Australopithecus. However, while the teeth continued to reduce, the jaw size did not keep pace, leaving Neanderthals with an oversized jaw for their teeth, and a gap between their final molar and the end of their jaw. This gap is called a retromolar gap.
The projecting occipital bone present in other Archaic Homo sapiens is also more prominent in Neanderthals, extending the trend found in Archaics. Among Neanderthals, this projection of bone is easily identified by its bun shape on the back of the skull and is known as an occipital bun. This projection appears quite similar to a dinner roll in size and shape. Its purpose, if any, remains unknown.
Continuing the Archaic Homo sapiens trend, Neanderthal brow ridges are prominent but somewhat smaller in size than those of Homo erectus and earlier Archaic Homo sapiens. In Neanderthals, the brow ridges are also often slightly less arched than those of other Archaic Homo sapiens.
In addition to extending traits present in Archaic Homo sapiens, Neanderthals possess several distinct traits. Neanderthal infraorbital foramina, the holes in the maxillae or cheek bones through which blood vessels pass, are notably enlarged compared to other hominins. The Neanderthal postcrania are also unique in that they demonstrate increased robusticity in terms of the thickness of bones and body proportions that show a barrel-shaped chest and short, stocky limbs, as well as increased musculature. These body portions are seen across the spectrum of Neanderthals—in men, women, and children.
Traditionally, many of the unique traits that Neanderthals possess were seen as adaptations to the extreme cold, dry environments in which they often lived and which exerted strong selective forces. For example, Bergmann’s and Allen’s Rules dictate that an increased body mass and short, stocky limbs are common in animals that live in cold conditions. Neanderthals were said to have matched the predictions of Bergmann’s and Allen’s Rules perfectly (Churchill 2006). In addition, the Neanderthal skull also exhibits adaptations to the cold. Neanderthals’ large infraorbital foramina allow for larger blood vessels, increasing the volume of blood that is found closest to the skin, which helps to keep the skin warmer. Their enlarged noses resulted in longer nasal passages and mucus membranes that warmed and moistened cold air before it reached the lungs. The Neanderthals’ larger nose has long been thought to have acted as a humidifier, easing physical exertion in their climate, although research on this particular trait continues to be studied and debated (Rae et al. 2011).
New research, however, seems to suggest that these unique skeletal adaptations might not have been strict adaptations to cold weather (Evteev et al. 2017; Pearce et al. 2013). For example, large brow ridges might have served as a way to shade the face from the sun. The increased occipital portion of the brain, some researchers state, was to support a larger visual system present in Neanderthals. This visual system would have given them increased light sensitivity, which would have been useful in higher latitudes that had dark winters. And, while recent modeling of nostril airflow on reconstructed Neanderthal specimens supports the notion that Neanderthals had extensive mucus membranes inside their noses, the data shows that modern Homo sapiens are superior to Neanderthals in our ability to use our noses as a way to warm and cool air. However, Neanderthals were able to snort air through their noses better than we can. Why is this important? When combined with the fact that Neanderthals tended to prefer a more temperate, tundra-like environment, and that other physical traits suggest that Neanderthals had huge bodies that needed massive amounts of calories to sustain them, the picture gets clearer. Massive amounts of energy would have been required to power a Neanderthal body, and anything that might have made them more calorically efficient would have been favored. Efficient breathing, through larger noses into large lungs, meaning deeper breaths, would have been favored. To further save energy expenditure, body sizes might have been sacrificed as well. These same types of adaptations are similar to ones seen in children today who are born in high altitudes, not cold climates. Figure 11.7 provides a summary of these unique features of the Neanderthal.
|
Distinct Neanderthal Anatomical Features |
|
|
Brain Size |
1,500 cc average |
|
Skull Shape |
Long and low |
|
Brow Ridge Size |
Large |
|
Nose Size |
Large, with midfacial prognathism |
|
Dentition |
Reduced, but large jaw size, creating retromolar gap |
|
Occipital Region |
Enlarged occipital region, occipital bun |
|
Other Unique Cranial Features |
Large infraorbital foramina |
|
Postcranial Features |
Short and stocky body, increased musculature, barrel-shaped chest |
A classic example of a Neanderthal with all of the characteristics mentioned above is the nearly complete La Ferrassie 1 Neanderthal, from France. This is a male skeleton, with a brain size of around 1640cc, an extremely large nose and infraorbital foramina, brow ridges that are marked in size, and an overall robust skeleton (Figure 11.8).
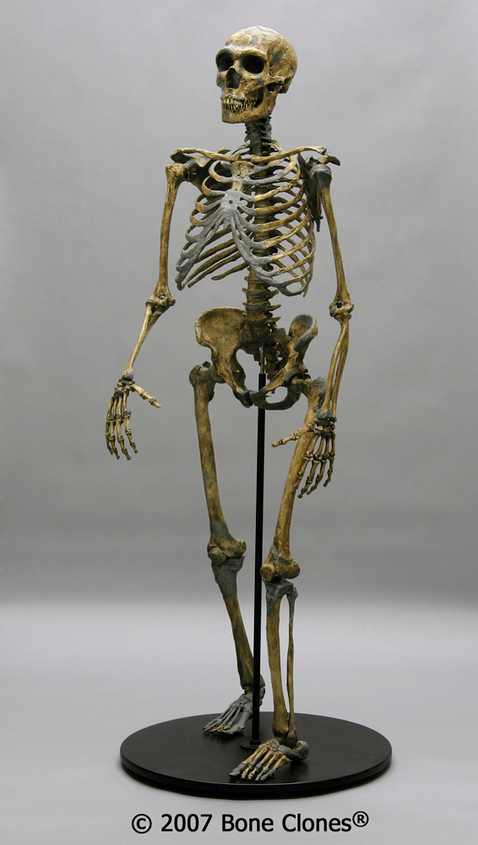
Neanderthal Culture: Tool Making and Use
One key Neanderthal adaptation was their cultural innovations, which are an important way that hominins adapt to their environment. As you recall, Homo erectus's tools, Acheulean handaxes, represented an increase in complexity over Oldowan tools, allowing more efficient removal of meat and possibly calculated scavenging. In contrast, Neanderthal tools mark a significant innovation in tool-making technique and use with Mousterian tools (named after the Le Moustier site in southwest France). These tools were significantly smaller, thinner, and lighter than Acheulean handaxes and formed a true toolkit. The materials used for Mousterian tools were of higher quality, which allowed for both more precise toolmaking and tool reworking when the tools broke or dulled after frequent reuse. The use of higher-quality materials is also indicative of required forethought and planning to acquire them for tool manufacture. It is noteworthy that the Neanderthals, unlike Homo erectus, saved and reused their tools, rather than making new ones each time a tool was needed.
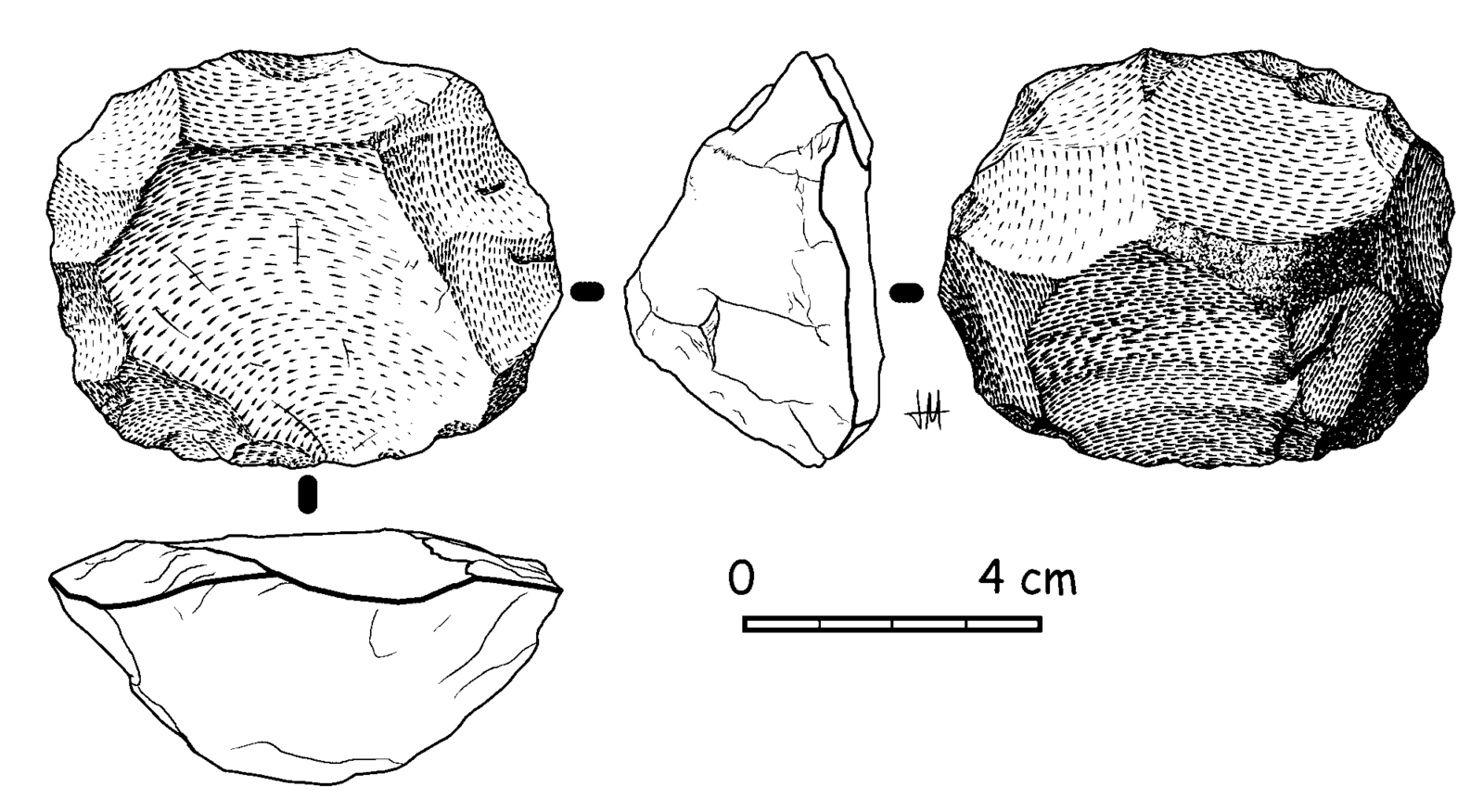
Mousterian tools are constructed in a very unique manner, utilizing the Levallois technique (Figure 11.9), named after the first finds of tools made with this technique, which were discovered in the Levallois-Perret suburb of Paris, France. The Levallois technique is a multistep process that requires preparing the core, or raw material, in a specific way that will yield flakes that are roughly uniform in dimension. The flakes are then turned into individual tools. The preparation of the core is akin to peeling a potato or carrot with a vegetable peeler—when peeling vegetables, you want to remove the skin in long, regular strokes, so that you are taking off the same amount of the vegetable all the way around. In the same way, the Levallois technique requires removing all edges of the cortex, or outside surface of the raw material, in a circle before removing the lid. The flakes, which will eventually be turned into the individual tools, can then be removed from the core. The potential yield of tools from one core would be many, as seen in Figure 11.10, compared to all previous tool-making processes, in which one core yielded a single tool. This manufacturing process might be considered the ultimate zero-waste tool-making technique (Delpiano et al. 2018).

Neanderthal tools were used for a variety of purposes, including cutting, butchering, woodworking or antler working, and hide working. Additionally, because the Mousterian tools were lighter than previous stone tools, Neanderthals could haft, or attach the tool onto a handle, as the stone would not have been too heavy (Degano et al. 2019). Neanderthals attached small stone blades onto short wood or antler handles to make knives or other small weapons, as well as attached larger blades onto longer shafts to make spears. New research examining tar-covered stones and black lumps at several Neanderthal sites in Europe suggests that Neanderthals may have been making tar by distilling it from birch tree bark, which could have been used to glue the stone tool onto its handle. If Neanderthals were, in fact, manufacturing tar to act as glue, this would predate modern humans in Africa using tree resin or similar adhesives by nearly 100,000 years.
Evidence shows that raw materials used by Neanderthals came from distances as far away as 100 km. This could indicate a variety of things regarding Neanderthal behavior, including a limited trade network with other Neanderthal groups or simply a large area scoured by Neanderthals when collecting raw materials. While research on specific applications continues, it should be clear from this brief discussion that Neanderthal tool manufacturing was much more complex than previous tool-making efforts, requiring technical expertise, patience, and skills beyond toolmaking to carry out.
Neanderthal Culture: Hunting and Diet
With their more sophisticated suite of tools and robust muscular bodies, Neanderthals were better armed for hunting than previous hominins. The animal remains in Neanderthal sites show that, unlike earlier Archaic Homo sapiens, Neanderthals were very effective hunters who were able to kill their own prey, rather than relying on scavenging. Though more refined than the tools of earlier hominins, the Neanderthal spear was not the kind of weapon that would have been thrown; rather, it would have been used in a jabbing fashion (Churchill 1998; Kortlandt 2002). This may have required Neanderthals to hunt in groups rather than individually and made it necessary to approach their prey quite closely (Gaudzinski-Windheuser et al. 2018). Remember, the animals living with Neanderthals were very large-bodied due to their adaptations to cold weather; this would have included species of deer, horses, and bovids (relatives of the cow).
Isotopes from Neanderthal bones show that meat was a significant component of their diet, similar to that seen in carnivores like wolves (Bocherens et al. 1999; Jaouen et al. 2019; Richards et al. 2000). In addition to large prey, their diet included ibex, seals, rabbits, and pigeons. Though red meat was a critical component of the Neanderthal diet, evidence shows that at times they also ate limpets, mussels, and pine nuts. Tartar examined from Neanderthal teeth in Iraq and Belgium reveal that they also ate plant material including wheat, barley, date palms, and tubers, first cooking them to make them palatable (Henry et al. 2010). While Neanderthals’ diet varied according to the specific environment in which they lived, meat comprised up to 80% of their diet (Wiẞin et al. 2015).
Neanderthal Culture: Caring for the Injured and Sick
While the close-range style of hunting used by Neanderthals was effective, it also had some major consequences. Many Neanderthal skeletons have been found with significant injuries, which could have caused paralysis or severely limited their mobility. Many of the injuries are to the head, neck, or upper body. Thomas Berger and Erik Trinkaus (1995) conducted a statistical comparative analysis of Neanderthal injuries compared to those recorded in modern-day workers’ compensation reports and found that the closest match was between Neanderthal injuries and those of rodeo workers. Rodeo professionals have a high rate of head and neck injuries that are similar to the Neanderthals’ injuries. What do Neanderthals and rodeo workers have in common? They were both getting very close to large, strong animals, and at times their encounters went awry.
The extensive injuries sustained by Neanderthals are evident in many fossil remains. Shanidar 1 (Figure 11.11), an adult male found at the Shanidar site in northern Iraq and dating to 45,000 ya, has a lifetime of injuries recorded in his bones (Stewart 1977). Shanidar 1 sustained—and healed from—an injury to the face that would have likely caused blindness. His lower right arm was missing and the right humerus shows severe atrophy, likely due to disuse. This pattern has been interpreted to indicate a substantial injury that required or otherwise resulted in amputation or wasting away of the lower arm. Additionally, Shanidar 1 suffered from bony growths in the inner ear that would have significantly impaired his hearing and severe arthritis in the feet. He also exhibited extensive anterior tooth wear, matching the pattern of wear found among modern populations who use their teeth as a tool. Rather than an anomaly, the type of injuries evident in Shanidar 1 are similar to those found in many other Neanderthal fossils, revealing injuries likely sustained from hunting large mammals as well as demonstrating a long life of physical activity.
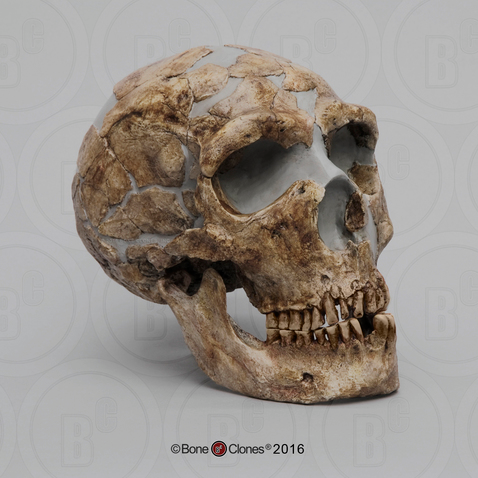

The pattern of injuries is as significant as the fact that Shanidar 1 and other injured Neanderthals show evidence of having survived their severe injuries. One of the earliest-known Neanderthal discoveries—the one on whom misinformed analysis shaped the stereotype of the species for nearly a century—is the La Chapelle-aux-Saints Neanderthal (Trinkaus 1985). The La Chapelle Neanderthal had a damaged eye orbit that likely caused blindness and suffered arthritis of the spine (Dawson and Trinkaus 1997). He had also lost most of his teeth, many of which he had lived without for so long that the mandibular and maxillary bones were partially reabsorbed due to lack of use. The La Chapelle Neanderthal was also thought to be at least in his mid-forties at death, an old age for the rough life of the Late Pleistocene—giving rise to his nickname, “the Old Man.” To have survived so long with so many injuries that obviously precluded successful large game hunting, he must have been taken care of by others. Such caretaking behavior is also evident in the survival of other seriously injured Neanderthals, such as Shanidar 1. Long thought to be a hallmark modern human characteristic, taking care of the injured and elderly, for example preparing or pre-chewing food for those without teeth, indicates strong social ties among Neanderthals.
Neanderthal Culture: Ritual Life
Such care practices may have been expressed upon death as well. Nearly complete Neanderthal skeletons are not uncommon in the fossil record, and most are well preserved within apparently deliberate burials that involve deep graves and bodies found in specific, often fetal or flexed positions (Harrold 1980). Discoveries of pollen in a grave at the Shanidar site in the 1960s led scientists to think that perhaps Neanderthals had placed flowering plants in the grave, an indication of ritual ceremony or spirituality so common in modern humans. But more recent investigations have raised some doubt about this conclusion (Pomeroy et al. 2020). The pollen may have been brought in by burrowing rodents. Claims of grave goods or other ornamentation in burials are similarly debated, although possible.
Some tantalizing evidence for symbolism, and debatably, ritual, is the frequent occurrence of natural pigments, such as ochre (red) and manganese dioxide (black) in Neanderthal sites that could have been used for art. However, the actual uses of pigments are unclear, as there is very little evidence of art or paintings in Mousterian sites. One exception may be the recent discovery in Spain of a perforated shell that appears to be painted with an orange pigment, which may be evidence of Neanderthal art and jewelry. However, many pigments also have properties that make them good emulsifiers in adhesive (like for attaching a stone tool to a wooden handle) or useful in tanning hides. So the presence of pigment may or may not be associated with symbolic thought; however, it definitely does show a technological sophistication beyond that of earlier Archaic hominins.
The Lasting Gift of Neanderthals: Tantalizing New Directions for Research
Examining the more recent time period in which Neanderthals lived and the extensive excavations completed across Europe allows for a much more complete archaeological record from this time period. Additionally, the increased cultural complexity such as complex tools and ritual behaviors expressed by Neanderthals left a more detailed record than previous hominins. Intentional burials enhanced preservation of the dead and potentially associated ritual behaviors. Such evidence allows for a more complete and nuanced picture of this species.

Additional analyses are possible on many Neanderthal finds, due to increased preservation of bone, the amount of specimens that have been uncovered, and the recency in which Neanderthals lived. We should be cautious, however, to consider the potential bias of many Neanderthal sites. Overwhelmingly, Neanderthal skeletons are found complete, with injuries or evidence of disease in caves. Does this mean all Neanderthals lived a tough, disease-wrought life? Probably not. It does, however, indicate that the sick were cared for by others, and that they lived in environments that preserved their bodies incredibly well. These additional studies include the examination of dental calculus and even DNA analysis. While limited, samples of Neanderthal DNA have been successfully extracted and analyzed. Research thus far has identified specific genetic markers that show some Neanderthals were light-skinned and probably red-haired with light eyes. Genetic analyses, different from the typical hominin reconstruction done with earlier species, allow scientists to further investigate soft tissue markers of Neanderthals and other more recent hominin species. These studies offer striking conclusions regarding Neanderthal traits, their physical appearance, and their culture, as reflected in these artists’ reconstructions (Figure 11.12).

Dr. Svante Pääbo (Figure 11.13), of the Max Planck Institute for Evolutionary Anthropology, has been at the forefront of much of this new research, largely in the form of genomic studies (The Nobel Prize 2022). Awarded the Nobel Prize for Physiology or Medicine in 2022, Pääbo is known primarily for his work with ancient DNA. He has successfully sequenced mitochondrial DNA (mtDNA) as well as the entire Neanderthal genome from nuclear DNA. His genomic work has led to the realization that Denisovans are genetically distinct from Neanderthals, as well as the recent identification of a Neanderthal father and teenage daughter, which he discovered by looking for unique DNA markers in the fossil record. Additionally, Pääbo’s genomic work has provided researchers with additional lines of evidence regarding the connections between hominin fossils (such as Neanderthals) and modern people, their time of divergence, and current genetic overlap. The work of Pääbo has even formalized a new field of study within anthropology—paleogenomics. To stay up to date with Dr. Svante Pääbo’s work, be sure to follow his lab’s website.
Neanderthal Culture: Communicating through Speech
To successfully live in groups and to foster cultural innovations, Neanderthals would have required at least a basic form of communication in order to function, possibly using a speech-based communication system. The challenge with this line of research is that speech, of course, is not preserved, so indirect evidence must be used to support this conclusion. It is thought that Neanderthals would have possessed some basic speech, as evidenced from a variety of sources, including throat anatomy and genetic evidence (Lieberman 1971). There is only one bone in the human body that could demonstrate if a hominin was able to speak, or produce clear vocalizations like modern humans, and that is the hyoid, a U-shaped bone that is found in the throat and is associated with the ability to precisely control the vocal cords. Very few hyoid bones have been found in the archaeological record; however, a few have been uncovered in Neanderthal burials. The shape of the Neanderthal hyoid is nearly identical to that of modern humans, pointing to the likelihood that they had the same vocal capabilities as modern humans. In addition, geneticists have uncovered a mutation present in both modern humans and Neanderthals—the FOXP2 gene—that is possibly linked to the ability to speak. However, other scientists argue that we cannot make sweeping conclusions that the FOXP2 gene accounts for speech due to small sample size. Finally, scientists have also pointed to the increasingly complex cultural behavior of Neanderthals as a sign that symbolic communication, likely through speech, would have been the only way to pass down the skills needed to make, for example, a Levallois blade or to position a body for intentional burial.
Neanderthal Intelligence
One of the enduring questions about Neanderthals centers on their intelligence, specifically in comparison to modern humans. Brain volume indicates that Neanderthals certainly had a large brain, but it continues to be debated if Neanderthals were of equal intelligence to modern humans. Remember, creatures with larger body sizes tend to have larger brains; however, scaling of the brain is not always associated with greater intelligence (Alex 2018). Brain volume (cranial capacity), cultural complexity, tool use, and compassion toward their kind all point to an increase in intellect among Neanderthals when compared to previous hominins.
Yet, new research is suggesting additional differences between Neanderthal brains and our own. For example, Euluned Pearce and colleagues (2013), from the University of Oxford, noted the frontal lobes of Neanderthals and modern humans are almost identical. However, Neanderthals had a larger visual cortex—the portion of the brain involved in processing visual information. This would have left Neanderthals with less brain tissue for other functions, including those that would have aided them in dealing with large social groupings, one of the differences that has been suggested to exist between Neanderthals and modern humans. Other differences were found when geneticist John Blangero, from the Texas Biomedical Research Institute, compared data from the Neanderthal genome against data from modern study participants. Blangero and his colleagues (Blangero et al. 2014) discovered that some Neanderthal brain components were very different, and smaller, than those in the modern sample. Differences were found in areas associated with the processing of information and controlling emotion and motivation, as well as overall brain connectivity. In short, as Blangero stated, “Neanderthals were certainly cognitively adept,” although their specific abilities may have differed from modern humans’ in key areas (qtd. in Wong 2015). This point has been echoed in other recent genetic studies comparing Neanderthal and anatomically modern human brains (el-Showk 2019).
Finally, scientists are fairly certain that Neanderthal brain development after birth was not the same as that of modern humans. After birth, anatomically modern Homo sapiens babies go through a critical period of brain expansion and cognitive development. It appears that Neanderthal babies’ brains and bodies did not follow the same developmental pattern (Smith et al. 2010; Zollikofer and Ponce de León 2013). Modern humans enjoy an extended period of childhood, which allows children to engage in imaginative play and develop creativity that fosters cognitive skills. Neanderthals had a more limited childhood, with less development of the creative mind that may have affected their species’ success (Nowell 2016).
The exact nature of Neanderthal intelligence remains under investigation, however. Some studies disagree with the idea that Neanderthal intelligence had limitations compared to our own, noting the extensive evidence of Neanderthals having limb asymmetry. Their tools also have wear marks indicating that they were hand-dominant. This is further supported by marks on Neanderthal teeth that demonstrate hand dominance. The Neanderthal “stuff-and-cut method” of eating, noted by David Frayer and colleagues (Frayer et al. 2012), would have seen Neanderthals hold a piece of meat in their teeth, while pulling it taut with one hand, and then using the other hand, their dominant one, to cut the meat off of the larger slab being held in their teeth. When looking at 17 Neanderthals and their tooth wear, only two do not show markings associated with a right-hand dominant individual eating in this manner. Further, it has been established that favoring the right hand is a key marker between modern humans and chimpanzees, and that handedness also relates to language development, in the form of bilateral brain development. That Neanderthals likely were hand-dominant suggests they had an indicator of bilateral brain development and a precondition for human speech.
The Middle Stone Age: Neanderthal Contemporaries in Africa
While Neanderthals made their home on and adapted to the European and Asian continents, evidence of fossil humans in Africa show they were also adapting to their local environments. These populations in Africa exhibit many more similarities to modern humans than Neanderthals, as well as overall evolutionary success. While the African fossil sample size is smaller and more fragmentary than the number of Neanderthal specimens across Europe and Asia, the African sample is interesting in that it represents a longer time period and larger geographical area. This group of fossils—often represented by the name “Middle Stone Age,” or MSA—dates to between 300,000 and 30,000 years ago across the entire continent of Africa. As with Archaic Homo sapiens, there is much variability seen in this African set of fossils. There are also a few key consistent elements: none of them exhibit Neanderthal skeletal features; instead, they demonstrate features that are increasingly consistent with anatomically modern Homo sapiens.
Similarities to Neanderthals and MSA contemporaries in Africa are seen, however, in their behavioral adaptations, including stone tools and other cultural elements. The tools associated with the specimens living in Africa during this time period are, like their physical features, varied. In some parts of Africa, namely Northern Africa, stone tools from this time so closely resemble Neanderthal tools that they are classified as Mousterian. In sub-Saharan Africa, the stone tools associated with these specimens are labeled as MSA. Some scholars argue that these could also be a type of Mousterian tools, but they are still typically subdivided based on geographical location.
Recall that Mousterian tools were much more advanced than their Acheulean predecessors in terms of how the stone tools were manufactured, the quality of the stones used, and the ultimate use of the tools that were made. In addition, recent evidence suggests that MSA tools may also have been heat treated—to improve the quality of the stone tool produced (Stolarczyk and Schmidt 2018). Evidence for heat treating is seen not only through advanced analysis of the tool itself but also through the residue of fires from this time period. Fire residues show a shift over time from small, short fires fueled by grasses (probably intended for cooking) to larger, more intensive fires that required the exploitation of dry wood, exactly the type of fire that would have been needed for heat treating stone tools (Esteban et al. 2018).
Other cultural elements seen with MSA specimens include the use of marine (sea-based) resources for their diet (Parkington 2003), manufacture of bone tools, use of adhesive and compound tools (e.g., hafted tools), shell bead production, engraving, use of pigments (such as ochre), and other more advanced tool-making technology (e.g., microlithics). While many of these cultural elements are also seen to a limited extent among Neanderthals, developments at MSA sites appear more complex. This MSA cultural expansion may have been a response to climate change or an increased use of language, complex communication, and/or symbolic thought. Others have suggested that the MSA cultural expansion was due to the increase of marine resources in their diet, which included more fatty acids that may have aided their cognitive development. Still others have suggested that the increased cultural complexity was due to increased interaction among groups, which spurred competition to innovate. Recent studies suggest that perhaps the best explanation for the marked cultural complexity of MSA cultural artifacts is best explained by the simple fact that they lived in diverse habitats (Kandel et al. 2015). This would have necessitated a unique set of cultural adaptations for each habitat type (for example, specialized marine tools would have been needed along coastal sites but not at inland locations). Simply put, the most useful adaptation of MSA was their flexibility of behavior and adaptability to their local environment. As noted previously in this chapter, flexibility of behavior and physical traits, rather than specialization, seems to be a feature that was favored in hominin evolution at this time.
Where Did They Go? The End of Neanderthals
While MSA specimens were increasingly successful and ultimately transitioned into modern Homo sapiens, Neanderthals disappeared from the fossil record by around 40,000 years ago. What happened to them? We know, based on genetics, that modern humans come largely from the modern people who occupied Africa around 300,000 to 100,000 years ago, at the same time that Neanderthals were living in northern Europe and Asia. As you will learn in Chapter 12, modern humans expanded out of Africa around 150,000 years ago, rapidly entering areas of Europe and Asia inhabited by Neanderthals and other Archaic hominins. Despite intense interest and speculation in fictional works about possible interactions between these two groups, there is very little direct evidence of either peaceful coexistence or aggressive encounters. It is clear, though, that these two closely related hominins shared Europe for thousands of years, and recent DNA evidence suggests that they occasionally interbred (Fu et al. 2015). Geneticists have found traces of Neanderthal DNA (as much as 1% to 4%) in modern humans of European and Asian descent not present in modern humans from sub-Saharan Africa. This is indicative of limited regional interbreeding with Neanderthals.
While some interbreeding likely occurred, as a whole, Neanderthals did not survive. What is the cause for their extinction? This question has fascinated many researchers and several possibilities have been suggested, including:
- At the time that Neanderthals were disappearing from the fossil record, the climate went through both cooling and warming periods—each of which posed challenges for Neanderthal survival (Defleur and Desclaux 2019; Staubwasser et al. 2018). It has been argued that as temperatures warmed, large-bodied animals, well adapted to cold weather, moved farther north to find colder environments or faced extinction. A shifting resource base could have been problematic for continued Neanderthal existence, especially as additional humans, in the form of modern Homo sapiens, began to appear in Europe and compete for a smaller pool of available resources.
- It has been suggested that the eruption of a European volcano 40,000 years ago could have put a strain on available plant resources (Golovanova et al. 2010). The eruption would have greatly affected local microclimates, reducing the overall temperature enough to alter the growing season.
- Possible differences in cognitive development may have limited Neanderthals in terms of their creative problem solving. As much as they were biologically specialized for their environment, the nature of their intelligence might not have offered them the creative problem-solving skills to innovate ways to adapt their culture when faced with a changing environment (Pearce et al. 2013).
- CRISPR gene-editing technology has been used in studies to evaluate potential differences between human and Neanderthal brains, based on differences in the genetic code. Potential differences include a Neanderthal propensity for mutations related to brain development that could account for more rapid brain development, maturation, synapse misfires, and less-orderly neural processes (Mora-Bermúdez et al. 2022; Trujillo et al. 2021). Fundamental differences in brain function at the cellular level may account for the differential survival rates of Neanderthal and modern human populations.
- There is evidence that suggests reproduction may have posed challenges for Neanderthals. Childbirth was thought to have been at least as difficult for female Neanderthals as anatomically modern Homo sapiens (Weaver and Hublin 2009). Female Neanderthals may have become sexually mature at an older age, even older than modern humans. This delayed maturation could have kept the Neanderthal population size small. A recent study has further suggested that male Neanderthals might have had a genetic marker on the Y chromosome that could have caused incompatibility between the fetus and mother during gestation; this would have had severe consequences for birth rate and survival (Mendez et al. 2016). Even a small but continuous decrease in fertility would have been enough to result in the extinction of Neanderthals (Degioanni et al. 2019).
- As mentioned above, the end of Neanderthal existence overlaps with modern human expansion into northern Europe and Asia. There is no conclusive direct evidence to indicate that Neanderthals and modern humans lived peacefully side by side, nor that they engaged in warfare, but by studying modern societies and the tendencies of modern humans, it has been suggested that modern humans may not have warmly embraced their close but slightly odd-looking cousins when they first encountered them (Churchill et al. 2009). Nevertheless, direct competition with modern humans for the same resources may have contributed to the Neanderthals’ decline (Gilpin et al. 2016); it may also have exposed them to new diseases, brought by modern humans (Houldcroft and Underdown 2016), which further decimated their population. Estimates of energy expenditures suggest Neanderthals had slightly higher caloric needs than modern humans (Venner 2018). When competing for similar resources, the slightly greater efficiency of modern humans might have helped them experience greater success in the face of competition—at a cost to Neanderthals.
As Neanderthal populations were fairly small to begin with (estimated between 5,000 and 70,000 individuals; Bocquet-Appel and Degioanni 2013), one or a combination of these factors could have easily led to their demise. As more research is conducted, we will likely get a better picture of exactly what led to Neanderthal extinction.
Denisovans

While Neanderthals represent one regionally adapted branch of the Archaic Homo sapiens family tree, recent discoveries in Siberia and the Tibetan Plateau surprised paleoanthropologists by revealing yet another population that was contemporary with Archaic Homo sapiens, Neanderthals, and modern Homo sapiens. The genetic analysis of a child’s finger bone (Figure 11.14) and an adult upper third molar (Figure 11.15) from Denisova Cave in the Altai Mountains in Siberia by a team including Svante Pääbo discovered that the mitochondrial and nuclear DNA sequences reflected distinct genetic differences from all known Archaic populations. Dubbed “Denisovans” after the cave in which the bones were found, this population is more closely related to Neanderthals than modern humans, suggesting the two groups shared an ancestor who split from modern humans first, then the Neanderthal-Denisovan line diverged more recently (Reich et al. 2010).
Denisovans share up to 5% of their DNA with modern Melanesians, aboriginal Australians, and Polynesians, and 0.2% of their DNA with other modern Asian populations and Native Americans. Additional studies have suggested one (Vernot et al. 2018) or two (Browning et al. 2018) separate points of time when interbreeding occurred between modern humans and Denisovans.
Genetic analysis reveals that Denisovans (potentially three distinct populations) had adaptations for life at high altitudes that prevented them from developing altitude sickness and hypoxia in extreme environments such as Tibet, where the average annual temperature is close to 0℃ and the altitude is more than a kilometer (about 4,000 feet) above sea level. Through protein analysis of a jawbone, one study (Chen et al. 2019) has placed Denisovans in Tibet as early as 160,000 years ago. Genetic evidence of interbreeding has linked modern Tibetan populations with Denisovans 30,000 to 40,000 years ago, which implies that the unique high-altitude adaptations seen in modern Tibetans may have originated with Denisovans (Huerta-Sanchez et al. 2014).
Other research suggests tantalizing new directions regarding Denisovans. Stone tools similar to those found in Siberia have been uncovered in the Tibetan plateau suggesting a connection between the Denisovan populations in those two areas (Zhang et al. 2018). The molar of a young girl, possibly Denisovan, has been found in Laos and shows strong similarities to specimens from China (Demeter et al. 2022). And DNA sequencing from discoveries in the Denisova Cave have yielded a genome that has been interpreted as the first-generation offspring of a Denisovan father and Neanderthal mother (Slon et al. 2018). While this research is not yet conclusive and is still being interpreted, exciting new possibilities are being revealed. To stay up-to-date with new discoveries, consider following organizations such as the Smithsonian’s Human Origins Program on social media.
How Do These Fit In? Homo naledi and Homo floresiensis
Recently, some fossils have been unearthed that have challenged our understanding of the hominin lineage. The fossils of Homo naledi and Homo floresiensis are significant for several reasons but are mostly known for how they don’t fit the previously held patterns of hominin evolution. While we examine information about these species, we ask you to consider the evidence presented in this chapter and others to draw your own conclusions regarding the significance and placement of these two unusual fossil species in the hominin lineage.
Homo naledi
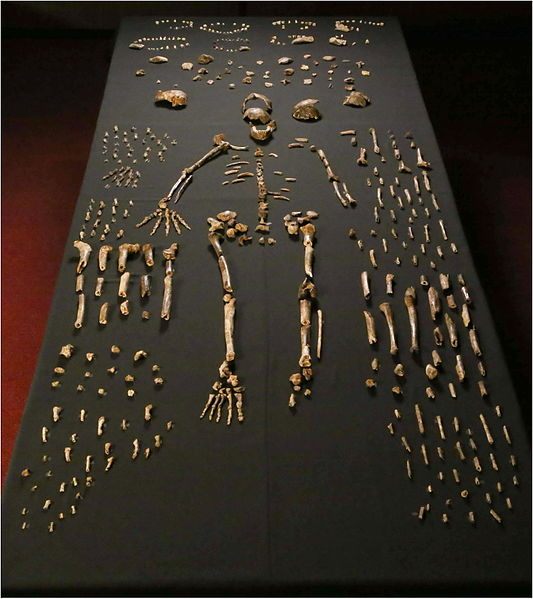
In 2013 recreational spelunkers uncovered a collection of bones deep in a cave network in Johannesburg, South Africa. The cave system, known as Rising Star, had been well documented by other cavers; however, it appears few people had ever gone as far into the cave as these spelunkers did. Lee Berger, paleoanthropologist at University of Witwatersrand, in Johannesburg, immediately put out a call for what he termed “underground astronauts” to begin recovery and excavation of the fossil materials. Unlike other excavations, Berger and most other paleoanthropologists would not be able to access the elusive site, as it was incredibly difficult to reach, and at some points there was only eight inches of space through which to navigate. The underground astronauts, all petite, slender female anthropologists, were the only ones who were able to access this remarkable site. Armed with small excavation tools and a video camera, which streamed the footage up to the rest of the team at the surface, the team worked together and uncovered a total of 1,550 bones, representing at least 15 individuals, as seen in Figure 11.16. Later, an additional 131 bones, including an almost-complete cranium, were found in a nearby chamber of the cave, representing three more individuals (Figure 11.17). Berger called in a team of specialists to participate in what was dubbed “Paleoanthropology Summer Camp.” Each researcher specialized in a different portion of the hominin skeleton. With various specialists working simultaneously, more rapid analysis was possible of Homo naledi than most fossil discoveries.
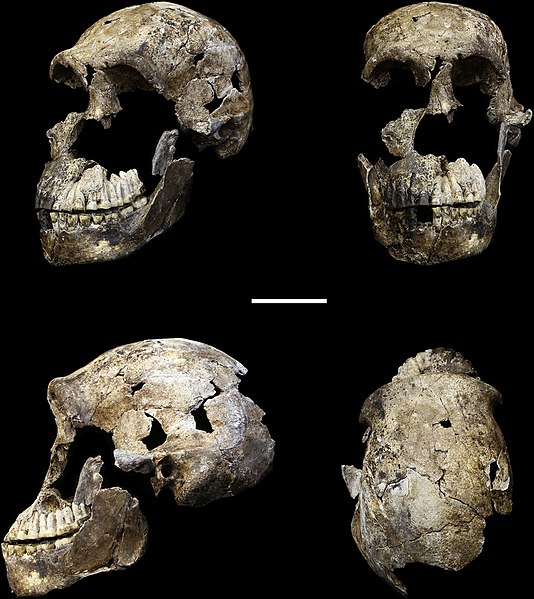
While access to the site, approximately 80 m from any known cave entrance or opening, was treacherous for researchers, it must have been difficult for Homo naledi as well. The route included moving through a portion that is just 25 cm wide at some points, known as “Superman’s Crawl.” The only way to get through this section is by crawling on your stomach with one arm by your side and the other raised above your head. Past Superman’s Crawl, a jagged wall known as the Dragon’s Back would have been very difficult to traverse. Below that, a narrow vertical chute would have eventually led down to the area where the fossils were discovered. While geology changes over time and the cave system likely has undergone its fair share, it is not likely that these features arose after Homo naledi lived (Dirks et al. 2017). This has made scientists curious as to how the bones ended up in the bottom of the cave system in the first place. It has been suggested that Homo naledi deposited the bones there, one way or another. If Homo naledi did deposit the bones, either through random disposal or intentional burial, this raises questions regarding their symbolic behavior and other cultural traits, including the use of fire, to access a very dark cave system. Another competing idea is that a few individuals may have entered the cave system to escape a predator and then got stuck. To account for the sheer number of fossils, this would have had to happen multiple times.
The features of Homo naledi are well-documented due to the fairly large sample, which represents individuals of all sexes and a wide range of ages. The skull shape and features are very much like other members of the genus Homo—including a sagittal keel and large brow, like Homo erectus, and a well-developed frontal lobe, similar to modern humans—yet the brain size is significantly smaller than its counterparts, at approximately 500 cc (560 cc for males and 465 cc for females). The teeth also exhibit features of later members of the genus Homo, such as Neanderthals, including a reduction in overall tooth size. Homo naledi also had unique shoulder anatomy and curved fingers, indicating similarities to tree-dwelling primates, which is very different from any other hominin yet found. Perhaps the greatest shock of all is that Homo naledi has been dated to 335,000 to 236,000 years ago, placing it as a contemporary to modern Homo sapiens, despite its very primitive features. An additional specimen of a child, found in 2021, not only shares many of the unique features found in the adult specimen but will also add insight into the growth and development of individuals of this species (Brophy et al. 2021).
Homo floresiensis
In a small cave called Liang Bua, on the island of Flores, in Indonesia, a small collection of fossils were discovered beginning in 2003 (Figure 11.18). The fossil fragments represent as many as nine individuals, including a nearly complete female skeleton. The features of the skull are very similar to that of Homo erectus, including the presence of a sagittal keel, an arching brow ridges and nuchal torus, and the lack of a chin (Figure 11.19). Homo floresiensis, as the new species is called, had a brain size that was remarkably small at 400 cc, and recent genetic studies suggest a common ancestor with modern humans that predates Homo erectus.

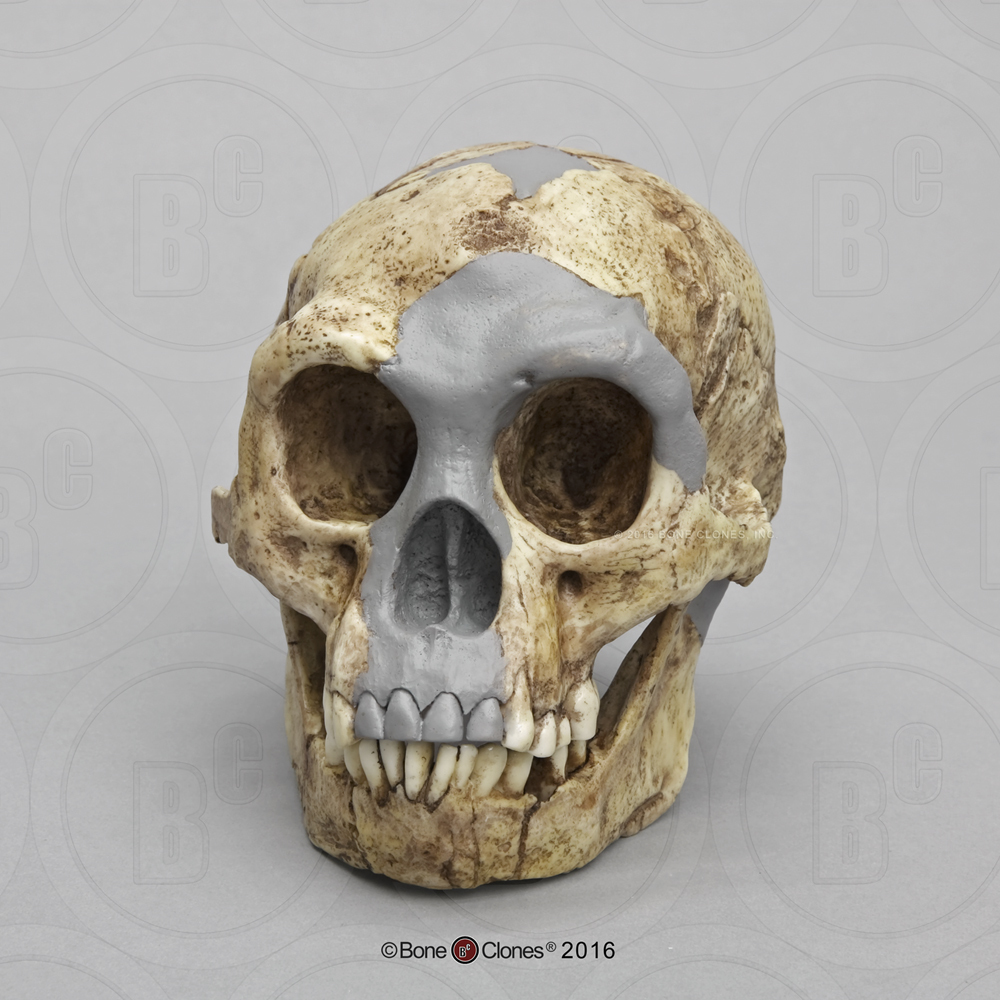
The complete female skeleton, who was an adult, was approximately a meter tall and would have weighed just under 30 kg, which is significantly shorter and just a few kilograms more than the average, modern, young elementary-aged child. A reconstructed comparison between an anatomically modern human and Homo floresiensis can be seen in Figure 11.20. The small size of the fossil has earned the species the nickname “the Hobbit.” Many questions have been asked about the stature of this species, as all of the specimens found also show evidence of diminutive stature and small brain size. Some explanations include pathology; however, this seems unlikely as all fossils found thus far demonstrate the same pattern. Another possible explanation lies in a biological phenomena seen in other animal species also found on the island, which date to a similar time period. This phenomenon, called insular dwarfing, is due to limited food resources on an island, which can create a selective pressure for large-bodied species to be selected for smaller size, as an island would not have been able to support their larger-bodied cousins for a long period of time. This phenomenon is the cause of other unique species known to have lived on the island at the same time, including the miniature stegodon, a dwarf elephant species.
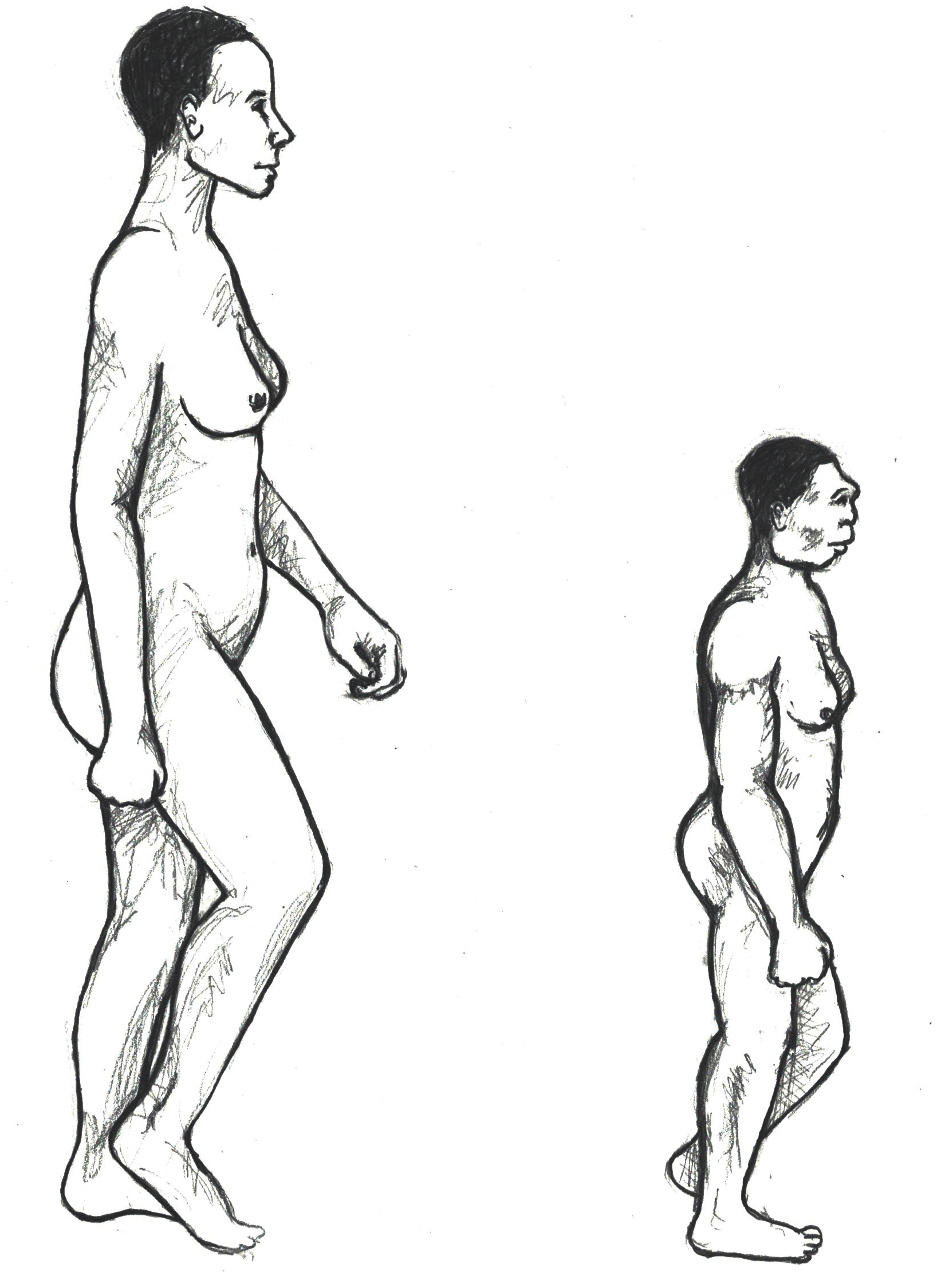
There is ongoing research and debate regarding Homo floresiensis’ dates of existence, with some researchers concluding that they lived on Flores until perhaps as recently as 17,000 years ago, although they are more often dated to 100,000 to 60,000 years ago. Stone tools from that time period uncovered at the site are similar to other hominin stone tools found on the island of Flores. Homo floresiensis would have hunted a wide range of animals, including the miniature stegodon, giant rats, and other large rodents. Other animals on the island that could have threatened them include the giant komodo dragon. An interesting note about this island chain is that ancestors of Homo floresiensis would have had to traverse the open ocean in order to get there, as the nearest island is almost 10 km away, and there is little evidence to support that a land bridge connecting mainland Asia or Australia to the island would have been present. This separation from the mainland would also have limited the number of other animals, including predators and human species, that would have had access to the island. Anatomically modern Homo sapiens arrived on the island around 30,000 years ago and, if some researchers’ later dates for Homo floresiensis are correct, both species may have lived on Flores at the same time. The modern population living on the island of Flores today believes that their ancestors came from the Liang Bua cave; however, recent genetic studies have determined they are not related to Homo floresiensis (Tucci et al. 2018).
Conclusion
Research presented in the chapter contributes to why scientists have taken to nicknaming this time period “the muddle in the middle.” We know that the Middle Pleistocene picks up from Homo erectus and ends with the appearance of anatomically modern Homo sapiens. While the start and the end are clear, it’s the middle that is messy. As more research is conducted and more data is collected, rather than clarifying our understanding of the hominin lineage during this time period, it only inspires more questions, particularly about the relationships between hominins during this time period, including the oft-misunderstood Neanderthal. Research is painting a more detailed picture of Neanderthal intelligence and both biological and behavioral adaptations. At the same time, their relationship to other Middle Pleistocene hominins, including Denisovans, as well as modern humans, remains unclear.
Homo naledi and Homo floresiensis are clear outliers when compared to their contemporary hominin species. Each has surprised paleoanthropologists for both their archaic traits in relatively modern times and their unique combination of traits seen in archaic species and modern humans. While these finds have been exciting, they have also completely upended the assumed trajectory of the human lineage, causing scientists to re-examine assumptions about hominin evolution and what it means to be modern. Add this to the developments being made using ancient DNA, other new fossil discoveries, and other innovations in paleoanthropology, and you see that our understanding of Archaic Homo sapiens and others living during this time period is rapidly developing and changing. This is a true testament to the nature of science and the scientific method.
Clearly, hominins of the Middle Pleistocene are distinct from our species today. Yet, understanding the hominins that directly preceded our species and clarifying the evolutionary relationships between us is important to better understanding our own place in nature.
Hominin Species Summaries
|
Hominin |
Archaic Homo sapiens |
|
Dates |
600,000–200,000 years ago (although some regional variation) |
|
Region(s) |
Africa, Europe, and Asia |
|
Famous discoveries |
Broken Hill (Zambia), Atapuerca (Spain) |
|
Brain size |
1,200 cc average |
|
Dentition |
Slightly smaller teeth in back of mouth, larger front teeth |
|
Cranial features |
Emerging forehead, no chin, projecting occipital region |
|
Postcranial features |
Robust skeleton |
|
Culture |
Varied regionally, but some continue to use Acheulean handaxe, others adopt Mousterian tool culture |
|
Other |
Lots of regional variation in this species |
|
Species |
Homo naledi |
|
Dates |
335,000–236,000 years ago |
|
Region(s) |
South Africa |
|
Famous discoveries |
Rising Star Cave |
|
Brain size |
500 cc average |
|
Dentition |
Reduced tooth size |
|
Cranial features |
Sagittal keel, large brow, well-developed frontal region |
|
Postcranial features |
Suspensory shoulder |
|
Culture |
unknown |
|
Other |
N/A |
|
Hominin |
Neanderthals |
|
Dates |
150,000–40,000 years ago |
|
Region(s) |
Western Europe, Middle East, and Western Asia only |
|
Famous discoveries |
Shanidar (Iraq), La Chapelle-aux-Saints (France) |
|
Brain size |
1500 cc average |
|
Dentition |
Retromolar gap |
|
Cranial features |
Large brow ridge, midfacial prognathism, large infraorbital foramina, occipital bun |
|
Postcranial features |
Robust skeleton with short and stocky body, increased musculature, barrel chest |
|
Culture |
Mousterian tools often constructed using the Levallois technique |
|
Other |
N/A |
|
Species |
Homo floresiensis |
|
Dates |
100,000–60,000 years ago, perhaps as recently as 17,000 years ago |
|
Region(s) |
Liang Bua, island of Flores, Indonesia |
|
Famous discoveries |
“The Hobbit” |
|
Brain size |
400 cc average |
|
Dentition |
unknown |
|
Cranial features |
Sagittal keel, arching brow ridges, nuchal torus, no chin |
|
Postcranial features |
Very short stature (approximately 3.5 ft.) |
|
Culture |
Tools similar to other tools found on the island of Flores |
|
Other |
N/A |
|
Hominin |
Denisovans |
|
Dates |
100,000–30,000 years ago |
|
Region(s) |
Siberia |
|
Famous discoveries |
Child’s finger bone and adult molar |
|
Brain size |
unknown |
|
Dentition |
Large molars (from limited evidence) |
|
Cranial features |
unknown |
|
Postcranial features |
unknown |
|
Culture |
unknown |
|
Other |
Closely related to Neanderthals (genetically) |
Review Questions
- What physical and cultural features are unique to Archaic Homo sapiens? How are Archaic Homo sapiens different in both physical and cultural characteristics from Homo erectus?
- Describe the specific changes to the brain and skull first seen in Archaic Homo sapiens. Why does the shape of the skull change so dramatically from Homo erectus?
- What role did the shifting environment play in the adaptation of Archaic Homo sapiens, including Neanderthals? Discuss at least one physical feature and one cultural feature that would have assisted these groups in surviving the changing environment.
- What does the regional variation in Archaic Homo sapiens represent in terms of the broader story of our species’ evolution?
- Describe the issues raised by the discoveries of Homo naledi and Homo floresiensis in the understanding of the story of the evolution of Homo sapiens.
Key Terms
Allele: Each of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome.
Anthropocentrism: A way of thinking that assumes humans are the most important species and leads to interpreting the world always through a human lens. Species-centric science and thought.
Cortex: The outside, or rough outer covering, of a rock. Usually the cortex is removed during the process of stone tool creation.
Ethnocentric: Applying negative judgments to other cultures based on comparison to one’s own.
Exogenous DNA: DNA that originates from sources outside of the specimen you are trying to sequence.
Flexed position: Fetal position, in which the legs are drawn up to the middle of the body and the arms are drawn toward the body center. Intentional burials are often found in the flexed body position.
Foraminifera: Microscopic single-celled organisms with a shell that are common in all marine environments. The fossil record of foraminifera extends back well over 500 million years.
Glaciation: A glacial period, or time when a large portion of the world is covered by glaciers and ice sheets.
Globular: Round-shaped, like a globe.
Grave goods: Items included with a body at burial. Items may signify occupation or hobbies, social status, or level of importance in the community, or they may be items believed necessary for the afterlife.
Haft: A handle. Also used as a verb—to attach a handle to an item, such as a stone tool.
Infraorbital foramina: Small holes on the maxilla bone of the face that allows nerves and blood to reach the skin.
Insular dwarfing: A form of dwarfism that occurs when a limited geographic region, such as an island, causes a large-bodied animal to be selected for a smaller body size.
Interglacial: A warmer period between two glacial time periods.
Levallois technique: A distinctive technique of stone tool manufacturing used by Archaic Homo sapiens, including Neanderthals. The technique involves the preparation of a core and striking edges off in a regular fashion around the core. Then a series of similarly sized pieces can be removed, which can then be turned into different tools.
Midfacial prognathism: A forward projection of the nose or the middle facial region. Usually associated with Neanderthals.
Mousterian tools: The stone tool industry of Neanderthals and their contemporaries in Africa and Western Asia. Mousterian tools are known for a diverse set of flake tools, which is different from the large bifacial tools of the Acheulean industry.
Nasal aperture: The opening for the nose visible on a skull. Often pear- or heart-shaped.
Occipital bun: A prominent bulge or projection on the back of the skull, specifically the occipital bone. This is a feature present only on Neanderthal skulls.
Ochre: A natural clay pigment mixed with ferric oxide and clay and sand. Ranges in color from brown to red to orange.
Retracted face: A face that is flatter.
Retromolar gap: A space behind the last molar and the end of the jaw. This is a feature present only on Neanderthals. It also occurs through cultural modification in modern humans who have had their third molars, or wisdom teeth, removed.
About the Authors
 Amanda Wolcott Paskey, M.A.
Amanda Wolcott Paskey, M.A.
Cosumnes River College, paskeya@crc.losrios.edu
Amanda Wolcott Paskey is an anthropology professor at Cosumnes River College in Sacramento, California. She earned her B.A. and M.A. in anthropology from the University of California, Davis. Her speciality in anthropology is archaeology; however, she was trained in a holistic program and most of her teaching load is in biological anthropology. She is currently working on analyzing a post–gold rush era archaeological site, in Sacramento, with colleagues and students. This project has given her many opportunities to engage in sharing archaeology with a public audience, including local school children and Sacramentans interested in local history.
 AnnMarie Beasley Cisneros, M.A.
AnnMarie Beasley Cisneros, M.A.
American River College, beaslea@arc.losrios.edu
AnnMarie Beasley Cisneros is an anthropology professor at American River College in Sacramento, California. Trained as a four-field anthropologist, she earned her B.A. and M.A. in anthropology from California State University, Sacramento. She regularly teaches biological anthropology, among other courses, and is currently engaged in applied anthropology work in community development with historically underserved communities. She most recently has particularly enjoyed facilitating her students’ involvement in projects serving Sacramento’s Latino and immigrant Mexican populations.
For Further Exploration
Anne and Bernard Spitzer Hall of Human Origins—American Museum of Natural History.
“Dawn of Humanity,” PBS documentary, 2015
“DNA Clues to Our Inner Neanderthal,” TED Talk by Svante Pääbo, 2011.
“The Dirt” Podcast, Episode 30, “The Human Family Tree (Shrub? Crabgrass? Tumbleweed?), Part 3: Very Humany Indeed”.
Frank, Rebecca. 2021. “The Genus Homo.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Hobbits on Flores, Indonesia - Smithsonian Human Origins.
Lumping or Splitting in the Fossil Record - UC Berkeley Understanding Evolution.
Neandertals and More - Max Planck Institute for Evolutionary Anthropology.
Neanderthals: Body of Evidence - SAPIENS.
Perash, Rose L., and Kristen A. Broehl. 2021. “Hominin Review: Evolutionary Trends.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Perkl, Bradley. “Brain, Language, Lithics.” In Explorations: Lab and Activity Manual, edited by. Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. CC BY-NC. Arlington, VA: American Anthropological Association.
Shanidar 3 - Neanderthal Skeleton - Smithsonian Human Origins.
Species - Smithsonian Human Origins.
Smithsonian Human Origins Program Facebook page (@smithsonian.humanorigins).
Paleoartist Brings Human Evolution to Life - Elisabeth Daynés.
References
Adler, Daniel S., Timothy J. Prindiville, and Nicholas J. Conard. 2003. “Patterns of Spatial Organization and Land Use During the Eemian Interglacial in the Rhineland: New Data from Wallertheim, Germany.” Eurasian Prehistory 1(2): 25–78.
Alex, Bridget. 2018. “Neanderthal Brains: Bigger, Not Necessarily Better.” Discover, September 21, 2018. https://www.discovermagazine.com/planet-earth/neanderthal-brains-bigger-not-necessarily-better.
Ashton, Nick M. 2002. “Absence of Humans in Britain during the Last Interglacial Period (Oxygen Isotope Stage 5e).” Publications du CERP 8: 93–103.
Berger, Lee R., John Hawks, Darryl J. de Ruiter, Steven E. Churchill, Peter Schmid, Lucas K. Delezene, Tracy L. Kivell, et al. 2015. “Homo naledi, a New Species of the Genus Homo from the Dinaledi Chamber, South Africa.” eLife 4:e09560. https://doi.org/10.7554/eLife.09560.
Berger, Thomas D., and Erik Trinkaus. 1995. “Patterns of Trauma among the Neanderthals.” Journal of Archaeological Science 22 (6): 841–852.
Blangero, J., E.E. Quillen, M.A. Almeida, D.R. McKay, J.M. Peralta, S. Williams-Blangero, J.E. Curran, R. Duggirala, D.C. Glahn. 2014. “Genomic Reconstruction of Neandertal Brain Structure and Function.” Paper presented at the American Association of Physical Anthropologists Annual Meeting, Calgary, Alberta, Canada, 12 April 2014.
Bocherens, Hervé, Daniel Billiou, André Mariotti, Marylène Patou-Mathis, Marcel Otte, Dominique Bonjean, and Michel Toussaint. 1999. “Palaeoenvionmental and Palaeodietary Implications of Isotopic Biogeochemistry of Last Interglacial Neanderthal and Mammal Bones in Scladina Cave (Belgium).” Journal of Archaeological Science 26 (6): 599–607.
Bocquet-Appel, Jean-Pierre and Anna Degioanni. 2013. “Neanderthal Demographic Estimates.” Current Anthropology 54 (S8): S202–S213.
Boettger, Tatjana, Elena Yu. Novenko, Andrej A. Velichko, Olga K. Borisova, Konstantin V. Kremenetski, Stefan Knetsch, and Frank W. Junge. 2009. “Instability of Climate and Vegetation Dynamics in Central and Eastern Europe during the Final Stage of the Last Interglacial (Eemian, Mikulino) and Early Glaciation.” Quaternary International 207 (1-2): 137–144. https://doi.org/10.1016/j.quaint.2009.05.006.
Brophy, Juliet K., Marina C. Elliott, Darryl J. De Ruiter, Debra R. Bolter, Steven E. Churchill, Christopher S. Walker, John Hawks, and Lee R. Berger. 2021. “Immature Hominin Craniodental Remains from a New Locality in the Rising Star Cave System, South Africa.” PaleoAnthropology 1: 1–14. https://doi.org/10.48738/2021.iss1.64.
Browning, Sharon R., Brian L. Browning, Ying Zhou, Serena Tucci, and Josh M. Akey. 2018. “Analysis of Human Sequence Data Reveals Two Pulses of Archaic Denisovan Admixture.” Cell 173 (1): 53–61.
Chen, Fahu, Frido Welker, Chuan-Chou Shen, Shara E. Bailey, Inga Bergmann, Simon Davis, Huan Xia, et al. 2019. “A Late Middle Pleistocene Denisovan Mandible from the Tibetan Plateau.” Nature 569: 409–412. https://doi.org/10.1038/s41586-019-1139-x.
Churchill, Steven E. 1998. “Cold Adaptations, Heterochrony, and Neanderthals.” Evolutionary Anthropology 7 (2): 46–60.
Churchill, Steven E. 2006. “Bioenergetic Perspective on Neanderthal Thermoregulatory and Activity Budgets.” In Neanderthals Revisited: New Approaches and Perspectives, edited by K. Havarti and T. Harrison, pp. 113–134. Dordrecht, Germany: Springer.
Churchill, Steven E., Robert G. Franciscus, Hilary A. McKean-Peraza, Julie A. Daniel, and Brittany R. Warren. 2009. “Shanidar 3 Neanderthal Rib Puncture Wound and Paleolithic Weaponry.” Journal of Human Evolution 57 (2): 163–178.
Cook, Rebecca W., Antonio Vazzana, Rita Sorrentino, Stefano Benazzi, Amanda L. Smith, David S. Strait, and Justin A. Ledogar. 2021. “The Cranial Biomechanics and Feeding Performance of Homo floresiensis.” Interface Focus 11 (5). https://doi.org/10.1098/rsfs.2020.0083.
Dawson, James E., and Erik Trinkaus. 1997. “Vertebral Osteoarthritis of La Chapelle-Aux-Saints Neanderthal.” Journal of Archaeological Science 24 (11): 1015-1021. https://doi.org/10.1006/jasc.1996.0179.
Defleur, Alban R., and Emmanuel Desclaux. 2019. “Impact of the Last Interglacial Climate Change on Ecosystems and Neanderthals Behavior at Baume Moula-Guercy, Ardèche, France.” Journal of Archaeological Science 104: 114–124. https://doi.org/10.1016/j.jas.2019.01.002.
Degano, Ilaria, Sylvain Soriano, Paola Villa, Luca Pollarolo, Jeannette J. Lucejko, Zenobia Jacobs, Katerina Douka, Silvana Vitagliano, and Carlo Tozzi. 2019. “Hafting of Middle Paleolithic Tools in Latium (Central Italy): New Data from Fossellone and Sant’Agostino Caves.” PLoS ONE 14(10): 1–29. https://doi.org/10.1371/journal.pone.0213473.
Degioanni Anna, Chritophe Bonenfant, Sandrine Cabut, and Silvana Condemi. 2019. Living on the Edge: Was Demographic Weakness the Cause of Neanderthal Demise? PLoS ONE 14 (5): e0216742.
https://doi.org/10.1371/journal.pone.0216742.
Delpiano, Davide, Kristin Heasley, and Marci Peresani. 2018. “Assessing Neanderthal Land Use and Lithic Raw Material Management in Discoid Technology.” Journal of Anthropological Sciences 96: 89–110. https://doi.org/10.4436/jass.96006.
Demeter, Fabrice, Clément Zanolli, Kira E. Westaway, Renaud Joannes-Boyau, Phillippe Duringer, Mike W. Morley, Frido Welker, et al. 2022. “A Middle Pleistocene Denisovan Molar from the Annamite Chain of Northern Laos.” Nature Communications 13 (1): 2557. https://doi.org/10.7554/eLife.24231.
Dirks, Paul H. G. M., Eric M. Roberts, Hannah Hilbert-Wolf, Jan D. Kramers, John Hawks, Anthony Dosseto, Mathieu Duval, et al. 2017. “The Age of Homo naledi and Associated Sediments in the Rising Star Cave, South Africa.” eLife 6: e24231. https://doi.org/10.7554/eLife.24231.
el-Showk, Sedeer. 2019. “Neanderthal Clues to Brain Evolution in Humans.” Nature 571: S10–S11. https://doi.org/10.1038/d41586-019-02210-6.
Esteban, Irene, Curtis W. Marean, Erich C. Fisher, Panagiotis Karkanas, Dan Carbanes, and Rosa M. Albert. 2018. “Phytoliths as an Indicator of Early Modern Humans’ Plant-Gathering Strategies, Fire, Fuel, and Site-Occupation Intensity During the Middle Stone Age at Pinnacle Point 5-6 (South Coast, South Africa).” PLoS One 13 (6): 1–33.
Evteev, Andrej A., Alla A. Movsesian, and Alexandra N. Grosheva. 2017. “The Association between Mid-facial Morphology and Climate in Northeast Europe Differs from That in North Asia: Implications for Understanding the Morphology of Late Pleistocene Homo sapiens.” Journal of Human Evolution 107: 36–48. https://doi.org/10.1016/j.jhevol.2017.02.008.
Frayer, David W., Marina Lozano, Jose M. Bermudez de Castro, Eudald Carbonell, Juan-Luis Arsuaga, Jakov Radovcic, Ivana Fiore, and Luca Bondioli. 2012. “More Than 500,000 Years of Right-handedness in Europeans.” Laterality 17 (1): 51–69. https://doi.org/10.1080/1357650X.2010.529451.
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216. https://doi.org/10.1038/nature14558.
Gaudzinski-Windheuser, Sabine, Elizabeth S. Noack, Eduard Pop, Constantin Herbst, Johannes Pfleging, Jonas Buchli, Arne Jacob, et al. 2018. “Evidence for Close-Range Hunting by Last Interglacial Neanderthals.” Nature Ecology and Evolution 2: 1087–1092. https://doi.org/10.1038/s41559-018-0596-1.
Gilpin, William., Marcus W. Feldman, and Kenichi Aoki. 2016. “An Ecocultural Model Predicts Neanderthal Extinction through Competition with Modern Humans.” Proceedings of the National Academy of Sciences 113 (8): 2134–2139. https://doi.org/10.1073/pnas.1524861113.
Golovanova, Liubov. Vitaliena, Vladimir B. Doronichev, Naomi E. Cleghorn, Marianna A. Koulkova, Tatiana V. Sapelko, and M. Steven Shackley. 2010. “Significance of Ecological Factors in the Middle to Upper Paleolithic Transition.” Current Anthropology 51 (5): 655–691. https://doi.org.10.1086/656185.
Harrold, Francis B. 1980. “A Comparative Analysis of Eurasian Paleolithic Burials.” World Archaeology 12 (2): 195–211.
Hawks, John, Marina Elliott, Peter Schmid, Steven E. Churchill, Darryl J. de Ruiter, Eric M. Roberts, Hannah Hilbert-Wolf, et al. 2017. "New Fossil Remains of Homo naledi from the Lesedi Chamber, South Africa.” eLife 6: e24232. https://doi.org/10.7554/eLife.24232.
Henry, Amanda G., Alison S. Brooks, and Dolores R. Piperno. 2010. “Microfossils and Calculus Demonstrate Consumption of Plants and Cooked Foods in Neanderthal Diets (Shanidar III, Iraq; Spy I and II, Belgium).” Proceedings of the National Academy of Sciences USA 108 (2): 486–491. https://doi.org/10.1073/pnas.1016868108.
Houldcroft, Charlotte J., and Simon J. Underdown. 2016. “Neanderthal Genomics Suggests a Pleistocene Time Frame for the First Epidemiologic Transition.” American Journal of Physical Anthropology 160 (3): 379–388.
Huerta-Sánchez, Emilia, Xin Jin, Asan, Zhuoma Bianba, Benjamin M. Peter, Nicolas Vinckenbosch, Yu Liang, et al. 2014. “Altitude Adaptation in Tibetans Caused by Introgression of Denisovan-like DNA.” Nature 512 (7513): 194-197.
Jaouen, Klervia, Michael P. Richards, and Adeline Le Cabec. 2019. “Exceptionally High δ15N Values in Collagen Single Amino Acids Confirm Neanderthals as High-Trophic Level Carnivores.” Proceedings of the National Academy of Sciences 116 (11): 4928–4933. https://doi.org/10.1073/pnas.1814087116.
Kandel, Andrew W., Michael Bolus, Knut Bretzke, Angela A. Bruch, Miriam N. Haidle, Christine Hertler, and Michael Märker. 2015. “Increasing Behavioral Flexibility? An Integrative Macro-Scale Approach to Understanding the Middle Stone Age of Southern Africa.” Journal of Archaeological Method and Theory 23: 623–668. https://doi.org/10.1007/s10816-015-9254-y.
Kortlandt, Adriaan. 2002. “Neanderthal Anatomy and the Use of Spears.” Evolutionary Anthropology 11 (5): 183–184.
Kruger, A., P. Randolph-Quinney, and M. Elliott. 2016. “Multimodal Spatial Mapping and Visualization of Dinaledi Chamber and Rising Star Cave.” South African Journal of Science 112 (5–6). https://doi.org/10.17159/sajs.2016/20160032.
Lieberman, Philip, and Edmund S. Crelin. 1971. “On the Speech of Neanderthal Man.” Linguistic Inquiry 2 (2): 203–222.
Mendez, Fernando L., G. David Poznik, Sergi Castellano, and Carlos D. Bustamante. 2016. “The Divergence of Neanderthal and Modern Human Y Chromosomes.” American Journal of Human Genetics 98 (4): 728–734.
Mora-Bermúdez, Felipe, Philipp Kanis, Dominik Macak, Jula Peters, Ronald Naumann, Lei Xing, Mihail Sarov, et al. 2022. “Longer Metaphase and Fewer Chromosome Segregation Errors in Modern Human Than Neanderthal Brain Development.” Science Advances 8 (30). https://doi.org/10.1126/sciadv.abn7702.
Nicholson, Christopher M. 2017. “Eemian Paleoclimate Zones and Neanderthal Landscape Use: A GIS Model of Settlement Patterning during the Last Interglacial.” Quaternary International 438 (B): 144–157. https://doi.org/10.1016/j.quaint.2017.04.023.
Nobel Prize, The. 2022. “Press Release: The Nobel Prize in Physiology or Medicine 2022.” Nobelförsamlingen The Nobel Assembly at Karolinska Institutet. Press release, November 3, 2022. https://www.nobelprize.org/prizes/medicine/2022/press-release/.
Nowell, April. 2016. “Childhood, Play and the Evolution of Cultural Capacity in Neanderthals and Modern Humans.” In The Nature of Culture: Based on an Interdisciplinary Symposium “The Nature of Culture,” Tübingen, Germany, edited by M. N. Haidle, N. J. Conard, and M. Bolus, 87–97. Heidelberg, Germany: Springer. https://doi.org.10.1007/978-94-017-7426-0_9.
Parkington, John. 2003. “Middens and Moderns: Shellfishing and the Middle Stone Age of the Western Cape, South Africa.” South African Journal of Science 99 (5–6): 243–274.
Pearce, Eiluned, Chris Stringer, and R. I. M. Dunbar. 2013. “New Insights into Differences in Brain Organizations between Neanderthals and Anatomically Modern Humans.” Proceedings of the Royal Society B: Biological Sciences 280 (1758): 20130168. https://doi.org/10.1098/rspb.2013.0168.
Pomeroy, Emma, Paul Bennett, Chris O. Hunt, Tim Reynolds, Lucy Farr, Marine Frouin, James Holman, Ross Lane, Charles French, and Graeme Barker. 2020. “New Neanderthal Remains Associated with the ‘Flower Burial’ at Shanidar Cave.” Antiquity 94 (373): 11–26. https://doi.org/10.15184/aqy.2019.207.
Rae, Todd, Thomas Koppe, and Chris B. Stringer. 2011. “The Neanderthal Face Is Not Cold-Adapted.” Journal of Human Evolution 60 (2): 234–239. https://doi.org/10.1016/j.jhevol.2010.10.003.
Reich, David, Richard E. Green, Martin Kircher, Johannes Krause, Nick Patterson, Eric Y. Durand, Bence Viola, et al. 2010. “Genetic History of an Archaic Hominin Group from Denisova Cave in Siberia.” Nature 468: 1053–1060. https://doi.org/10.1038/nature09710.
Richards, Michael P., Paul B. Pettit, Erik Trinkaus, Fred H. Smith, Maja Paunović, and Ivor Karavanić. 2000. “Neanderthal Diet at Vindija and Neanderthal Predation: The Evidence from Stable Isotopes.” Proceedings of the National Academy of Sciences 97 (13): 7663–7666.
https://doi.org/10.1073/pnas.120178997.
Richter, Jürgen, 2006. “Neanderthals in Their Landscape: Neanderthals in Europe.” In Proceedings of the International Conference held in the Gallo-Roman Museum in Tongeren, ERAUL 117, edited by B. Demarsin and M. Otte, 17–32. Köln: Universität zu Köln.
Roksandic, Mirjana, Predrag Radovic, Xiu-Jie Wu, and Christopher J. Bae. 2021. “Resolving the ‘Muddle in the Middle’: The Case for Homo bodoensis.” Evolutionary Anthropology: Issues, News, and Reviews, 31 (1). https://doi.org/10.1002/evan.21929.
Slon, Viviane, Fabrizio Mafessoni, Benjamin Vernot, Cesare de Filippo, Steffi Grote, Bence Viola, Mateja Hajdinjak, et al. 2018. “The Genome of the Offspring of a Neanderthal Mother and a Denisovan Father.” Nature 561 (7721): 113–116.
Smith, Tanya M., Paul Tafforeau, Donald J. Reid, Joanne Pouech, Vincent Lazzari, John P. Zermeno, Debbi Guatelli-Steinberg, et al. 2010. “Dental Evidence for Ontogenetic Differences Between Modern Humans and Neanderthals.” Proceedings for the National Academy of Sciences 107 (49): 20923–20928.
Staubwasser, Michael, Virgil Drăguʂin, Bogdan P. Onac, Sergey Assonov, Vasile Ersek, Dirk L. Hoffman, and Daniel Veres. 2018. “Impact of Climate Change on the Transition of Neanderthals to Modern Humans in Europe.” Proceedings of the National Academy of Sciences 115 (37): 9116–9121. https://doi.org/10.1073/pnas.1808647115.
Stewart, T. D. 1977. “The Neanderthal Skeletal Remains from Shanidar Cave, Iraq: A Summary of Findings to Date.” Proceedings of the American Philosophical Society 121 (2): 121–165.
Stolarczyk, Regine E., and Patrick Schmidt. 2018. “Is Early Silcrete Heat Treatment a New Behavioural Proxy in the Middle Stone Age?” PLoS One 13 (10): 1–21.
Trinkaus, E. 1985. “Pathology and Posture of the La-Chapelle-aux-Saints Neanderthal.” American Journal of Physical Anthropology 67 (1): 19–41.
Trujillo Cleber A., Edward S. Rice, Nathan K. Schaefer, Isaac A. Chaim, Emily C. Wheeler, Assael A. Madrigal, Justin Buchanan, et al. 2021. “Reintroduction of the Archaic Variant of NOVA1 in Cortical Organoids Alters Neurodevelopment.” Science 371 (6530): eaax2537. https://doi.org/10.1126/science.aax2537.
Tucci, Serena, Samuel H. Vohr, Rajiv C. McCoy, Benjamin Vernot, Matthew R. Robinson, Chiara Barbieri, Brad J. Nelson, et al. 2018. “Evolutionary History and Adaptation of a Human Pygmy Population of Flores Island, Indonesia.” Science 361 (6401): 511–516.
Van Andel, T. H., and P. C. Tzedakis. 1996. “Paleolithic Landscapes of Europe and Environs, 150,000–25,000 Years Ago: An Overview.” Quaternary Science Reviews 15 (5–6): 481–500.
Venner, Stephen J. 2018. “A New Estimate for Neanderthal Energy Expenditure.” CUNY Academic Works.
Vernot, Benjamin, Serena Tucci, Janet Kelso, Joshua G. Schraiber, Aaron B. Wolf, Rachel M. Gittelman, Michael Danneman, et al. 2016. “Excavating Neanderthal and Denisovan DNA from the Genomes of Melanesian Individuals.” Science 352 (6282): 235–239.
Weaver, T. D., and J. Hublin. 2009. “Neanderthal Birth Canal Shape and the Evolution of Human Childbirth.” Proceedings of the National Academy of Sciences 106 (20): 8151–8156.
Wiẞing, Christoph, Hélène Rougier, Isabelle Crevecoer, Mietje Germonpré, Yuichi Naito, Patrick Semal, and Hervé Bocherens. 2015. “Isotopic Evidence for Dietary Ecology of Late Neanderthals in Northwestern Europe.” Quaternary International 411 (A): 327–345. https://doi.org/10.1016/j.quaint.2015.09.091.
Wong, Kate. 2015. “Neanderthal Minds.” Scientific American (January): 312(2): 36-43. https://doi.org/10.1038/scientificamerican0215-36.
Zhang, X. L., B. B. Ha, S. J. Wang, Z. J. Chen, J. Y. Ge, H. Long, W. He, et al. 2018. “The Earliest Human Occupation of the High-Altitude Tibetan Plateau 40 Thousand to 30 Thousand Years Ago.” Science 362 (6418): 1049–1051. https://doi.org/10.1126/sciadv.add5582.
Zilhão João, Diego E. Angelucci, Ernestina Badal-García, Francesco d'Errico, Floréal Daniel, Laure Dayet, Katerina Douka, et al. 2010. “Symbolic Use of Marine Shells and Mineral Pigments by Iberian Neandertals.” Proceedings of the National Academy of Sciences 107 (3): 1023–1028. https://doi.org/10.1073/pnas.0914088107.
Zollikofer, Christopher Peter Edwards, and Marcia Silvia Ponce de León. 2013. “Pandora’s Growing Box: Inferring the Evolution and Development of Hominin Brains from Endocasts.” Evolutionary Anthropology 22 (1): 20–33. https://doi.org/10.1002/evan.21333.
Acknowledgments
The authors would like to extend their thanks to Cassandra Gilmore and Anna Goldfield for thoughtful and insightful suggestions on the first edition of this chapter.
Keith Chan, Ph.D., Grossmont-Cuyamaca Community College District and MiraCosta College
This chapter is a revision from "Chapter 12: Modern Homo sapiens” by Keith Chan. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Identify the skeletal and behavioral traits that represent modern Homo sapiens.
- Critically evaluate different types of evidence for the origin of our species in Africa and our expansion around the world.
- Understand how the human lifestyle changed when people transitioned from foraging to agriculture.
- Hypothesize how human evolutionary trends may continue into the future.
The walls of a pink limestone cave in the hillside of Jebel Irhoud jutted out of the otherwise barren landscape of the Moroccan desert (Figure 12.1). Miners had excavated the cave in the 1960s, revealing some fossils. In 2007, a re-excavation of the site became a momentous occasion for science. A fossil cranium unearthed by a team of researchers was barely visible to the untrained eye. Just the fossil’s robust brows were peering out of the rock. This research team from the Max Planck Institute for Evolutionary Anthropology was the latest to explore the ancient human presence in this part of North Africa after a find by miners in 1960. Excavating near the first discovery, the researchers wanted to learn more about how Homo sapiens lived far from East Africa, where we thought our species originated.

The scientists were surprised when they analyzed the cranium, named Irhoud 10, and other fossils. Statistical comparisons with other human crania concluded that the Irhoud face shapes were typical of recent modern humans while the braincases matched ancient modern humans. Based on the findings of other scientists, the team expected these modern Homo sapiens fossils to be around 200,000 years old. Instead, dating revealed that the cranium had been buried for around 315,000 years.
Together, the modern-looking facial dimensions and the older date reshaped the interpretation of our species: modern Homo sapiens. Some key evolutionary changes from the archaic Homo sapiens (described in Chapter 11) to our species today happened 100,000 years earlier than we had thought and across the vast African continent rather than concentrated in its eastern region.
This revelation in the study of modern Homo sapiens is just one of the latest in this continually advancing area of biological anthropology. Researchers today are still discovering amazing fossils and ingenious ways to collect data and test hypotheses about our past. Through the collective work of many scientists, we are building an overall theory of modern human origins. In this chapter, we will first cover the skeletal changes from archaic Homo sapiens to modern Homo sapiens. Next, we will track how modern Homo sapiens expanded around the world. Lastly, we will cover the development of agriculture and how it changed human culture.
Defining Modernity
What defines modern Homo sapiens when compared to archaic Homo sapiens? Modern humans, like you and me, have a set of derived traits that are not seen in archaic humans or any other hominin. As with other transitions in hominin evolution, such as increasing brain size and bipedal ability, modern traits do not appear fully formed or all at once. In other words, the first modern Homo sapiens was not just born one day from archaic parents. The traits common to modern Homo sapiens appeared in a mosaic manner: gradually and out of sync with one another. There are two areas to consider when tracking the complex evolution of modern human traits. One is the physical change in the skeleton. The other is behavior inferred from the size and shape of the cranium and material culture evidence.
Skeletal Traits
The skeleton of modern Homo sapiens is less robust than that of archaic Homo sapiens. In other words, the modern skeleton is gracile, meaning that the structures are thinner and smoother. Differences related to gracility in the cranium are seen in the braincase, the face, and the mandible. There are also broad differences in the rest of the skeleton.
Cranial Traits
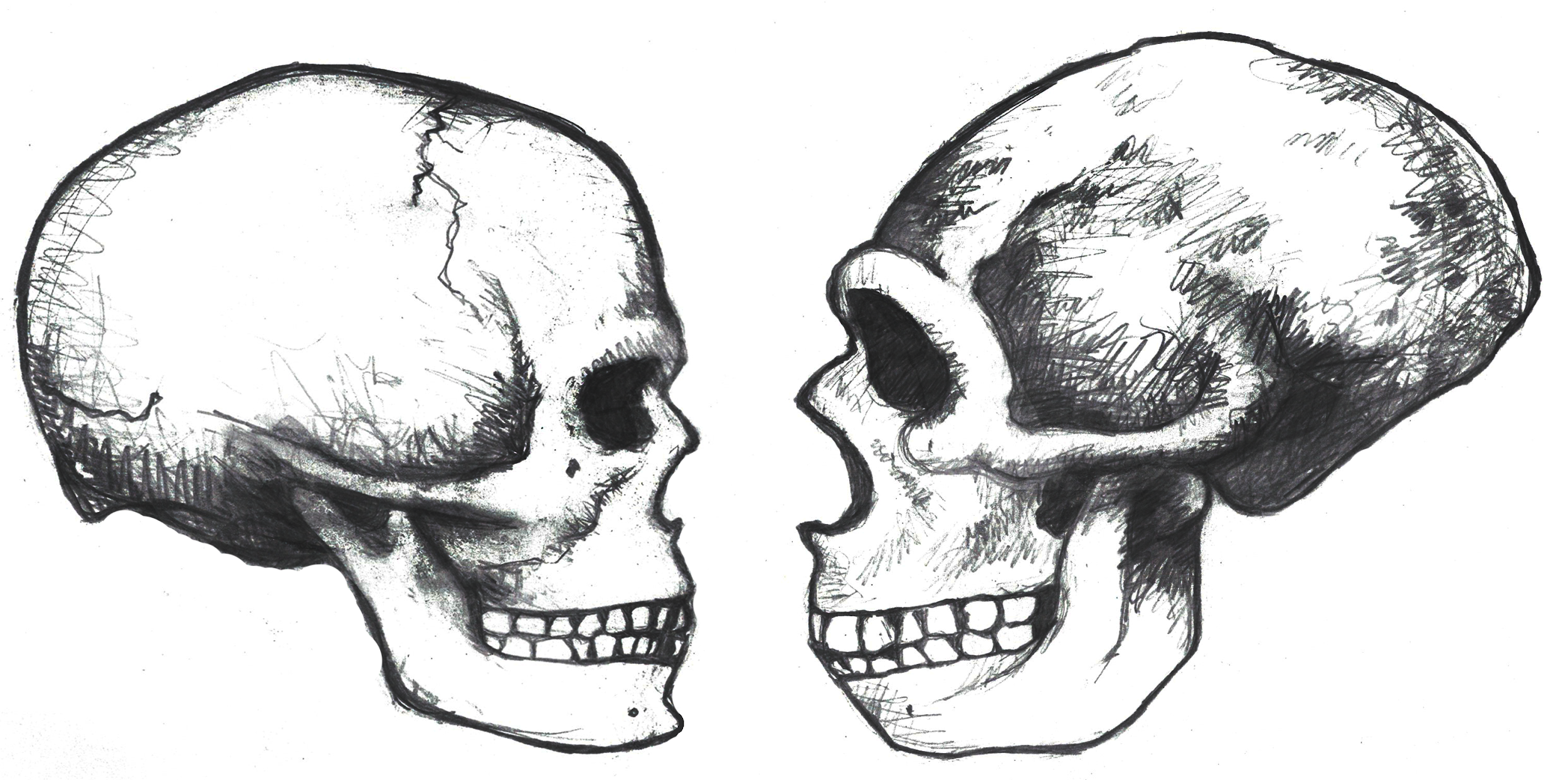
Several elements of the braincase differ between modern and archaic Homo sapiens. Overall, the shape is much rounder, or more globular, on a modern skull (Lieberman, McBratney, and Krovitz 2002; Neubauer, Hublin, and Gunz 2018; Pearson 2008; Figure 12.2). You can feel the globularity of your own modern human skull. Feel the height of your forehead with the palm of your hand. Viewed from the side, the tall vertical forehead of a modern Homo sapiens stands out when compared to the sloping archaic version. This is because the frontal lobe of the modern human brain is larger than the one in archaic humans, and the skull has to accommodate the expansion. The vertical forehead reduces a trait that is common to all other hominins: the brow ridge or supraorbital torus. The parietal lobes of the brain and the matching parietal bones on either side of the skull both bulge outward more in modern humans. At the back of the skull, the archaic occipital bun is no longer present. Instead, the occipital region of the modern human cranium has a derived tall and smooth curve, again reflecting the globular brain inside.
The trend of shrinking face size across hominins reaches its extreme with our species as well. The facial bones of a modern Homo sapiens are extremely gracile compared to all other hominins (Lieberman, McBratney, and Krovitz 2002). Continuing a trend in hominin evolution, technological innovations kept reducing the importance of teeth in reproductive success (Lucas 2007). As natural selection favored smaller and smaller teeth, the surrounding bone holding these teeth also shrank.
Related to smaller teeth, the mandible is also gracile in modern humans when compared to archaic humans and other hominins. Interestingly, our mandibles have pulled back so far from the prognathism of earlier hominins that we gained an extra structure at the most anterior point, called the mental eminence. You know this structure as the chin. At the skeletal level, it resembles an upside-down “T” at the centerline of the mandible (Pearson 2008). Looking back at archaic humans, you will see that they all lack a chin. Instead, their mandibles curve straight back without a forward point. What is the chin for and how did it develop? Flora Gröning and colleagues (2011) found evidence of the chin’s importance by simulating physical forces on computer models of different mandible shapes. Their results showed that the chin acts as structural support to withstand strain on the otherwise gracile mandible.
Postcranial Gracility
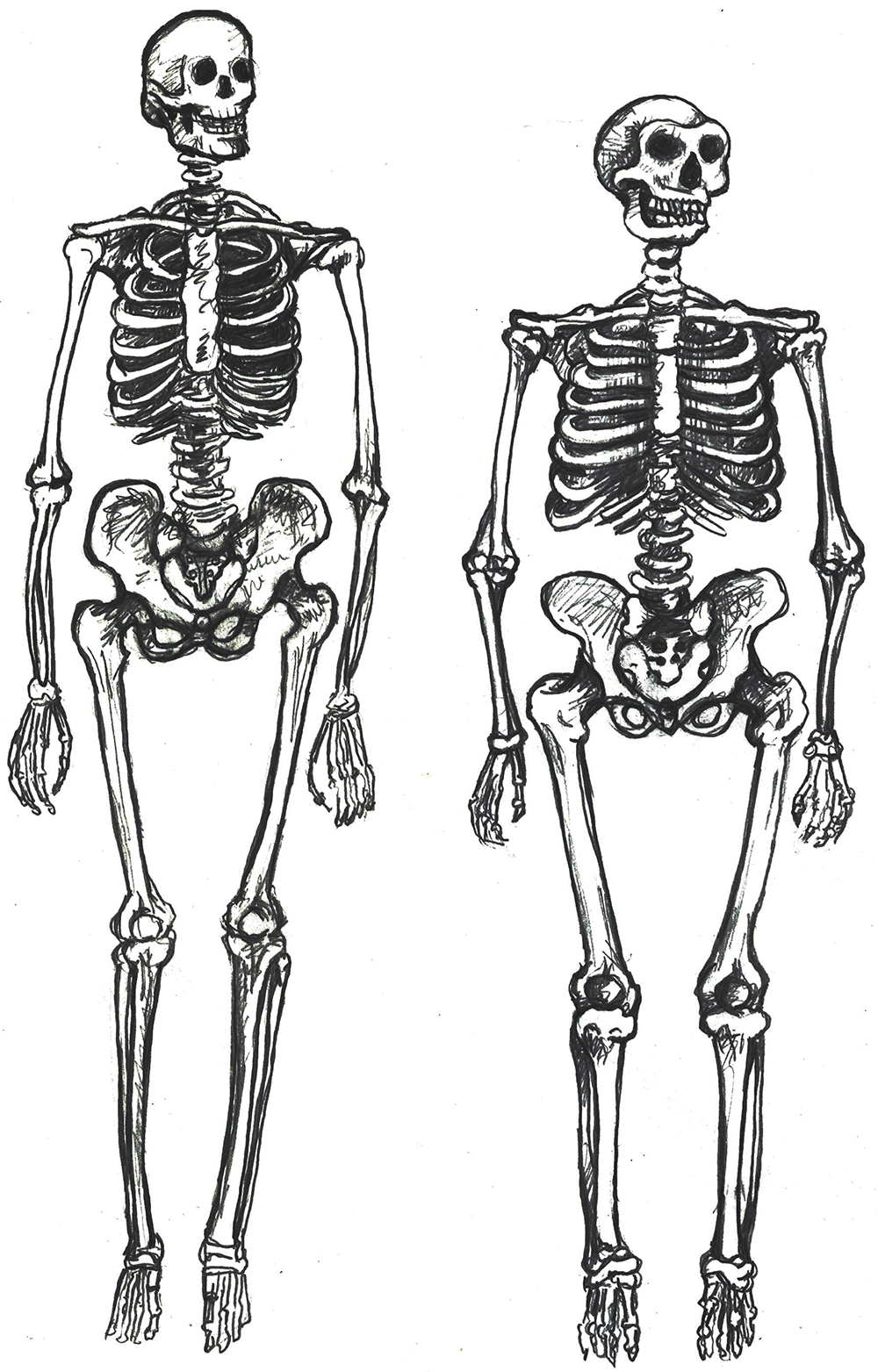
The rest of the modern human skeleton is also more gracile than its archaic counterpart. The differences are clear when comparing a modern Homo sapiens with a cold-adapted Neanderthal (Sawyer and Maley 2005), but the trends are still present when comparing modern and archaic humans within Africa (Pearson 2000). Overall, a modern Homo sapiens postcranial skeleton has thinner cortical bone, smoother features, and more slender shapes when compared to archaic Homo sapiens (Figure 12.3). Comparing whole skeletons, modern humans have longer limb proportions relative to the length and width of the torso, giving us lankier outlines.
Why is our skeleton so gracile compared to those of other hominins? Natural selection can drive the gracilization of skeletons in several ways (Lieberman 2015). A slender frame is adapted for the efficient long-distance running ability that started with Homo erectus. Furthermore, slenderness is a genetic adaptation for cooling an active body in hotter climates, which aligns with the ample evidence that Africa was the home continent of our species.
Behavioral Modernity
Aside from physical differences in the skeleton, researchers have also uncovered evidence of behavioral changes associated with increased cultural complexity from archaic to modern humans. How did cultural complexity develop? Two investigations into this question are archaeology and the analysis of reconstructed brains.
Archaeology tells us much about the behavioral complexity of past humans by interpreting the significance of material culture. In terms of advanced culture, items created with an artistic flair, or as decoration, speak of abstract thought processes (Figure 12.4). The demonstration of difficult artistic techniques and technological complexity hints at social learning and cooperation as well. According to paleoanthropologist John Shea (2011), one way to track the complexity of past behavior through artifacts is by measuring the variety of tools found together. The more types of tools constructed with different techniques and for different purposes, the more modern the behavior. Researchers are still working on an archaeological way to measure cultural complexity that is useful across time and place.

The interpretation of brain anatomy is another promising approach to studying the evolution of human behavior. When looking at investigations on this topic in modern Homo sapiens brains, researchers found a weak association between brain size and test-measured intelligence (Pietschnig et al. 2015). Additionally, they found no association between intelligence and biological sex. These findings mean that there are more significant factors that affect tested intelligence than just brain size. Since the sheer size of the brain is not useful for weighing intelligence within a species, paleoanthropologists are instead investigating the differences in certain brain structures. The differences in organization between modern Homo sapiens brains and archaic Homo sapiens brains may reflect different cognitive priorities that account for modern human culture. As with the archaeological approach, new discoveries will refine what we know about the human brain and apply that knowledge to studying the distant past.
Taken together, the cognitive abilities in modern humans may have translated into an adept use of tools to enhance survival. Researchers Patrick Roberts and Brian A. Stewart (2018) call this concept the generalist-specialist niche: our species is an expert at living in a wide array of environments, with populations culturally specializing in their own particular surroundings. The next section tracks how far around the world these skeletal and behavioral traits have taken us.
First Africa, Then the World
What enabled modern Homo sapiens to expand its range further in 300,000 years than Homo erectus did in 1.5 million years? The key is the set of derived biological traits from the last section. The gracile frame and neurological anatomy allowed modern humans to survive and even flourish in the vastly different environments they encountered. Based on multiple types of evidence, the source of all of these modern humans was Africa. Instead of originating from just one location, evidence shows that modern Homo sapiens evolution occurred in a complex gene flow network across Africa, a concept called African multiregionalism (Scerri et al. 2018).
This section traces the origin of modern Homo sapiens and the massive expansion of our species across all of the continents (except Antarctica) by 12,000 years ago. While modern Homo sapiens first shared geography with archaic humans, modern humans eventually spread into lands where no human had gone before. Figure 12.5 shows the broad routes that our species took expanding around the world. I encourage you to make your own timeline with the dates in this part to see the overall trends.
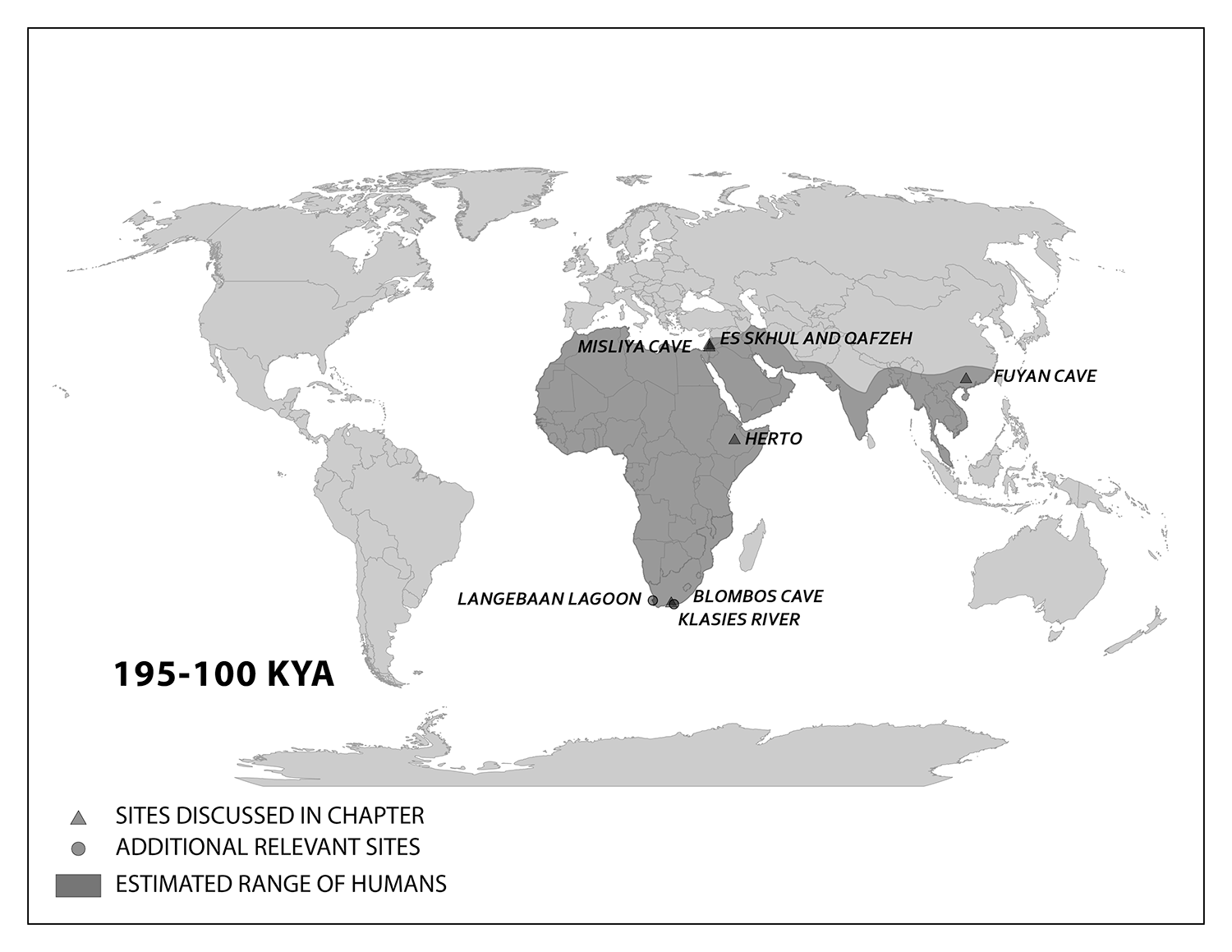
Modern Homo sapiens Biology and Culture in Africa
We start with the ample fossil evidence supporting the theory that modern humans originated in Africa during the Middle Pleistocene, having evolved from African archaic Homo sapiens. The earliest dated fossils considered to be modern actually have a mosaic of archaic and modern traits, showing the complex changes from one type to the other. Experts have various names for these transitional fossils, such as Early Modern Homo sapiens or Early Anatomically Modern Humans. However they are labeled, the presence of some modern traits means that they illustrate the origin of the modern type. Three particularly informative sites with fossils of the earliest modern Homo sapiens are Jebel Irhoud, Omo, and Herto.
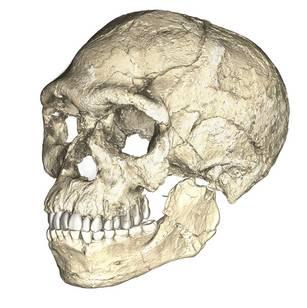
Recall from the start of the chapter that the most recent finds at Jebel Irhoud are now the oldest dated fossils that exhibit some facial traits of modern Homo sapiens. Besides Irhoud 10, the cranium that was dated to 315,000 years ago (Hublin et al. 2017; Richter et al. 2017), there were other fossils found in the same deposit that we now know are from the same time period. In total there are at least five individuals, representing life stages from childhood to adulthood. These fossils form an image of high variation in skeletal traits. For example, the skull named Irhoud 1 has a primitive brow ridge, while Irhoud 2 and Irhoud 10 do not (Figure 12.6). The braincases are lower than what is seen in the modern humans of today but higher than in archaic Homo sapiens. The teeth also have a mix of archaic and modern traits that defy clear categorization into either group.
Research separated by nearly four decades uncovered fossils and artifacts from the Kibish Formation in the Lower Omo Valley in Ethiopia. These Omo Kibish hominins were represented by braincases and fragmented postcranial bones of three individuals found kilometers apart, dating back to around 233,000 years ago (Day 1969; McDougall, Brown, and Fleagle 2005; Vidal et al. 2022). One interesting finding was the variation in braincase size between the two more-complete specimens: while the individual named Omo I had a more globular dome, Omo II had an archaic-style long and low cranium.
Also in Ethiopia, a team led by Tim White (2003) excavated numerous fossils at Herto. There were fossilized crania of two adults and a child, along with fragments of more individuals. The dates ranged between 160,000 and 154,000 years ago. The skeletal traits and stone-tool assemblage were both intermediate between the archaic and modern types. Features reminiscent of modern humans included a tall braincase and thinner zygomatic (cheek) bones than those of archaic humans (Figure 12.7). Still, some archaic traits persisted in the Herto fossils, such as the supraorbital tori. Statistical analysis by other research teams concluded that at least some cranial measurements fit just within the modern human range (McCarthy and Lucas 2014), favoring categorization with our own species.

The timeline of material culture suggests a long period of relying on similar tools before a noticeable diversification of artifacts types. Researchers label the time of stable technology shared with archaic types the Middle Stone Age, while the subsequent time of diversification in material culture is called the Later Stone Age.
In the Middle Stone Age, the sites of Jebel Irhoud, Omo, and Herto all bore tools of the same flaked style as archaic assemblages, even though they were separated by almost 150,000 years. The consistency in technology may be evidence that behavioral modernity was not so developed. No clear signs of art dating back this far have been found either. Other hypotheses not related to behavioral modernity could explain these observations. The tool set may have been suitable for thriving in Africa without further innovation. Maybe works of art from that time were made with media that deteriorated or perhaps such art was removed by later humans.
Evidence of what Homo sapiens did in Africa from the end of the Middle Stone Age to the Later Stone Age is concentrated in South African cave sites that reveal the complexity of human behavior at the time. For example, Blombos Cave, located along the present shore of the Cape of Africa facing the Indian Ocean, is notable for having a wide variety of artifacts. The material culture shows that toolmaking and artistry were more complex than previously thought for the Middle Stone Age. In a layer dated to 100,000 years ago, researchers found two intact ochre-processing kits made of abalone shells and grinding stones (Henshilwood et al. 2011). Marine snail shell beads from 75,000 years ago were also excavated (Figure 12.8; d’Errico et al. 2005). Together, the evidence shows that the Middle Stone Age occupation at Blombos Cave incorporated resources from a variety of local environments into their culture, from caves (ochre), open land (animal bones and fat), and the sea (abalone and snail shells). This complexity shows a deep knowledge of the region’s resources and their use—not just for survival but also for symbolic purposes.
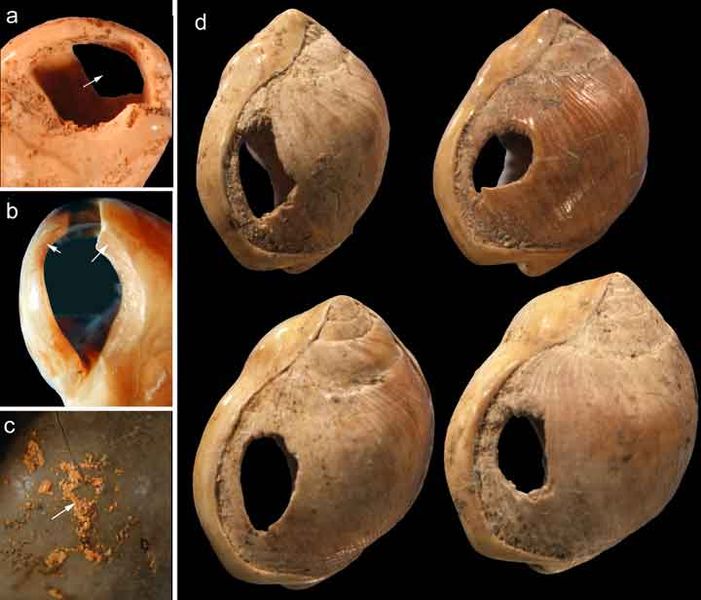
On the eastern coast of South Africa, Border Cave shows new African cultural developments at the start of the Later Stone Age. Paola Villa and colleagues (2012) identified several changes in technology around 43,000 years ago. Stone-tool production transitioned from a slower process to one that was faster and made many microliths, small and precise stone tools. Changes in decorations were also found across the Later Stone Age transition. Beads were made from a new resource: fragments of ostrich eggs shaped into circular forms resembling present-day breakfast cereal O’s (d’Errico et al. 2012). These beads show a higher level of altering one’s own surroundings and a move from the natural to the abstract in terms of design.
Summary of Modern H. sapiens in Africa
The combined fossil evidence paints a picture of diversity in geography and traits. Instead of evolving in just East Africa, the Jebel Irhoud find revealed that early modern Homo sapiens had a wide range across Middle Pleistocene Africa. Supporting this explanation, fossils have different mosaics of archaic and modern traits in different places and even within the same area. The high level of diversity from just these fossils shows that the modern traits took separate paths toward the set we have today. The connections were convoluted, involving fluctuating gene flow among small groups of regional nomadic foragers across a large continent over a long time.
African culture experienced a long constant phase called the Middle Stone Age until a faster burst of change produced innovation and new styles. The change was not one moment but rather an escalation in development. Later Stone Age culture introduced elements seen across many regions, including the construction of composite tools and even the use of strung decorations such as beads. These developments appear in the Later Stone Age of other regions, such as Europe. Based on the early date of the African artifacts, Later Stone Age culture may have originated in Africa and passed from person to person and region to region, with people adapting the general technique to their local resources and viewing the meaning in their own way.
Expansion into the Middle East and Asia
While modern Homo sapiens lived across Africa, some members eventually left the continent. These pioneers could have used two connections to the Middle East or West Asia. From North Africa, they could have crossed the Sinai Peninsula and moved north to the Levant, or eastern Mediterranean. Finds in that region show an early modern human presence. Other finds support the Southern Dispersal model, with a crossing from East Africa to the southern Arabian Peninsula through the Straits of Bab-el-Mandeb. It is tempting to think of one momentous event in which people stepped off Africa and into the Middle East, never to look back. In reality, there were likely multiple waves of movement producing gene flow back and forth across these regions as the overall range pushed east. The expanding modern human population could have thrived by using resources along the southern coast of the Arabian Peninsula to South Asia, with side routes moving north along rivers. The maximum range of the species then grew across Asia.
Modern Homo sapiens in the Middle East
Geographically, the Middle East is the ideal place for the African modern Homo sapiens population to inhabit upon expanding out of their home continent. In the Eastern Mediterranean coast of the Levant, there is a wealth of skeletal and material culture linked to modern Homo sapiens. Recent discoveries from Saudi Arabia further add to our view of human life just beyond Africa.
The Caves of Mount Carmel in present-day Israel have preserved skeletal remains and artifacts of modern Homo sapiens, the first-known group living outside Africa. The skeletal presence at Misliya Cave is represented by just part of the left upper jaw of one individual, but it is notable for being dated to a very early time, between 194,000 and 177,000 years ago (Hershkovitz et al. 2018). Later, from 120,000 to 90,000 years ago, fossils of multiple individuals across life stages were found in the caves of Es-Skhul and Qafzeh (Shea and Bar-Yosef 2005). The skeletons had many modern Homo sapiens traits, such as globular crania and more gracile postcranial bones when compared to Neanderthals. Still, there were some archaic traits. For example, the adult male Skhul V also possessed what researchers Daniel Lieberman, Osbjorn Pearson, and Kenneth Mowbray (2000) called marked or clear occipital bunning. Also, compared to later modern humans, the Mount Carmel people were more robust. Skhul V had a particularly impressive brow ridge that was short in height but sharply jutted forward above the eyes (Figure 12.9). The high level of preservation is due to the intentional burial of some of these people. Besides skeletal material, there are signs of artistic or symbolic behavior. For example, the adult male Skhul V had a boar’s jaw on his chest. Similarly, Qafzeh 11, a juvenile with healed cranial trauma, had an impressive deer antler rack placed over his torso (Figure 12.10; Coqueugniot et al. 2014). Perforated seashells colored with ochre, mineral-based pigment, were also found in Qafzeh (Bar-Yosef Mayer, Vandermeersch, and Bar-Yosef 2009).
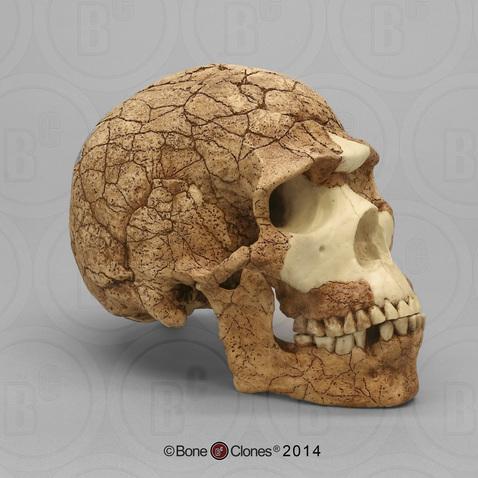

One remaining question is, what happened to the modern humans of the Levant after 90,000 years ago? Another site attributed to our species did not appear in the region until 47,000 years ago. Competition with Neanderthals may have accounted for the disappearance of modern human occupation since the Neanderthal presence in the Levant lasted longer than the dates of the early modern Homo sapiens. John Shea and Ofer Bar-Yosef (2005) hypothesized that the Mount Carmel modern humans were an initial expansion from Africa that failed. Perhaps they could not succeed due to competition with the Neanderthals who had been there longer and had both cultural and biological adaptations to that environment.
Modern Homo sapiens of China
A long history of paleoanthropology in China has found ample evidence of modern human presence. Four notable sites are the caves at Fuyan, Liujiang, Tianyuan, and Zhoukoudian. In the distant past, these caves would have been at least seasonal shelters that unintentionally preserved evidence of human presence for modern researchers to discover.
At Fuyan Cave in Southern China, paleoanthropologists found 47 adult teeth associated with cave formations dated to between 120,000 and 80,000 years ago (Liu et al. 2015). It is currently the oldest-known modern human site in China, though other researchers question the validity of the date range (Michel et al. 2016). The teeth have the small size and gracile features of modern Homo sapiens dentition.
The fossil Liujiang (or Liukiang) hominin (67,000 years ago) has derived traits that classified it as a modern Homo sapiens, though primitive archaic traits were also present. In the skull, which was found nearly complete, the Liujiang hominin had a taller forehead than archaic Homo sapiens but also had an enlarged occipital region (Figure 12.11; Brown 1999; Wu et al. 2008). Other parts of the skeleton also had a mix of modern and archaic traits: for example, the femur fragments suggested a slender length but with thick bone walls (Woo 1959).
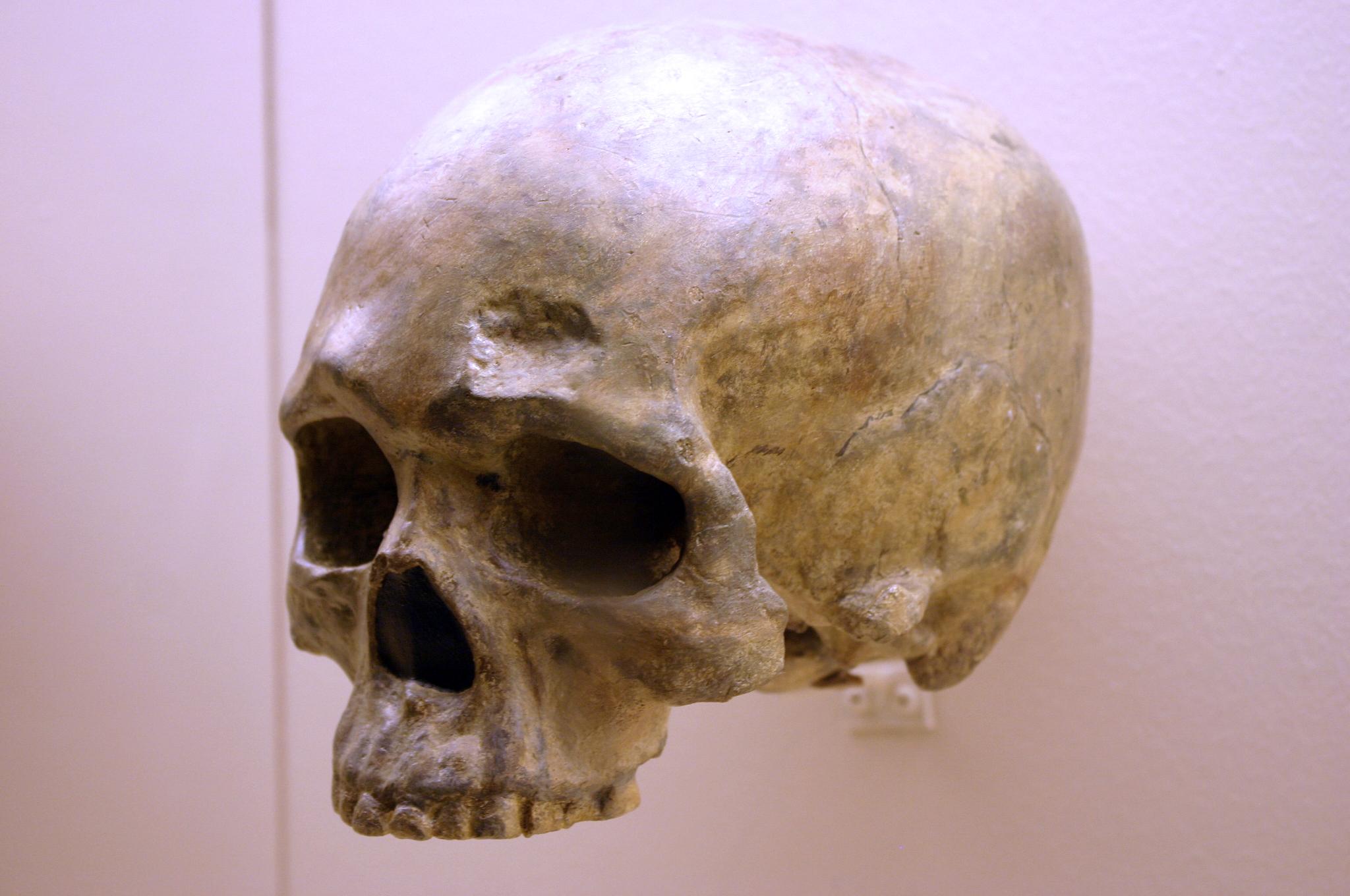
Another Chinese site to describe here is the one that has been studied the longest. In the Zhoukoudian Cave system (Figure 12.12), where Homo erectus and archaic Homo sapiens have also been found, there were three crania of modern Homo sapiens. These crania, which date to between 34,000 and 10,000 years ago, were all more globular than those of archaic humans but still lower and longer than those of later modern humans (Brown 1999; Harvati 2009). When compared to one another, the crania showed significant differences from one another. Comparison of cranial measurements to other populations past and present found no connection with modern East Asians, again showing that human variation was very different from what we see today.

Summary of Modern H. sapiens in the Middle East and Asia
As in Africa, the finds of the Middle East have shown that humans were biologically diverse and had complex relationships with their environment. Work in the Levant showed an initial expansion north from the Sinai Peninsula that did not last. Away from the Levant, expansion continued. Local resources were used to make lithics and decorative items.
The early Asian presence of modern Homo sapiens was complex and varied as befitting the massive continent. What the evidence shows is that people adapted to a wide array of environments that were far removed from Africa. From the Levant to China, humans with modern anatomy used caves that preserved signs of their presence. Faunal and floral remains found in these shelters speak to the flexibility of the human omnivorous diet as local wildlife and foliage became nourishment. Decorative items, often found as burial goods in planned graves, show a flourishing cultural life.
Eventually, modern humans at the southeastern fringe of the geographical range of the species found their way southeast until some became the first humans in Australia.
Crossing to Australia
Expansion of the first modern human Asians, still following the coast, eventually entered an area that researchers call Sunda before continuing on to modern Australia. Sunda was a landmass made up of the modern-day Malay Peninsula, Sumatra, Java, and Borneo. Lowered sea levels connected these places with land bridges, making them easier to traverse. Proceeding past Sunda meant navigating Wallacea, the archipelago that includes the Indonesian islands east of Borneo. In the distant past, there were many megafauna, large animals that migrating humans would have used for food and materials (such as utilizing animals’ hides and bones). Further southeast was another landmass called Sahul, which included New Guinea and Australia as one contiguous continent. Based on fossil evidence, this land had never seen hominins or any other primates before modern Homo sapiens arrived. Sites along this path offer clues about how our species handled the new environment to live successfully as foragers.

The skeletal remains at Lake Mungo, land traditionally owned by Mutthi Mutthi, Ngiampaa, and Paakantji peoples, are the oldest known in the continent. The now-dry lake was one of a series located along the southern coast of Australia in New South Wales, far from where the first people entered from the north (Barbetti and Allen 1972; Bowler et al. 1970). Two individuals dating to around 40,000 years ago show signs of artistic and symbolic behavior, including intentional burial. The bones of Lake Mungo 1 (LM1), an adult female, were crushed repeatedly, colored with red ochre, and cremated (Bowler et al. 1970). Lake Mungo 3 (LM3), a tall, older male with a gracile cranium but robust postcranial bones, had his fingers interlocked over his pelvic region (Brown 2000).
Kow Swamp, within traditional Yorta Yorta land also in southern Australia, contained human crania that looked distinctly different from the ones at Lake Mungo (Durband 2014; Thorne and Macumber 1972). The crania, dated between 9,000 and 20,000 years ago, had extremely robust brow ridges and thick bone walls, but these were paired with globular features on the braincase (Figure 12.13).
While no fossil humans have been found at the Madjedbebe rock shelter in the North Territory of Australia, more than 10,000 artifacts found there show both behavioral modernity and variability (Clarkson et al. 2017). They include a diverse array of stone tools and different shades of ochre for rock art, including mica-based reflective pigment (similar to glitter). These impressive artifacts are as far back as 56,000 years old, providing the date for the earliest-known presence of humans in Australia.
Summary of Modern H. sapiens in Australia
The overall view of the first modern humans in Australia from a biological perspective shows a high amount of skeletal diversity. This is similar to the trends seen earlier in Africa, the Middle East, and East Asia. The earliest-known arrivals brought with them a multifaceted suite of cultural practices as seen in their material culture.
From the Levant to Europe
The first modern human expansion into Europe occurred after other members of our species settled East Asia and Australia. As the evidence from the Levant suggests, modern human movement to Europe may have been hampered by the presence of Neanderthals. Another obstacle was that the colder climate was incompatible with the biology of African modern Homo sapiens, which was adapted for exposure to high temperature and ultraviolet radiation. Still, by 40,000 years ago, modern Homo sapiens had a detectable presence. This time was also the start of the Later Stone Age or Upper Paleolithic, when there was an expansion in cultural complexity. There is a wealth of evidence from this region due to a Western bias in research, the proximity of these findings to Western scientific institutions, and the desire of Western scientists to explore their own past. This section will cover key evidence of early modern human life in Europe, and the typologies used to view cultural changes in this region.
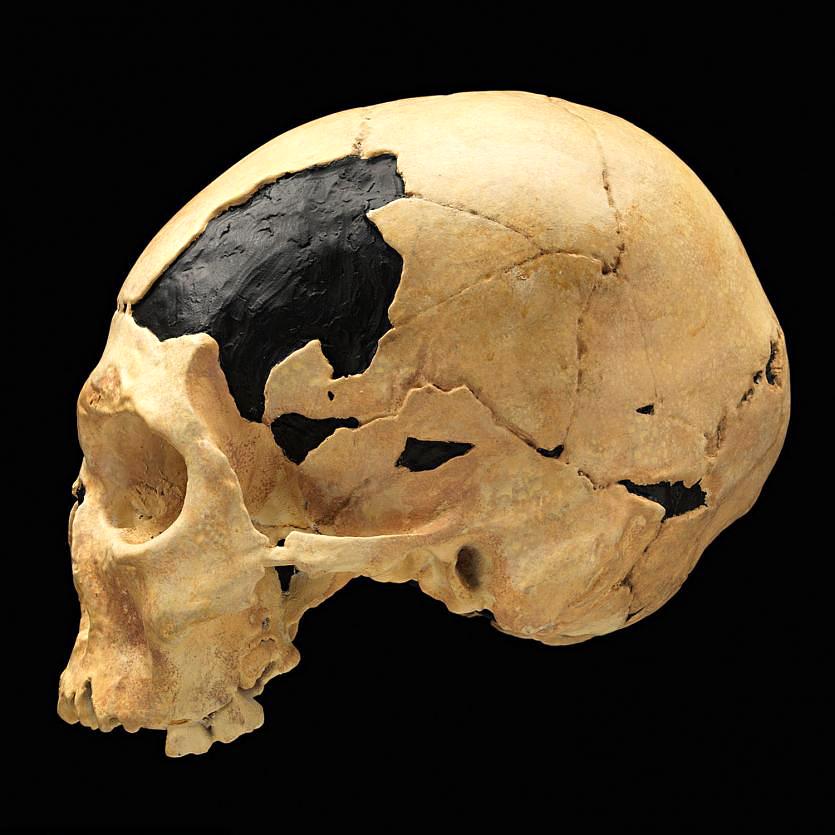
In Romania, the site of Peștera cu Oase (Cave of Bones) had the oldest-known remains of modern Homo sapiens in Europe, dated to around 40,000 years ago (Trinkaus et al. 2003a). Among the bones and teeth of many animals were the fragmented cranium of one person and the mandible of another (the two bones did not fit each other). Both bones have modern human traits similar to the fossils from the Middle East, but they also had Neanderthal traits. Oase 1, the mandible, had a mental eminence but also extremely large molars (Trinkaus et al. 2003b). This mandible has yielded DNA that surprisingly is equally similar to DNA from present-day Europeans and Asians (Fu et al. 2015). This means that Oase 1 was not the direct ancestor of modern Europeans. The Oase 2 cranium has the derived traits of reduced brow ridges along with archaic wide zygomatic cheekbones and an occipital bun (Figure 12.14; Rougier et al. 2007).
Dating to around 26,000 years ago, Předmostí near Přerov in the Czech Republic was a site where people buried over 30 individuals along with many artifacts. Eighteen individuals were found in one mass burial area, a few covered by the scapulae of woolly mammoths (Germonpré, Lázničková-Galetová, and Sablin 2012). The Předmostí crania were more globular than those of archaic humans but tended to be longer and lower than in later modern humans (Figure 12.15; Velemínská et al. 2008). The height of the face was in line with modern residents of Central Europe. There was also skeletal evidence of dog domestication, such as the presence of dog skulls with shorter snouts than in wild wolves (Germonpré, Lázničková-Galetová, and Sablin et al. 2012). In total, Předmostí could have been a settlement dependent on mammoths for subsistence and the artificial selection of early domesticated dogs.
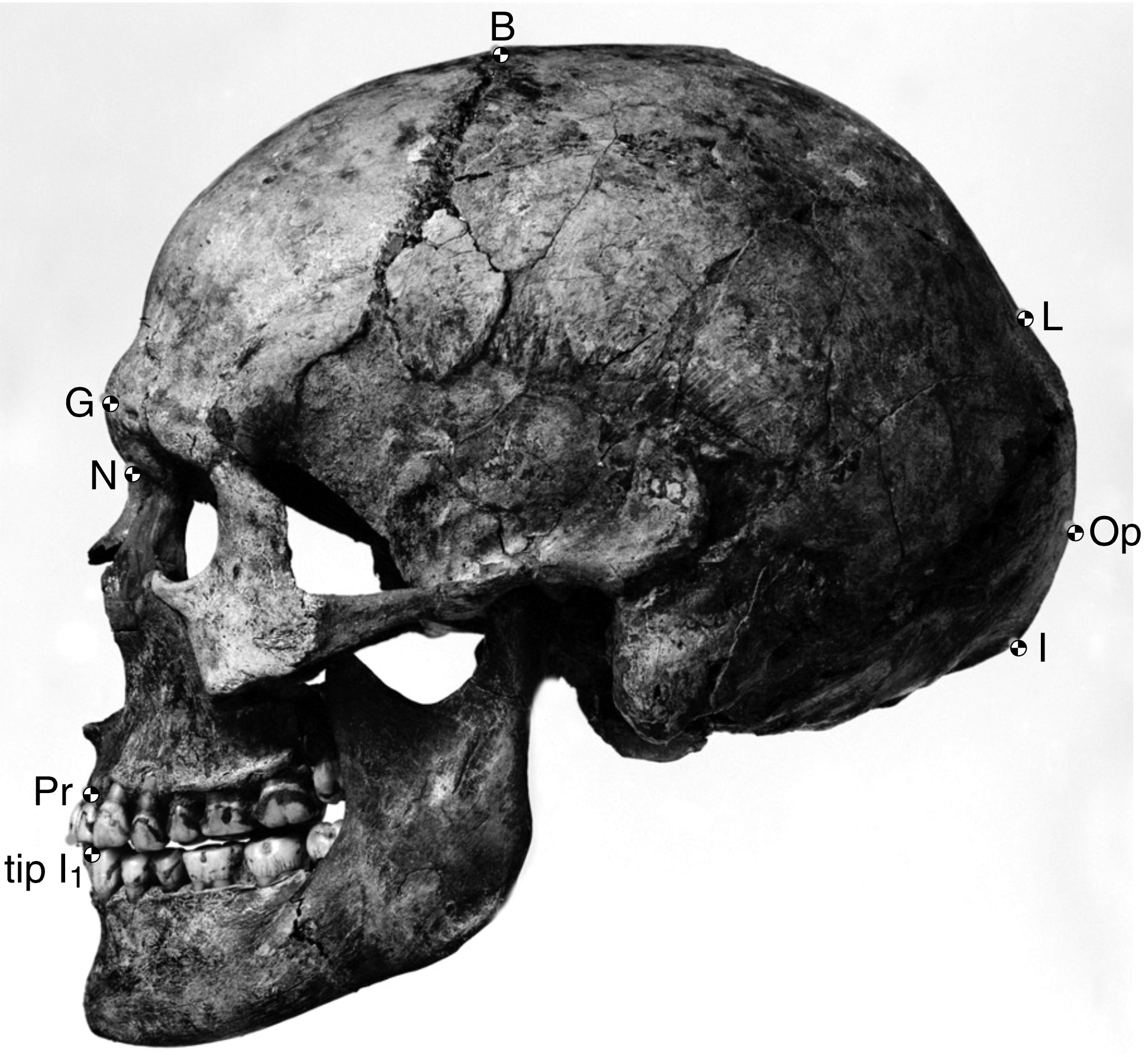
The sequence of modern Homo sapiens technological change in the Later Stone Age has been thoroughly dated and labeled by researchers working in Europe. Among them, the Gravettian tradition of 33,000 years to 21,000 years ago is associated with most of the known curvy female figurines, often assumed to be “Venus” figures. Hunting technology also advanced in this time with the first known boomerang, atlatl (spear thrower), and archery. The Magdalenian tradition spread from 17,000 to 12,000 years ago. This culture further expanded on fine bone tool work, including barbed spearheads and fishhooks (Figure 12.16).
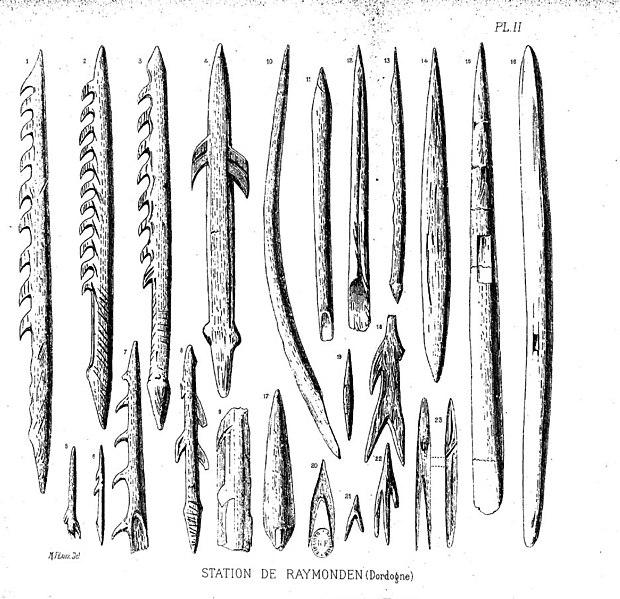
Among the many European sites dating to the Later Stone Age, the famous cave art sites deserve mention. Chauvet-Pont-d'Arc Cave in southern France dates to separate Aurignacian occupations 31,000 years ago and 26,000 years ago. Over a hundred art pieces representing 13 animal species are preserved, from commonly depicted deer and horses to rarer rhinos and owls. Another French cave with art is Lascaux, which is several thousand years younger at 17,000 years ago in the Magdalenian period. At this site, there are over 6,000 painted figures on the walls and ceiling (Figure 12.17). Scaffolding and lighting must have been used to make the paintings on the walls and ceiling deep in the cave. Overall, visiting Lascaux as a contemporary must have been an awesome experience: trekking deeper in the cave lit only by torches giving glimpses of animals all around as mysterious sounds echoed through the galleries.

Summary of Modern H. sapiens in Europe
Study of Europe in the Upper Paleolithic gives a more detailed view of the general pattern of biological and cultural change linked with the arrival of modern Homo sapiens. The modern humans experienced a rapidly changing culture that went through waves of complexity and refinement. Skeletally, the increasing globularity of the cranium and the gracility of the rest of the skeleton continued, though with unique regional traits, too. The cave art sites showed a deeper exploration of creativity though the exact meaning is unclear. With survival dependent on the surrounding ecology, painting the figures may have connected people to important and impressive wildlife at both a physical and spiritual level. Both reverence for animals and the use of caves for an enhanced sensory experience are common to cultures past and present.
Peopling of the Americas
By 25,000 years ago, our species was the only member of Homo left on Earth. Gone were the Neanderthals, Denisovans, Homo naledi, and Homo floresiensis. The range of modern Homo sapiens kept expanding eastward into—using the name given to this area by Europeans much later—the Western Hemisphere. This section will address what we know about the peopling of the Americas, from the first entry to these continents to the rapid spread of Indigenous Americans across its varied environments.
While evidence points to an ancient land bridge called Beringia that allowed people to cross from what is now northeastern Siberia into modern-day Alaska, what people did to cross this land bridge is still being investigated. For most of the 20th century, the accepted theory was the Ice-Free Corridor model. It stated that northeast Asians (East Asians and Siberians) first expanded across Beringia inland through a passage between glaciers that opened into the western Great Plains of the United States, just east of the Rocky Mountains, around 13,000 years ago (Swisher et al. 2013). While life up north in the cold environment would have been harsh, migrating birds and an emerging forest might have provided sustenance as generations expanded through this land (Potter et al. 2018).
However, in recent decades, researchers have accumulated evidence against the Ice-Free Corridor model. Archaeologist K. R. Fladmark (1979) brought the alternate Coastal Route model into the archaeological spotlight; researcher Jon M. Erlandson has been at the forefront of compiling support for this theory (Erlandson et al. 2015). The new focus is the southern edge of the land bridge instead of its center: About 16,000 years ago, members of our species expanded along the coastline from northeast Asia, east through Beringia, and south down the Pacific Coast of North America while the inland was still sealed off by ice. The coast would have been free of ice at least part of the year, and many resources would have been found there, such as fish (e.g., salmon), mammals (e.g., whales, seals, and otters), and plants (e.g., seaweed).
South through the Americas
When the first modern Homo sapiens reached the Western Hemisphere, the spread through the Americas was rapid. Multiple migration waves crossed from North to South America (Posth et al. 2018). Our species took advantage of the lack of hominin competition and the bountiful resources both along the coasts and inland. The Americas had their own wide array of megafauna, which included woolly mammoths (Figure 12.18), mastodons, camels, horses, ground sloths, giant tortoises, and—a favorite of researchers—a two-meter-tall beaver. The reason we cannot see these amazing animals today may be that resources gained from these fauna were crucial to the survival for people over 12,000 years ago (Araujo et al. 2017). Several sites are notable for what they add to our understanding of the distant past in the Americas, including interactions with megafauna and other elements of the environment.

A 2019 discovery may allow researchers to improve theories about the peopling of the Americas. In White Sands National Park, New Mexico, 60 human footprints have been astonishingly dated to around 22,000 years ago (Bennett et al. 2021). This date and location do not match either the Ice-Free Corridor or Coastal Route models. Researchers are now working to verify the find and adjust previous models to account for the new evidence. This groundbreaking find is sparking new theories; it is another example of the fast pace of research performed on our past.
Monte Verde is a landmark site that shows that the human population had expanded down the whole vertical stretch of the Americas to Chile by 14,600 years ago, only a few thousand years after humans first entered the Western Hemisphere from Alaska. The site has been excavated by archaeologist Tom D. Dillehay and his team (2015). The remains of nine distinct edible species of seaweed at the site shows familiarity with coastal resources and relates to the Coastal Route model by showing a connection between the inland people and the sea.

Named after the town in New Mexico, the Clovis stone-tool style is the first example of a widespread culture across much of North America, between 13,400 and 12,700 years ago (Miller, Holliday, and Bright 2013). Clovis points were fluted with two small projections, one on each end of the base, facing away from the head (Figure 12.19). The stone points found at this site match those found as far as the Canadian border and northern Mexico, and from the west coast to the east coast of the United States. Fourteen Clovis sites also contained the remains of mammoths or mastodons, suggesting that hunting megafauna with these points was an important part of life for the Clovis people. After the spread of the Clovis style, it diversified into several regional styles, keeping some of the Clovis form but also developing their own unique touches.
Summary of Modern H. sapiens in the Americas
Research in Native American origins found some surprising details, refining older models. Genetically, the migration can be considered one long period of movement with splits into regional populations. This finding matches the sudden appearance and diversification of the homegrown Clovis culture. A few thousand years after arrival into the hemisphere, people had already covered the Americas from north to south.
The peopling of the Americas also had a lot of elements in common with the prior spread of humans across Africa, Europe, Asia, and Australia. In all of these expansions, these pioneers explored new lands that tested their ability to adapt, both culturally and biologically. Besides stone-tool technology, the use of ochre as decoration was seen from South Africa to South America. The coasts and rivers were likely avenues in the movement of people, artifacts, and ideas, outlining the land masses while providing access to varied environments. The presence of megafauna aided human success, but this resource was eventually depleted in many parts of the world.
The Big Picture: The Assimilation Hypothesis
How do researchers make sense of all of these modern Homo sapiens discoveries that cover over 300,000 years of time and stretch across every continent except Antarctica? How was modern Homo sapiens related to archaic Homo sapiens?
The Assimilation hypothesis proposes that modern Homo sapiens evolved in Africa first and expanded out but also interbred with the archaic Homo sapiens they encountered outside Africa (Figure 12.20). This hypothesis is powerful since it explains why Africa has the oldest modern human fossils, why early modern humans found in Europe and Asia bear a resemblance to the regional archaics, and why traces of archaic DNA can be found in our genomes today (Dannemann and Racimo 2018; Reich et al. 2010; Reich et al. 2011; Slatkin and Racimo 2016; Smith et al. 2017; Wall and Yoshihara Caldeira Brandt 2016).
While researchers have produced a model that satisfies the data, there are still a lot of questions for paleoanthropologists to answer regarding our origins. What were the patterns of migration in each part of the world? Why did the archaic humans go extinct? In what ways did archaic and modern humans interact? The definitive explanation of how our species started and what our ancestors did is still out there to be found. You are now in a great place to welcome the next discovery about our distant past—maybe you’ll even contribute to our understanding as well.
The Chain Reaction of Agriculture
While it may be hard to imagine today, for most of our species’ existence we were nomadic: moving through the landscape without a singular home. Instead of a refrigerator or pantry stocked with food, we procured nutrition and other resources as needed based on what was available in the environment. Instead of collecting and displaying shelves of stuff, we kept our possessions small for mobility. This section gives an overview of how the foraging lifestyle enabled the expansion of our species and how the invention of a new way of life caused a chain reaction of cultural change.
The Foraging Tradition
There are a variety of possible subsistence strategies, or methods of finding sustenance and resources. To understand our species is to understand the subsistence strategy of foraging, or the search for resources in the environment. While most (but not all) humans today live in cultures that practice agriculture (whereby we greatly shape the environment to mass produce what we need), we have spent far more time as nomadic foragers than as settled agriculturalists. As such, our traits have evolved to be primarily geared toward foraging. For instance, our efficient bipedalism allows persistence-hunting across long distances as well as movement from resource to resource.
How does human foraging, also known as hunting and gathering, work? Anthropologists have used all four fields to answer this question (see Ember n.d.). Typically, people formed bands, or kin-based groups of around 50 people or less (rarely over 100). A band’s organization would be egalitarian, with a flexible hierarchy based on an individual’s age, level of experience, and relationship with others. Everyone would have a general knowledge of the skills assigned to their gender roles, rather than specializing in different occupations. A band would be able to move from place to place in the environment, using knowledge of the area to forage (Figure 12.21). In varied environments—from savannas to tropical forests, deserts, coasts, and the Arctic circle—people found sustenance needed for survival. Our species’s omnivorousness and cultural abilities led us to excel in the generalist-specialist niche.

Humans made extensive use of the foraging subsistence strategy, but this lifestyle did have limitations. The ease of foraging depended on the richness of the environment. Due to the lack of storage, resources had to be dependably found when needed. While a bountiful environment would require just a few hours of foraging a day and could lead to a focus on one location, the level and duration of labor increased greatly in poor or unreliable environments. Labor was also needed to process the acquired resources, which contributed to the foragers’ daily schedule (Crittenden and Schnorr 2017).
The adaptations to foraging found in modern Homo sapiens may explain why our species became so successful both within Africa and in the rapid expansion around the world. Overcoming the limitations, each generation at the edge of our species’s range would have found it beneficial to expand a little further, keeping contact with other bands but moving into unexplored territory where resources were more plentiful. The cumulative effect would have been the spread of modern Homo sapiens across continents and hemispheres.
Why Agriculture?
After hundreds of thousands of years of foraging, some groups of people around 12,000 years ago started to practice agriculture. This transition, called the Neolithic Revolution, occurred at the start of the Holocene epoch. While the reasons for this global change are still being investigated, two likely co-occurring causes are a growing human population and natural global climate change.
Overcrowding could have affected the success of foraging in the environment, leading to the development of a more productive subsistence strategy (Cohen 1977). Foraging works best with low population densities since each band needs a lot of space to support itself. If too many people occupy the same environment, they deplete the area faster. The high population could exceed the carrying capacity, or number of people a location can reliably support. Reaching carrying capacity on a global level due to growing population and limited areas of expansion would have been an increasingly pressing issue after the expansion through the major continents by 14,600 years ago.
A changing global climate immediately preceded the transition to agriculture, so researchers have also explored a connection between the two events. Since the Last Glacial Maximum of 23,000 years ago, the Earth slowly warmed. Then, from 13,000 to 11,700 years ago, the temperature in most of the Northern Hemisphere dropped suddenly in a phenomenon called the Younger Dryas. Glaciers returned in Europe, Asia, and North America. In Mesopotamia, which includes the Levant, the climate changed from warm and humid to cool and dry. The change would have occurred over decades, disrupting the usual nomadic patterns and subsistence of foragers around the world. The disruption to foragers due to the temperature shift could have been a factor in spurring a transition to agriculture. Researchers Gregory K. Dow and colleagues (2009) believe that foraging bands would have clustered in the new resource-rich places where people started to direct their labor to farming the limited area. After the Younger Dryas ended, people expanded out of the clusters with their agricultural knowledge (Figure 12.22).
The double threat of the limitation of human continental expansion and the sudden global climate change may have placed bands in peril as more populations outpaced their environment’s carrying capacity. Not only had a growing population led to increased competition with other bands, but environments worldwide shifted to create more uncertainty. As people in different areas around the world faced this unpredictable situation, they became the independent inventors of agriculture.
Agriculture around the World
Due to global changes to the human experience starting from 12,000 years ago, cultures with no knowledge of each other turned toward intensely farming their local resources (see Figure 12.22). The first farmers engaged in artificial selection of their domesticates to enhance useful traits over generations. The switch to agriculture took time and effort with no guarantee of success and constant challenges (e.g. fires, droughts, diseases, and pests). The regions with the most widespread impact in the face of these obstacles became the primary centers of agriculture (Figure 12.23; Fuller 2010):
- Mesopotamia: The Fertile Crescent from the Tigris and Euphrates rivers through the Levant was where bands started to domesticate plants and animals around 12,000 years ago. The connection between the development of agriculture and the Younger Dryas was especially strong here. Farmed crops included wheat, barley, peas, and lentils. This was also where cattle, pigs, sheep, and goats were domesticated.
- South and East Asia: Multiple regions across this land had varieties of rice, millet, and soybeans by 10,000 years ago. Pigs were farmed with no connection to Mesopotamia. Chickens were also originally from this region, bred for fighting first and food second.
- New Guinea: Agriculture started here 10,000 years ago. Bananas, sugarcane, and taro were native to this island. Sweet potatoes were brought back from voyages to South America around the year C.E. 1000. No known animal farming occurred here.
- Mesoamerica: Agriculture from Central Mexico to northern South America also occurred from 10,000 years ago; it was also only plant based. Maize was a crop bred from teosinte grass, which has become one of the global staples. Beans, squash, and avocados were also grown in this region.
- The Andes: Starting around 8,000 years ago, local domesticated plants started with squash but later included potatoes, tomatoes, beans, and quinoa. Maize was brought down from Mesoamerica. The main farm animals were llamas, alpacas, and guinea pigs.
- Sub-Saharan Africa: This region went through a change 5,000 years ago called the Bantu expansion. The Bantu agriculturalists were established in West Central Africa and then expanded south and east. Native varieties of rice, yams, millet, and sorghum were grown across this area. Cattle were also domesticated here.
- Eastern North America: This region was the last major independent agriculture center, from 4,000 years ago. Squash and sunflower are the produce from this region that are most known today, though sumpweed and pitseed goosefoot were also farmed. Hunting was still the main source of animal products.

By 5,000 years ago, our species was well within the Neolithic Revolution. Agriculturalists spread to neighboring parts of the world with their domesticates, further expanding the use of this subsistence strategy. From this point, the human species changed from being primarily foragers to primarily agriculturalists with skilled control of their environments. The planet changed from mostly unaffected by human presence to being greatly transformed by humans. The revolution took millennia, but it was a true revolution as our species’ lifestyle was dramatically reshaped.
Cultural Effects of Agriculture
The worldwide adoption of agriculture altered the course of human culture and history forever. The core change in human culture due to agriculture is the move toward not moving: rather than live a nomadic lifestyle, farmers had to remain in one area to tend to their crops and livestock. The term for living bound to a certain location is sedentarism. This led to new aspects of life that were uncommon among foragers: the construction of permanent shelters and agricultural infrastructure, such as fields and irrigation, plus the development of storage technology, such as pottery, to preserve extra resources in case of future instability.

The high productivity of successful agriculture sparked further changes (Smith 2009). Since successful agriculture produced a much-greater amount of food and other resources per unit of land compared to foraging, the population growth rate skyrocketed. The surplus of a bountiful harvest also provided insurance for harder times, reducing the risk of famine. Changes happened to society as well. With a few farming households producing enough food to feed many others, other people could focus on other tasks. So began specialization into different occupations such as craftspeople, traders, religious figures, and artists, spurring innovation in these areas as people could now devote time and effort toward specific skills. These interdependent people would settle an area together for convenience. The growth of these settlements led to urbanization, the founding of cities that became the foci of human interaction (Figure 12.24).
The formation of cities led to new issues that sparked the growth of further specializations, called institutions. These are cultural constructs that exist beyond the individual and have wide control over a population. Leadership of these cities became hierarchical with different levels of rank and control. The stratification of society increased social inequality between those with more or less power over others. Under leadership, people built impressive monumental architecture, such as pyramids and palaces, that embodied the wealth and power of these early cities. Alliances could unite cities, forming the earliest states. In several regions of the world, state organization expanded into empires, wide-ranging political entities that covered a variety of cultures.
Urbanization brought new challenges as well. The concentration of sedentary peoples was ideal for infectious diseases to thrive since they could jump from person to person and even from livestock to person (Armelagos, Brown, and Turner 2005). While successful agriculture provided a large surplus of food to thwart famine, the food produced offered less diverse food sources than foragers’ diets (Cohen and Armelagos 1984; Cohen and Crane-Kramer 2007). This shift in nutrition caused other diseases to flourish among those who adopted farming, such as dental cavities and malocclusion (the misalignment of teeth caused by soft, agricultural diets). The need to extract “wisdom teeth” or third molars seen in agricultural cultures today stems from this misalignment between the environment our ancestors adapted to and our lifestyles today.
As the new disease trends show, the adoption of agriculture and the ensuing cultural changes were not entirely positive. It is also important to note that this is not an absolutely linear progression of human culture from simple to complex. In many cases, empires have collapsed and, in some cases, cities dispersed to low-density bands that rejected institutions. However, a global trend has emerged since the adoption of agriculture, wherein population and social inequality have increased, leading to the massive and influential nation-states of today.
The rise of states in Europe has a direct impact on many of this book’s topics. Science started as a European cultural practice by the upper class that became a standardized way to study the world. Education became an institution to provide a standardized path toward producing and gaining knowledge. The scientific study of human diversity, embroiled in the race concept that still haunts us today, was connected to the European slave trade and colonialism.
Also starting in Europe, the Industrial Revolution of the 19th century turned cities into centers of mass manufacturing and spurred the rapid development of inventions (Figure 12.25). In the technologically interconnected world of today, human society has reached a new level of complexity with globalization. In this system, goods are mass-produced and consumed in different parts of the world, weakening the reliance on local farms and factories. The imbalanced relationship between consumers and producers of goods further increases economic inequality.

As states based on agriculture and industry keep exerting influence on humanity today, there are people, like the Hadzabe of Tanzania, who continue to live a lifestyle centered on foraging. Due to the overwhelming force that agricultural societies exert, foragers today have been marginalized to live in the least habitable parts of the world—the areas that are not conducive to farming, such as tropical rainforests, deserts, and the Arctic (Headland et al. 1989). Foragers can no longer live in the abundant environments that humans would have enjoyed before the Neolithic Revolution. Interactions with agriculturalists are typically imbalanced, with trade and other exchanges heavily favoring the larger group. One of anthropology’s important roles today is to intelligently and humanely manage equitable interactions between people of different backgrounds and levels of influence.
Special Topic: Indigenous Land Management
Insight into the lives of past modern humans has evolved as researchers revise previous theories and establish new connections with Indigenous knowledge holders.
The outdated view of foraging held that people lived off of the land without leaving an impact on the environment. Accompanying this idea was anthropologist Marshall Sahlins’s (1968) proposal that foragers were the “original affluent society” since they were meeting basic needs and achieving satisfaction with less work hours than agriculturalists and city-dwellers. This view countered an earlier idea that foragers were always on the brink of starvation. Sahlins’s theory took hold in the public eye as an attractive counterpoint to our busy contemporary lives in which we strive to meet our endless wants.
A fruitful type of study involving researchers collaborating with Indigenous experts has found that foragers did not just live off the land with minimal effort nor were they barely surviving in unchanging environments. Instead, they shaped the landscape to their needs using labor and strategies that were more subtle than what European colonizers and subsequent researchers were used to seeing. Research from two regions shows the latest developments in understanding Indigenous land management.
In British Columbia, Canada, the bridging of scientific and Indigenous perspectives has shown that the forests of the region are not untouched wilderness but, rather, have been crafted by Indigenous peoples thousands of years ago. Forest gardens adjacent to archaeological sites show higher plant diversity than unmanaged places even after 150 years (Armstrong et al. 2021). On the coast, 3,500-year-old archaeological sites are evidence of constructed clam gardens, according to Indigenous experts (Lepofsky et al. 2015). Another project, in consultation with Elders of the T’exelc (William Lakes First Nation) in British Columbia, introduced researchers to explanations of how forests were managed before the practice was disrupted by European colonialism (Copes-Gerbitz et al. 2021). Careful management of controlled fires reduced the density of the forest to favor plants such as raspberries and allow easier movement through the landscape.
Similarly, the study of landscapes in Australia, in consultation with Aboriginal Australians today, shows that areas previously considered wilderness by scientists were actually the result of controlling fauna and fires. The presence of grasslands with adjacent forests were purposely constructed to attract kangaroos for hunting (Gammage 2008). People also managed other animal and insect life, from emus to caterpillars. In Tasmania, a shift from productive grassland to wildfire-prone rainforest occurred after Aboriginal Australian land management was replaced by British colonial rule (Fletcher, Hall, and Alexander 2021). The site of Budj Bim of the Gunditjmara people has archaeological features of aquaculture, or the farming of fish, that date back 6,600 years (McNiven et al. 2012; McNiven et al. 2015). These examples show that Indigenous knowledge of how to manipulate the environment may be invaluable at the state level, such as by creating an Aboriginal ranger program to guide modern land management.
Conclusion
Modern Homo sapiens is the species that took the hominin lifestyle the furthest to become the only living member of that lineage. The largest factor that allowed us to persist while other hominins went extinct was likely our advanced ability to culturally adapt to a wide variety of environments. Our species, with its skeletal and behavioral traits, was well-suited to be generalist-specialists who successfully foraged across most of the world’s environments. The biological basis of this adaptation was our reorganized brain that facilitated innovation in cultural adaptations and intelligence for leveraging our social ties and finding ways to acquire resources from the environment. As the brain’s ability increased, it shaped the skull by reducing the evolutionary pressure to have large teeth and robust cranial bones to produce the modern Homo sapiens face.
Our ability to be generalist-specialists is seen in the geographical range that modern Homo sapiens covered in 300,000 years. In Africa, our species formed from multiregional gene flow that loosely connected archaic humans across the continent. People then expanded out to the rest of the continental Eurasia and even further to the Americas.
For most of our species’s existence, foraging was the general subsistence strategy within which people specialized to culturally adapt to their local environment. With omnivorousness and mobility, people found ways to extract and process resources, shaping the environment in return. When resource uncertainty hit the species, people around the world focused on agriculture to have a firmer control of sustenance. The new strategy shifted human history toward exponential growth and innovation, leading to our high dependence on cultural adaptations today.
While a cohesive image of our species has formed in recent years, there is still much to learn about our past. The work of many driven researchers shows that there are amazing new discoveries made all the time that refine our knowledge of human evolution. Technological innovations such as DNA analysis enable scientists to approach lingering questions from new angles. The answers we get allow us to ask even more insightful questions that will lead us to the next revelation. Like the pink limestone strata at Jebel Irhoud, previous effort has taken us so far and you are now ready to see what the next layer of discovery holds.
Special Topic: The Future of Humanity
A common question stemming from understanding human evolution is: What will the genetic and biological traits of our species be hundreds of thousands of years in the future? When faced with this question, people tend to think of directional selection. Maybe our braincases will be even larger, resembling the large-headed and small-bodied aliens of science fiction (Figure 12.26). Or, our hands could be specialized for interacting with our touch-based technology with less risk of repetitive injury. These ideas do not stand up to scrutiny. Since natural selection is based on adaptations that increase reproductive success, any directional change must be due to a higher rate of producing successful offspring compared to other alleles. Larger brains and more agile fingers would be convenient to possess, but they do not translate into an increase in the underlying allele frequencies.
Scientists are hesitant to professionally speculate on the unknowable, and we will never know what is in store for our species one thousand or one million years from now, but there are two trends in human evolution that may carry on into the future: increased genetic variation and a reduction in regional differences.
Rather than a directional change, genetic variation in our species could expand. Our technology can protect us from extreme environments and pathogens, even if our biological traits are not tuned to handle these stressors. The rapid pace of technological advancement means that biological adaptations will become less and less relevant to reproductive success, so nonbeneficial genetic traits will be more likely to remain in the gene pool. Biological anthropologist Jay T. Stock (2008) views environmental stress as needing to defeat two layers of protection before affecting our genetics. The first layer is our cultural adaptations. Our technology and knowledge can reduce pressure on one’s genotype to be “just right” to pass to the next generation. The second defense is our flexible physiology, such as our acclimatory responses. Only stressors not handled by these powerful responses would then cause natural selection on our alleles. These shields are already substantial, and cultural adaptations will only keep increasing in strength.
The increasing ability to travel far from one’s home region means that there will be a mixing of genetic variation on a global level in the future of our species. In recent centuries, gene flow of people around the world has increased, creating admixture in populations that had been separated for tens of thousands of years. For skin color, this means that populations all around the world could exhibit the whole range of skin colors, rather than the current pattern of decreasing melanin pigment farther from the equator. The same trend of intermixing would apply to all other traits, such as blood types. While our genetics will become more varied, the variation will be more intermixed instead of regionally isolated.
Our distant descendants will not likely be dextrous ultraintellectuals; more likely, they will be a highly variable and mobile species supported by novel cultural adaptations that make up for any inherited biological limitations. Technology may even enable the editing of DNA directly, changing these trends. With the uncertainty of our future, these are just the best-educated guesses for now. Our future is open and will be shaped little by little by the environment, our actions, and the actions of our descendants.
Hominin Species Summary
|
Hominin |
Modern Homo sapiens |
|
Dates |
315,000 years ago to present |
|
Region(s) |
Starting in Africa, then expanding around the world |
|
Famous discoveries |
Cro-Magnon individuals, discovered 1868 in Dordogne, France. Otzi the Ice Man, discovered 1991 in the Alps between Austria and Italy. Kennewick man, discovered 1996 in Washington state. |
|
Brain size |
1400 cc average |
|
Dentition |
Extremely small with short cusps. |
|
Cranial features |
An extremely globular brain case and gracile features throughout the cranium. The mandibular symphysis forms a chin at the anterior-most point. |
|
Postcranial features |
Gracile skeleton adapted for efficient bipedal locomotion at the expense of the muscular strength of most other large primates. |
|
Culture |
Extremely extensive and varied culture with many spoken and written languages. Art is ubiquitous. Technology is broad in complexity and impact on the environment. |
|
Other |
The only living hominin. Chimpanzees and bonobos are the closest living relatives. |
Review Questions
- What are the skeletal and behavioral traits that define modern Homo sapiens? What are the evolutionary explanations for its presence?
- What are some creative ways that researchers have learned about the past by studying fossils and artifacts?
- How do the discoveries mentioned in “First Africa, Then the World” fit the Assimilation model?
- What is foraging? What adaptations do we have for this subsistence strategy? Could you train to be a skilled forager?
- What are aspects of your life that come from dependence on agriculture and its cultural effects? Where did the ingredients of your favorite foods originate from?
Key Terms
African multiregionalism: The idea that modern Homo sapiens evolved as a complex web of small regional populations with sporadic gene flow among them.
Agriculture: The mass production of resources through farming and domestication.
Aquaculture: The farming of fish using techniques such as trapping, channels, and artificial ponds.
Assimilation hypothesis: Current theory of modern human origins stating that the species evolved first in Africa and interbred with archaic humans of Europe and Asia.
Atlatl: A handheld spear thrower that increased the force of thrown projectiles.
Band: A small group of people living together as foragers.
Beringia: Ancient landmass that connected Siberia and Alaska. The ancestors of Indigenous Americans would have crossed this area to reach the Americas.
Carrying capacity: The amount of organisms that an environment can reliably support.
Coastal Route model: Theory that the first Paleoindians crossed to the Americas by following the southern coast of Beringia.
Early Modern Homo sapiens, Early Anatomically Modern Human: Terms used to refer to transitional fossils between archaic and modern Homo sapiens that have a mosaic of traits. Humans like ourselves, who mostly lack archaic traits, are referred to as Late Modern Homo sapiens and simply Anatomically Modern Humans.
Egalitarian: Human organization without strict ranks. Foraging societies tend to be more egalitarian than those based on other subsistence strategies.
Foraging: Lifestyle consisting of frequent movement through the landscape and acquiring resources with minimal storage capacity.
Generalist-specialist niche: The ability to survive in a variety of environments by developing local expertise. Evolution toward this niche may have been what allowed modern Homo sapiens to expand past the geographical range of other human species.
Globalization: A recent increase in the interconnectedness and interdependence of people that is facilitated with long-distance networks.
Globular: Having a rounded appearance. Increased globularity of the braincase is a trait of modern Homo sapiens.
Gracile: Having a smooth and slender quality; the opposite of robust.
Holocene: The epoch of the Cenozoic Era starting around 12,000 years ago and lasting arguably through the present.
Ice-Free Corridor model: Theory that the first Native Americans crossed to the Americas through a passage between glaciers.
Institutions: Long-lasting and influential cultural constructs. Examples include government, organized religion, academia, and the economy.
Last Glacial Maximum: The time 23,000 years ago when the most recent ice age was the most intense.
Later Stone Age: Time period following the Middle Stone Age with a diversification in tool types, starting around 50,000 years ago.
Levant: The eastern coast of the Mediterranean. The site of early modern human expansion from Africa and later one of the centers of agriculture.
Megafauna: Large ancient animals that may have been hunted to extinction by people around the world.
Mental eminence: The chin on the mandible of modern H. sapiens. One of the defining traits of our species.
Microlith: Small stone tool found in the Later Stone Age; also called a bladelet.
Middle Stone Age: Time period known for Mousterian lithics that connects African archaic to modern Homo sapiens.
Monumental architecture: Large and labor-intensive constructions that signify the power of the elite in a sedentary society. A common type is the pyramid, a raised crafted structure topped with a point or platform.
Mosaic: Composed from a mix or composite of traits.
Neolithic Revolution: Time of rapid change to human cultures due to the invention of agriculture, starting around 12,000 years ago.
Ochre: Iron-based mineral pigment that can be a variety of yellows, reds, and browns. Used by modern human cultures worldwide since at least 80,000 years ago.
Sahul: Ancient landmass connecting New Guinea and Australia.
Sedentarism: Lifestyle based on having a stable home area; the opposite of nomadism.
Southern Dispersal model: Theory that modern H. sapiens expanded from East Africa by crossing the Red Sea and following the coast east across Asia.
Subsistence strategy: The method an organism uses to find nourishment and other resources.
Sunda: Ancient Asian landmass that incorporated modern Southeast Asia.
Supraorbital torus: The bony brow ridge across the top of the eye orbits on many hominin crania.
Upper Paleolithic: Time period considered synonymous with the Later Stone Age.
Urbanization: The increase of population density as people settled together in cities.
Wallacea: Archipelago southeast of Sunda with different biodiversity than Asia.
Younger Dryas: The rapid change in global climate—notably a cooling of the Northern Hemisphere—13,000 years ago.
About the Author

Keith Chan, Ph.D.
Grossmont-Cuyamaca Community College District and MiraCosta College, drkeithcchan@gmail.com, Dr. Keith Chan is an instructor of anthropology at Grossmont-Cuyamaca Community College District and MiraCosta College in San Diego County. He reached this step of his anthropological path after many memorable experiences across the country and the hemisphere. He earned a bachelor’s degree in anthropology from the University of California, Berkeley, in 2001. As a graduate student at the University of Missouri, he traveled to Perú with teams of students to study skeletons in the archaeological record to understand the lives of ancient Andeans. He completed his dissertation and earned a Ph.D. in 2011. Inspired by many educators in his journey, Dr. Chan turned his career toward teaching anthropology and helping students understand and appreciate humanity.
For Further Exploration
Websites
First-person virtual tour of Lascaux cave with annotated cave art: Ministère de la Culture and Musée d’Archéologie Nationale. “Visit the cave” Lascaux website.
Online anthropology magazine articles related to paleoanthropology and human evolution: SAPIENS. “Evolution.” SAPIENS website.
Various presentations of information about hominin evolution: Smithsonian Institution. “What does it mean to be human?” Smithsonian National Museum of Natural History website.
Magazine-style articles on archaeology and paleoanthropology: ThoughtCo. “Archaeology.” ThoughtCo. Website.
Database of comparisons across hominins and primates: University of California, San Diego. “MOCA Domains.” Center for Academic Research & Training in Anthropogeny website.
Books
Engaging book that covers human-made changes to the environment with industrialization and globalization: Kolbert, Elizabeth. 2014. The Sixth Extinction: An Unnatural History. New York: Bloomsbury.
Overview of what human life was like among the environmental shifts of the Ice Age: Woodward, Jamie. 2014. The Ice Age: A Very Short Introduction. Oxford: OUP Press.
Articles
Recent review paper about the current state of paleoanthropology research: Stringer, C. 2016. “The Origin and Evolution of Homo sapiens.” Philosophical Transactions of the Royal Society B 371 (1698).
Overview of the history of American paleoanthropology and the many debates that have occurred over the years: Trinkaus, E. 2018. “One Hundred Years of Paleoanthropology: An American Perspective.” American Journal of Physical Anthropology 165 (4): 638–651.
Amazing magazine article that synthesizes hominin evolution and why it is important to study this subject: Wheelwright, Jeff. 2015. “Days of Dysevolution.” Discover 36 (4): 33–39.
Fascinating research on Ötzi, a mummy from 5,000 years ago: Wierer, Ursula, Simona Arrighi, Stefano Bertola, Günther Kaufmann, Benno Baumgarten, Annaluisa Pedrotti, Patrizia Pernter, and Jacques Pelegrin. 2018. “The Iceman’s Lithic Toolkit: Raw Material, Technology, Typology and Use.” PLOS One 13 (6): e0198292. https://doi.org/10.1371/journal.pone.0198292.
Documentaries
PBS NOVA series covering the expansion of modern Homo sapiens and interbreeding with archaic humans: Brown, Nicholas, dir. 2015. First Peoples. Edmonton: Wall to Wall Television. Amazon Prime Video.
PBS NOVA special featuring the footprints found in White Sands National Park: Falk, Bella, dir. 2016. Ice Age Footprints. Boston: Windfall Films. https://www.pbs.org/wgbh/nova/video/ice-age-footprints/.
PBS NOVA special about how modern humans evolved adaptations to different environments. Shows how present-day people live around the world: Thompson, Niobe, dir. 2016. Great Human Odyssey. Edmonton: Clearwater Documentary. http://www.pbs.org/wgbh/nova/evolution/great-human-odyssey.html.
References
Araujo, Bernardo B. A., Luiz Gustavo R. Oliveira-Santos, Matheus S. Lima-Ribeiro, José Alexandre F. Diniz-Filho, and Fernando A. S. Fernandez. 2017. “Bigger Kill Than Chill: The Uneven Roles of Humans and Climate on Late Quaternary Megafaunal Extinctions.” Quaternary International 431: 216–222.
Armelagos, George J., Peter J. Brown, and Bethany Turner. 2005. “Evolutionary, Historical, and Political Economic Perspectives on Health and Disease.” Social Science & Medicine 61 (4): 755–765.
Armstrong, C. G., J. E. D. Miller, A. C. McAlvay, P. M. Ritchie, and D. Lepofsky. 2021. “Historical Indigenous Land-Use Explains Plant Functional Trait Diversity. Ecology and Society 26 (2): 6.
Bar-Yosef Mayer, Daniella E., Bernard Vandermeersch, and Ofer Bar-Yosef. 2009. “Shells and Ochre in Middle Paleolithic Qafzeh Cave, Israel: Indications for Modern Behavior.” Journal of Human Evolution 56 (3): 307–314.
Barbetti, M., and H. Allen. 1972. “Prehistoric Man at Lake Mungo, Australia, by 32,000 Years Bp.” Nature 240 (5375): 46–48.
Bennett, M. R., D. Bustos, J. S. Pigati, K. B. Springer, T. M. Urban, V. T. Holliday, Sally C. Reynolds, et al. (2021). “Evidence of Humans in North America during the Last Glacial Maximum.” Science 373 (6562): 1528–1531.
Bowler, J. M., Rhys Jones, Harry Allen, and A. G. Thorne. 1970. “Pleistocene Human Remains from Australia: A Living Site and Human Cremation from Lake Mungo, Western New South Wales.” World Archaeology 2 (1): 39–60.
Brown, Peter. 1999. “The First Modern East Asians? Another Look at Upper Cave 101, Liujiang and Minatogawa 1.” In Interdisciplinary Perspectives on the Origins of the Japanese, edited by K. Omoto, 105–131. Kyoto: International Research Center for Japanese Studies.
Brown, Peter. 2000. “Australian Pleistocene Variation and the Sex of Lake Mungo 3.” Journal of Human Evolution 38 (5): 743–749.
Clarkson, Chris, Zenobia Jacobs, Ben Marwick, Richard Fullagar, Lynley Wallis, Mike Smith, Richard G. Roberts, et al. 2017. “Human Occupation of Northern Australia by 65,000 Years Ago.” Nature 547 (7663): 306–310.
Cohen, Mark Nathan. 1977. The Food Crisis in Prehistory: Overpopulation and the Origins of Agriculture. New Haven, CT: Yale University Press.
Cohen, Mark Nathan, and George J. Armelagos, eds. 1984. Paleopathology at the Origins of Agriculture. Orlando, FL: Academic Press.
Cohen, Mark Nathan, and Gillian M. M. Crane-Kramer, eds. 2007. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. Gainesville, FL: University Press of Florida.
Copes-Gerbitz, K., S. Hagerman, and L. Daniels. 2021. “Situating Indigenous Knowledge for Resilience in Fire-Dependent Social-Ecological Systems.” Ecology and Society 26(4): 25. https://www.ecologyandsociety.org/vol26/iss4/art25/.
Coqueugniot, Hélène, Olivier Dutour, Baruch Arensburg, Henri Duday, Bernard Vandermeersch, and Anne-Marie Tillier. 2014. “Earliest Cranio-Encephalic Trauma from the Levantine Middle Palaeolithic: 3-D Reappraisal of the Qafzeh 11 Skull, Consequences of Pediatric Brain Damage on Individual Life Condition and Social Care.” PLOS ONE 9 (7): e102822.
Crittenden, Alyssa N., and Stephanie L. Schnorr. 2017. “Current Views on Hunter‐Gatherer Nutrition and the Evolution of the Human Diet.” American Journal of Physical Anthropology 162 (S63): 84–109.
d’Errico, Francesco, Lucinda Backwell, Paola Villa, Ilaria Degano, Jeannette J. Lucejko, Marion K. Bamford, Thomas F. G. Higham, Maria Perla Colombini, and Peter B. Beaumont. 2012. “Early Evidence of San Material Culture Represented by Organic Artifacts from Border Cave, South Africa.” Proceedings of the National Academy of Sciences 109 (33): 13214–13219.
d’Errico, Francesco, Christopher Henshilwood, Marian Vanhaeren, and Karen Van Niekerk. 2005. “Nassarius Kraussianus Shell Beads from Blombos Cave: Evidence for Symbolic Behaviour in the Middle Stone Age.” Journal of Human Evolution 48 (1): 3–24.
Dannemann, Michael, and Fernando Racimo. 2018. “Something Old, Something Borrowed: Admixture and Adaptation in Human Evolution.” Current Opinion in Genetics & Development 53: 1–8.
Day, M. H. 1969. “Omo Human Skeletal Remains.” Nature 222: 1135–1138.
Dillehay, Tom D., Carlos Ocampo, José Saavedra, Andre Oliveira Sawakuchi, Rodrigo M. Vega, Mario Pino, Michael B. Collins, et al. 2015. “New Archaeological Evidence for an Early Human Presence at Monte Verde, Chile.” PLOS ONE 10 (11): e0141923. doi:10.1371/journal.pone.0141923.
Dow, Gregory K., Clyde G. Reed, and Nancy Olewiler. 2009. “Climate Reversals and the Transition to Agriculture.” Journal of Economic Growth 14 (1): 27–53.
Durband, Arthur C. 2014. “Brief Communication: Artificial Cranial Modification in Kow Swamp and Cohuna.” American Journal of Physical Anthropology 155 (1): 173–178.
Ember, Carol R. N.d. “Hunter-Gatherers.” Explaining Human Culture. Human Relations Area Files. Accessed March 4, 2023. http://hraf.yale.edu/ehc/summaries/hunter-gatherers.
Erlandson, Jon M., Todd J. Braje, Kristina M. Gill, and Michael H. Graham. 2015. “Ecology of the Kelp Highway: Did Marine Resources Facilitate Human Dispersal from Northeast Asia to the Americas?” The Journal of Island and Coastal Archaeology 10 (3): 392–411.
Fladmark, K. R. 1979. “Routes: Alternate Migration Corridors for Early Man in North America.” American Antiquity 44 (1): 55–69.
Fletcher, M. S., T. Hall, and A. N. Alexandra. 2021. “The Loss of an Indigenous Constructed Landscape Following British Invasion of Australia: An Insight into the Deep Human Imprint on the Australian Landscape.” Ambio 50(1): 138–149.
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216–219.
Fuller, Dorian Q. 2010. “An Emerging Paradigm Shift in the Origins of Agriculture.” General Anthropology 17 (2): 1, 8–11.
Gammage, B. 2008. “Plain Facts: Tasmania under Aboriginal Management.” Landscape Research 33 (2): 241–254.
Germonpré, Mietje, Martina Lázničková-Galetová, and Mikhail V. Sablin. 2012. “Palaeolithic Dog Skulls at the Gravettian Předmostí Site, the Czech Republic.” Journal of Archaeological Science 39 (1): 184–202.
Gröning, Flora, Jia Liu, Michael J. Fagan, and Paul O’Higgins. 2011. “Why Do Humans Have Chins? Testing the Mechanical Significance of Modern Human Symphyseal Morphology with Finite Element Analysis.” American Journal of Physical Anthropology 144 (4): 593–606.
Harvati, Katerina. 2009. “Into Eurasia: A Geometric Morphometric Reassessment of the Upper Cave (Zhoukoudian) Specimens.” Journal of Human Evolution 57 (6): 751–762.
Headland, Thomas N., Lawrence A. Reid, M. G. Bicchieri, Charles A. Bishop, Robert Blust, Nicholas E. Flanders, Peter M. Gardner, Karl L. Hutterer, Arkadiusz Marciniak, and Robert F. Schroeder. 1989. “Hunter-Gatherers and Their Neighbors from Prehistory to the Present.” Current Anthropology 30 (1): 43–66.
Henshilwood, Christopher S., Francesco d’Errico, Karen L. van Niekerk, Yvan Coquinot, Zenobia Jacobs, Stein-Erik Lauritzen, Michel Menu, and Renata García-Moreno. 2011. “A 100,000-Year-Old Ochre-Processing Workshop at Blombos Cave, South Africa.” Science 334 (6053): 219–222.
Hershkovitz, Israel, Gerhard W. Weber, Rolf Quam, Mathieu Duval, Rainer Grün, Leslie Kinsley, Avner Ayalon, et al. 2018. “The Earliest Modern Humans Outside Africa.” Science 359 (6374): 456–459.
Hublin, Jean-Jacques, Abdelouahed Ben-Ncer, Shara E. Bailey, Sarah E. Freidline, Simon Neubauer, Matthew M. Skinner, Inga Bergmann, et al. 2017. “New Fossils from Jebel Irhoud, Morocco, and the Pan-African Origin of Homo sapiens.” Nature 546 (7657): 289–292.
Lepofsky, D., N. F. Smith, N. Cardinal, J. Harper, M. Morris, M., Gitla (Elroy White), Randy Bouchard, et al. 2015. “Ancient Shellfish Mariculture on the Northwest Coast of North America.” American Antiquity 80 (2): 236–259.
Lieberman, Daniel E. 2015. “Human Locomotion and Heat Loss: An Evolutionary Perspective.” Comprehensive Physiology 5 (1): 99–117.
Lieberman, Daniel E., Brandeis M. McBratney, and Gail Krovitz. 2002. “The Evolution and Development of Cranial Form in Homo sapiens.” Proceedings of the National Academy of Sciences 99 (3): 1134–1139.
Lieberman, Daniel E., Osbjorn M. Pearson, and Kenneth M. Mowbray. 2000. “Basicranial Influence on Overall Cranial Shape.” Journal of Human Evolution 38 (2): 291–315.
Liu, Wu, María Martinón-Torres, Yan-jun Cai, Song Xing, Hao-wen Tong, Shu-wen Pei, Mark Jan Sier, Xiao-hong Wu, R. Lawrence Edwards, and Hai Cheng. 2015. “The Earliest Unequivocally Modern Humans in Southern China.” Nature 526 (7575): 696-699.
Lucas, Peter W. 2007. “The Evolution of the Hominin Diet from a Dental Functional Perspective.” In Evolution of the Human Diet: The Known, the Unknown, and the Unknowable, edited by Peter S. Ungar, 31–38 Oxford, UK: Oxford University Press.
McCarthy, Robert C., and Lynn Lucas. 2014. “A Morphometric Reassessment of Bou-Vp-16/1 from Herto, Ethiopia.” Journal of Human Evolution 74: 114–117.
McDougall, Ian, Francis H. Brown, and John G. Fleagle. 2005. “Stratigraphic Placement and Age of Modern Humans from Kibish, Ethiopia.” Nature 433 (7027): 733–736.
McNiven, I. J., J. Crouch, T. Richards, N. Dolby, and G. Jacobsen. 2012. “Dating Aboriginal Stone-Walled Fishtraps at Lake Condah, Southeast Australia.” Journal of Archaeological Science 39 (2): 268–286.
McNiven, I., J. Crouch, T. Richards, K. Sniderman, N. Dolby, and G. Mirring. 2015. “Phased Redevelopment of an Ancient Gunditjmara Fish Trap over the Past 800 Years: Muldoons Trap Complex, Lake Condah, Southwestern Victoria.” Australian Archaeology 81 (1): 44–58.
Michel, Véronique, Hélène Valladas, Guanjun Shen, Wei Wang, Jian-xin Zhao, Chuan-Chou Shen, Patricia Valensi, and Christopher J. Bae. 2016. “The Earliest Modern Homo sapiens in China?” Journal of Human Evolution 101: 101–104.
Miller, D. Shane, Vance T. Holliday, and Jordon Bright. 2013. “Clovis across the Continent.” In Paleoamerican Odyssey, edited by Kelly E. Graf, Caroline V. Ketron, and Michael R. Waters, 207–220. College Station: Texas A&M University Press.
Neubauer, Simon, Jean-Jacques Hublin, and Philipp Gunz. 2018. “The Evolution of Modern Human Brain Shape.” Science Advances 4 (1): eaao5961. https://doi.org/10.1126/sciadv.aao5961.
Pearson, Osbjorn M. 2000. “Postcranial Remains and the Origin of Modern Humans.” Evolutionary Anthropology 9: 229–247.
Pearson, Osbjorn M. 2008. “Statistical and Biological Definitions of ‘Anatomically Modern’ Humans: Suggestions for a Unified Approach to Modern Morphology.” Evolutionary Anthropology: Issues, News, and Reviews 17 (1): 38–48.
Pietschnig, Jakob, Lars Penke, Jelte M. Wicherts, Michael Zeiler, and Martin Voracek. 2015. “Meta-Analysis of Associations between Human Brain Volume and Intelligence Differences: How Strong Are They and What Do They Mean?” Neuroscience & Biobehavioral Reviews 57: 411–432.
Posth, Cosimo, Nathan Nakatsuka, Iosif Lazaridis, Pontus Skoglund, Swapan Mallick, Thiseas C. Lamnidis, Nadin Rohland, et al. 2018. “Reconstructing the Deep Population History of Central and South America.” Cell 175 (5): 1185–1197.
Potter, Ben A., James F. Baichtal, Alwynne B. Beaudoin, Lars Fehren-Schmitz, C. Vance Haynes, Vance T. Holliday, Charles E. Holmes, et al. 2018. “Current Evidence Allows Multiple Models for the Peopling of the Americas.” Science Advances 4 (8): eaat5473. https://doi.org/10.1126/sciadv.aat5473.
Reich, David, Richard E. Green, Martin Kircher, Johannes Krause, Nick Patterson, Eric Y. Durand, Bence Viola, et al. 2010. “Genetic History of an Archaic Hominin Group from Denisova Cave in Siberia.” Nature 468 (7327): 1053–1060.
Reich, David, Nick Patterson, Martin Kircher, Frederick Delfin, Madhusudan R. Nandineni, Irina Pugach, Albert Min-Shan Ko, et al. 2011. “Denisova Admixture and the First Modern Human Dispersals into Southeast Asia and Oceania.” American Journal of Human Genetics 89 (4): 516–528.
Richter, Daniel, Rainer Grün, Renaud Joannes-Boyau, Teresa E. Steele, Fethi Amani, Mathieu Rué, Paul Fernandes, et al. 2017. “The Age of the Hominin Fossils from Jebel Irhoud, Morocco, and the Origins of the Middle Stone Age.” Nature 546 (7657): 293–296.
Roberts, Patrick, and Brian A. Stewart. 2018. “Defining the ‘Generalist-Specialist’ Niche for Pleistocene Homo sapiens.” Nature Human Behaviour 2: 542–550.
Rougier, Helene, Ştefan Milota, Ricardo Rodrigo, Mircea Gherase, Laurenţiu Sarcinǎ, Oana Moldovan, João Zilhão, et al. 2007. “Peştera Cu Oase 2 and the Cranial Morphology of Early Modern Europeans.” Proceedings of the National Academy of Sciences 104 (4): 1165–1170.
Sahlins, Marshall. 1968. “Notes on the Original Affluent Society.” In Man the Hunter, edited by R. B. Lee and I. DeVore, 85–89. New York: Aldine Publishing Company.
Sawyer, G. J., and Blaine Maley. 2005. “Neanderthal Reconstructed.” The Anatomical Record (Part B: New Anat.) 283 (1): 23–31.
Scerri, Eleanor M. L., Mark G. Thomas, Andrea Manica, Philipp Gunz, Jay T. Stock, Chris Stringer, Matt Grove, et al. 2018. “Did Our Species Evolve in Subdivided Populations Across Africa, and Why Does It Matter?” Trends in Ecology & Evolution 33 (8): 582–594.
Shea, John J. 2011. “Refuting a Myth about Human Origins.” American Scientist 99 (2): 128–135.
Shea, John J., and Ofer Bar-Yosef. 2005. “Who Were the Skhul/Qafzeh People? An Archaeological Perspective on Eurasia’s Oldest Modern Humans.” Journal of the Israel Prehistoric Society 35: 451–468.
Slatkin, Montgomery, and Fernando Racimo. 2016. “Ancient DNA and Human History.” Proceedings of the National Academy of Sciences 113 (23): 6380–6387.
Smith, Fred H., James C. M. Ahern, Ivor Janković, and Ivor Karavanić. 2017. “The Assimilation Model of Modern Human Origins in Light of Current Genetic and Genomic Knowledge.” Quaternary International 450: 126–136.
Smith, Michael. 2009. “V. Gordon Childe and the Urban Revolution: A Historical Perspective on a Revolution in Urban Studies.” Town Planning Review 80 (1): 3–29.
Stock, Jay T. 2008. “Are Humans Still Evolving?” EMBO Reports 9 (Suppl 1): S51–S54.
Swisher, Mark E., Dennis L. Jenkins, Lionel E. Jackson Jr., and Fred M. Phillips. 2013. “A Reassessment of the Role of the Canadian Ice-Free Corridor in Light of New Geological Evidence.” Poster Symposium 5B: Geology, Geochronology and Paleoenvironments of the First Americans at the Paleoamerican Odyssey Conference, Santa Fe, New Mexico, October 16–19.
Thorne, A. G., and P. G. Macumber. 1972. “Discoveries of Late Pleistocene Man at Kow Swamp, Australia.” Nature 238 (5363): 316–319.
Trinkaus, Erik, Ştefan Milota, Ricardo Rodrigo, Gherase Mircea, and Oana Moldovan. 2003a. “Early Modern Human Cranial Remains from the Peştera Cu Oase, Romania.” Journal of Human Evolution 45 (3): 245–253.
Trinkaus, Erik, Oana Moldovan, Adrian Bîlgăr, Laurenţiu Sarcina, Sheela Athreya, Shara E Bailey, Ricardo Rodrigo, Gherase Mircea, Thomas Higham, and Christopher Bronk Ramsey. 2003b. “An Early Modern Human from the Peştera Cu Oase, Romania.” Proceedings of the National Academy of Sciences 100 (20): 11231–11236.
Velemínská, J., J. Brůzek, P. Velemínský, L. Bigoni, A. Sefcáková, and S. Katina. 2008. “Variability of the Upper-Palaeolithic Skulls from Predmostí Near Prerov (Czech Republic): Craniometric Comparison with Recent Human Standards.” Homo 59 (1): 1–26.
Vidal, Céline M., Christine S. Lane, Asfawossen Asrat, Dan N. Barfod, Darren F. Mark, Emma L. Tomlinson, Ambdemichael Zafu Tadesse, et al. (2022). “Age of the Oldest Known Homo sapiens from Eastern Africa. Nature 601 (7894): 579–583.
Villa, Paola, Sylvain Soriano, Tsenka Tsanova, Ilaria Degano, Thomas F. G. Higham, Francesco d’Errico, Lucinda Backwell, Jeannette J. Lucejko, Maria Perla Colombini, and Peter B. Beaumont. 2012. “Border Cave and the Beginning of the Later Stone Age in South Africa.” Proceedings of the National Academy of Sciences 109 (33): 13208–13213.
Wall, Jeffrey D., and Deborah Yoshihara Caldeira Brandt. 2016. “Archaic Admixture in Human History.” Current Opinion in Genetics & Development 41: 93–97.
White, Tim D., Berhane Asfaw, David DeGusta, Henry Gilbert, Gary D. Richards, Gen Suwa, and F. Clark Howell. 2003. “Pleistocene Homo sapiens from Middle Awash, Ethiopia.” Nature 423 (6941): 742–747.
Woo, Ju-Kang. 1959. “Human Fossils Found in Liukiang, Kwangsi, China.” Vertebrata PalAsiatica 3 (3): 109–118.
Wu, XiuJie, Wu Liu, Wei Dong, JieMin Que, and YanFang Wang. 2008. “The Brain Morphology of Homo Liujiang Cranium Fossil by Three-Dimensional Computed Tomography.” Chinese Science Bulletin 53 (16): 2513–2519.
Acknowledgments
I could not have undertaken this project without the help of many who got me to where I am today. I extend sincere thank yous to the many colleagues and former students who have inspired me to keep learning and talking about anthropology. Thank you also to all who are involved in this textbook project. The anonymous reviewers truly sparked improvements to the chapter. Lastly, the staff of Starbucks #5772 also contributed immensely to this text.
Michael B. C. Rivera, Ph.D., University of Cambridge
This chapter is a revision from "Chapter 13: Race and Human Variation” by Michael B. C. Rivera. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Illustrate the troubling history of “race” concepts.
- Explain human variation and evolution as the thematic roots of biological anthropology as a discipline.
- Critique earlier “race” concepts based on a contemporary understanding of the apportionment of human genetic variation.
- Explain how biological variation in humans is distributed clinally and in accordance with both isolation-by-distance and Out-of-Africa models.
- Identify phenotypic traits that reflect selective and neutral evolution.
- Extend this more-nuanced view of human variation to today’s research, the implications for biomedical studies, applications in forensic anthropology, and other social/cultural/political concerns.
Humans exhibit biological variation. Humans also have a universal desire to categorize other humans in order to make sense of the world around them. Since the birth of the discipline of biological anthropology (or physical anthropology, as referred to back then), we have been interested in studying how humans vary biologically and what the sources of this variation are. Before we tackle these big problems, first consider this question: Why should we study human variation?

There are certainly academic reasons for studying human variation. First, it is highly interesting and important to consider the evolution of our species (see Chapters 9–12) and how our biological variation may be similar to (or different from) that of other species (e.g., other primates and apes; see Chapters 5 and 6). Second, anthropologists study modern human variation to understand how different biological traits developed over evolutionary time (see Figure 13.1). Suppose we are able to grasp the evolutionary processes that produce the differences in biology, physiology, body chemistry, behavior, and culture (human variation). In that case, we can make more accurate inferences about evolution and adaptation among our hominin ancestors, complementing our study of fossil evidence and the archaeological record. Third, as will be discussed in more detail later on, it is important to consider that biological variation among humans has biomedical, forensic, and sociopolitical implications. For these reasons, the study of human variation and evolution has formed the basis of anthropological inquiry for centuries and continues to be a major source of intrigue and inspiration for scientific research conducted today.
An even-more-important role of the biological anthropologist is to improve public understanding of human evolution and variation—outside of academic circles. Terms such as race and ethnicity are used in everyday conversations and in formal settings within and outside academia. The division of humankind into smaller, discrete categories is a regular occurrence in day-to-day life. This can be seen regularly when governments acquire census data with a heading like “geographic origin” or “ethnicity.” Furthermore, such checkboxes and drop-down lists are commonly seen as part of the identifying information required for surveys and job applications.
According to professors of anthropology, ethnic studies, and sociology, race is often understood as rooted in biological differences, ranging from such familiar traits as skin color or eye shape to variations at the genetic level. However, race can also be studied as an “ideological construct” that goes beyond biological and genetic bases (Fuentes et al. 2019), at different times relating to our ethnicities, languages, religious beliefs, and cultural practices. Sometimes people associate racial identity with the concept of socioeconomic status or position, or they link ideas about race to what passport someone has, how long they have been in a country, or how well they have “integrated” into a population.
Some of these ideas about ethnicity have huge social and political impacts, and notions of race have been part of the motivation behind various forms of racism and prejudice today, as well as many wars and genocides throughout history. Racism manifests in many ways—from instances of bullying between kids on school playgrounds to underpaid minorities in the workforce, and from verbal abuse hurled at people of color to violent physical behaviors against those of a certain race. Prejudice can be characterized as negative views toward another group based on some perceived characteristic that makes all members of that group worthy of disdain, disrespect, or exclusion (not solely along racial lines but also according to [dis]ability, gender, sexual orientation, or socioeconomic background, for example). According to Shay-Akil McLean (2014), “Racism is not something particular to the United States and race is not the same everywhere in the world. Racial categories serve particular contextual purposes depending on the society they are used in, but generally follow the base logic of the supremacy of one type of human body over all others (ordering these human bodies in a hierarchical fashion).” Choosing which biological or nonbiological features to use when discussing race is always a social process (Omi and Winant 2014). Race concepts have no validity to them unless people continue to use them in their daily lives—and, in the worst cases, to use them to justify racist behaviors and problematic ideas about racial difference or superiority/inferiority. Recent work in anthropological genetics has revealed the similarities amongst humans on a molecular level and the relatively few differences that exist between populations (Omi and Winant 2014).
The role of the biological anthropologist becomes crucial in the public sphere, because we may be able to debunk myths surrounding human variation and shed light, for the nonanthropologists around us, on how human variation is actually distributed worldwide (see Figure 13.1). Rooted in scientific observations, our work can help nonanthropologists recognize how common ideas about “race” often have no biological or genetic basis. Many anthropologists work hard to educate students on the history of where race concepts come from, why and how they last in public consciousness, and how we become more conscious of racial issues and the need to fight against racism in our societies. Throughout this chapter, I will highlight how humans cannot actually be divided into discrete “races,” because most traits vary on a continuous basis and human biology is, in fact, very homogenous compared to the greater genetic variation we observe in closely related species. Molecular anthropology, or anthropological genetics, continues to add new layers to our understanding of human biological variation and the evolutionary processes that gave rise to the contemporary patterns of human variation. The study of human variation has not always been unbiased, and thinkers and scientists have always worked in their particular sociohistorical context. For this reason, this chapter opens with a brief overview of race concepts throughout history, many of which relied on unethical and unscientific notions about different human groups.
Special Topic: My Experiences as an Asian Academic

My name is Michael, and I am a biological anthropologist and archaeologist (Figure 13.2). What strikes me as most interesting to investigate is human biological variation today and the study of past human evolution. For instance, some of my research on ancient coastal populations has revealed positive effects of coastal living on dietary health and many unique adaptations in bones and teeth when living near rivers and beaches. I love talking to students and nonscientists about bioanthropologists’ work, through teaching, science communication events, and writing book chapters like this one. I grew up in Hong Kong, a city in southern China. My father is from the Philippines and my mother is from Hong Kong, which makes me a mixed Filipino-Chinese academic. Growing up, I noticed that people came in all shapes, sizes, and colors. My life is very different now in that I have gained the expertise to explain those differences, and I feel a great responsibility to guide those new to anthropology toward their own understandings of diversity.
Biological anthropology is not taught extensively in Hong Kong, so I moved to the United Kingdom to earn my bachelor’s, master’s, and doctorate degrees. It was fascinating to me that we could answer important questions about human variation and history using scientific methods. However, I did not have many minority academic role models to look up to while I was at university. My department was made up almost exclusively of white westerner faculty, and it was hard to imagine I could one day get a job at these western institutions. While pursuing my degrees, I also remember several instances of my research contributions being overlooked or dismissed. Sometimes professors and fellow students would make racist comments toward Asian scholars (including me) and other Black, Indigenous and researchers of color, making us greatly uncomfortable in those spaces. When one of us would work up the courage to tell university leaders our experiences of being stereotyped, dismissed, or insulted, we received little support and were further excluded from research and teaching activities. This is a common experience for Black, Indigenous, and other people of color who pursue biological anthropology, and we face the difficult choice between leaving the field or bearing with such unsafe spaces.
It became important to me at that time to find other academics of color with whom to share experiences and form community. I feel inspired by all my colleagues who advocate for greater representation and diversity in universities (whether they are minority academics or not). I admire many of my fellow researchers who are underrepresented and do a great job of representing minority groups through their cutting-edge research and quality teaching at the undergraduate and graduate levels. Although I no longer work in the West, it has remained my great hope that those in the West and the “Global North” will continue to improve university culture, and I support any efforts there to welcome all scholars.
My current work is based in Hong Kong, where I am deeply dedicated to helping develop biological anthropology in East and Southeast Asia and promoting research from our home regions on the international scene. The study of anthropology really highlights how we share a common humanity and history. Being somebody who is “mixed race” and Asian likely played a key role in steering me toward the study of human variation. As this chapter hopefully shows, there is a lot to discuss about race and ethnicity regarding the discipline’s history and current understandings of human diversity. Some scientific and technological advancements today are misused for reasons to do with money, politics, or the continuation of antiquated ideas. It is my belief, alongside many of my friends and fellow anthropologists, that science should be more about empathy toward all members of our species and contributing to the intellectual and technological nourishment of society. After speaking to many members of the public, as well as my own undergraduate students, I have received lovely messages from other individuals of color expressing thanks and appreciation for my presence and understanding as a minority scientist and mentor figure. Anthropology needs much more diversity as well as to make room for those who have traveled different routes into the discipline. All paths taken into anthropology are valid and valuable. I would encourage everyone to study anthropology—it is a field for understanding and celebrating the intricacies of human diversity.
The History of "Race" Concepts
“Race” in the Classical Era
The earliest classification systems used to understand human variation are evidenced by ancient manuscripts, scrolls, and stone tablets recovered through archaeological, historical, and literary research. The Ancient Egyptians had the Book of Gates, dated to the New Kingdom between 1550 B.C.E. and 1077 B.C.E (Figure 13.3). In one part of this tome dedicated to depictions of the underworld, scribes used pictures and hieroglyphics to illustrate a division of Egyptian people into the four categories known to them at the time: the Aamu (Asiatics), the Nehesu (Nubians), the Reth (Egyptians), and the Themehu (Libyans). Though not rooted in any scientific basis like our current understandings of human variation today, the Ancient Egyptians believed that each of these groups were made of a distinctive category of people, distinguishable by their skin color, place of origin, and even behavioral traits.
Another well-known early document is the Bible, where it is written that all humankind descends from one of three sons of Noah: Shem (the ancestor to all olive-skinned Asians), Japheth (the ancestor to pale-skinned Europeans), and Ham (the ancestor to darker-skinned Africans). Similar to the Ancient Egyptians, these distinctions were based on behavioral traits and skin color. More recent work in historiography and linguistics suggest that the branches of “Hamites,” “Japhethites,” and “Shemites” may also relate to the formation of three independent language groups some time between 1000 and 3000 B.C.E. With the continued proliferation of Christianity, this concept of approximately three racial groupings lasted until the Middle Ages and spread as far across Eurasia as crusaders and missionaries ventured at the time.
There is also the “Great Chain of Being,” conceived by ancient Greek philosophers like Plato (427‒348 B.C.E.) and Aristotle (384‒322 B.C.E.). They played a key role in laying the foundations of empirical science, whereby observations of everything from animals to humans were noted with the aim of creating taxonomic categories. Aristotle describes the Great Chain of Being as a ladder along which all objects, plants, animals, humans, and celestial bodies can be mapped in an overall hierarchy (in the order of existential importance, with humans placed near the top, just beneath divine beings; see Figure 13.4). When he writes about humans, Aristotle expressed the belief that certain people are inherently (or genetically) more instinctive rulers, while others are more natural fits for the life of a worker or enslaved person. Based on research by biological anthropologists, we currently recognize that these early systems of classification and hierarchization are unhelpful in studying human biological variation. Both behavioral traits and physical traits are coded for by multiple genes each, and how we exhibit those traits based on our genetics can vary significantly even between individuals of the same population.
“Race” during the Scientific Revolution
The 1400s to 1600s saw the beginnings of the Scientific Revolution in Western societies, with thinkers like Nicholas Copernicus, Galileo Galilei, and Leonardo Da Vinci publishing some of their most important findings. While by no means the first or only scholars globally to use observation and experimentation to understand the world around them, early scientists living at the end of the medieval period in Europe increasingly employed more experimentation, quantification, and rational thought in their work. This is the main difference between the work of the ancient Egyptians, Romans, and Greeks and that of later scientists such as Isaac Newton and Carl Linnaeus in the 1600s and 1700s.

Linnaeus is the author of Systema Naturae (1758), in which he classified all plants and animals under the formalized naming system known as binomial nomenclature (Figure 13.5). This system is typological, in that organisms are placed into groups according to how they are similar or different to others under study. What was most anthropologically notable about Linnaeus’s typological system was that he was one of the first to group humans with apes and monkeys, based on the anatomical similarities between humans and nonhuman primates. However, Linnaeus viewed the world in line with essentialism, a problematic concept that dictates that there are a unique set of characteristics that organisms of a specific kind must have and that would remove organisms from taxonomic categorizations if they lacked any of the required criteria.
Linnaeus subdivided the human species into four varieties, with overtly racist categories based on skin color and “inherent” behaviors. Some European scientists during this period were not aware of their own biases skewing their interpretations of biological variation, while others deliberately worked to shape public perceptions of human variation in ways that established “otherness” and enforced European domination and the subordination of non-European people. The conclusions and claims at which they arrived, consciously or subconsciously, often fit the times they were living through—the so-called Age of Discovery, when the superiority of European cultures over others was a pervasive idea throughout people’s social and political lives. Although much of Eurasia was linked by spice- and silk-trading routes, the European colonial period between the 1400s and 1700s was marked by many new and unfortunately violent encounters overseas (Figure 13.6). When Europeans arrived by ship on the shores of continents that were already inhabited, it was their first meeting with the Indigenous peoples of the Americas and Australasia, who looked, spoke, and behaved differently from peoples with whom they were familiar. Building on the idea of species and “subspecies,” natural historians of this time invented the term race, from the French rasse meaning “local strain.”

Another scientist of the times, Johann Friedrich Blumenbach (1752‒1840), classified humans into five races based on his observations of cranial form variation as well as skin color. He thus dubbed the “original” form of the human cranium the “Caucasian” form, with the idea that the ideal climate conditions for early humans would have been in the Caucasus region near the Caspian Sea. The key insight Blumenbach presented was that human variation in any particular trait should be more accurately viewed as falling along a gradation (Figure 13.7). While some of his theories were correct according to what we observe today with more knowledge in genetics, they erroneously believed that human “subspecies” were “degenerated” or “transformed” varieties of an ancestral Caucasian or European race. According to them, the Caucasian cranial dimensions were the least changed over human evolutionary time, while the other skull forms represented geographic variants of this “original.” As will be discussed in greater detail later in this chapter, we have genetic and craniometric evidence for sub-Saharan Africa being the origin of the human species instead (see Chapter 12 on the fossil record that places the origins of modern Homo sapiens in north and east Africa). Based on work that shows how most biological characteristics are coded for by nonassociated genes, it is not reasonable to draw links between individuals’ personalities and their skull shapes.
“Race” and the Dawn of Scientific Racism
Between the 1800s and mid-1900s, and contrary to what you might expect, an increased use of scientific methods to justify racial schemes developed in scholarship. Differing from earlier views, which saw all humans as environmentally deviated from one “original” humankind, classification systems after 1800 became more polygenetic (viewing all people as having separate origins) rather than monogenetic (viewing all people as having a single origin). Instead of moving closer to our modern-day understandings of human variation, there was increased support for the notion that each race was created separately and with different attributes (intelligence, temperament, and appearance).
The 1800s were an important precursor to modern biological anthropology as we know it, given that this was when the scientific measurement of human physical features (anthropometry) truly became popularized. However, empirical studies in the 1800s pushed even further the idea that Europeans were culturally and biologically superior to others. While considered one of the pioneers of American “physical” anthropology, Samuel George Morton (1799‒1851) was a scholar who had a large role in perpetuating 1800s scientific racism. By measuring cranial size and shape, he calculated that “Caucasians,” on average, have greater cranial volumes than other groups, such as the Indigenous peoples of the Americas and peoples Morton referred to collectively as “Negros.” Today, we know that cranial size variation depends on such factors as Allen’s and Bergmann’s rules, which give a more likely explanation: in colder environments, it is advantageous for those living there to have larger and rounder heads because they conserve heat more effectively than more slender heads (Beals et al. 1984). The leading figures in craniometry during the 1800s instead were linked heavily with powerful individuals and wealthy sociopolitical institutions and financial bodies. Theories in support of hierarchical racial schemes using “scientific” bases certainly helped continue the exploitative and unethical trafficking and enslavement of Africans between the 1500s and 1800s.
Morton went on to write in his publication Crania Americana (1839) a number of views that fit with a concept called biological determinism. The idea behind biological determinism is that an association exists between people’s physical characteristics and their behavior, intelligence, ability, values, and morals. If the idea is that some groups of people are essentially superior to others in cognitive ability and temperament, then it is easier to justify the unequal treatment of certain groups based on outward appearances. Another such problematic thinker was Paul Broca (1824‒1880), after whom a region of the frontal lobe related to language use is named (Broca’s area). Influenced by Morton, Broca likewise claimed that internal skull capacities could be linked with skin color and cognitive ability. He went on to justify the European colonization of other global territories by purporting it was noble for a biologically more “civilized” population to improve the “humanity” of more “barbaric” populations. Today, these theories of Morton, Broca, and others like them are known to have no scientific basis. If we could speak with them today, they would likely try to emphasize that their conclusions were based on empirical evidence and not a priori reasoning. However, we now can clearly see that their reasoning was biased and affected by prevailing societal views at the time.
“Race” and the Beginnings of Physical Anthropology
In the early 20th century, we saw a number of new figures coming into the science of human variation and shifting the theoretical focus within. Most notably, these included Aleš Hrdlička and Franz Boas.

Aleš Hrdlička (1869‒1943) was a Czech anthropologist who moved to the United States. In 1903, he established the physical anthropology section of the National Museum of Natural History (Figure 13.8). In 1918, he founded the American Journal of Physical Anthropology, which remains one of the foremost scientific journals disseminating bioanthropological research. As part of his work and the scope of the journal, he differentiated “physical anthropology” from other kinds of anthropology: he wrote that physical anthropology is “the study of racial anatomy, physiology, and pathology” and “the study of man’s variation” (Hrdlička 1918). In some ways, although the scope and technological capabilities of biological anthropologists have changed significantly, Hrdlička established an area of inquiry that has continued and prospered for over a hundred years.
Franz Boas (1858‒1942) was a German American anthropologist who established the four-field anthropology system in the United States and founded the American Anthropological Association in 1902. He argued that the scientific method should be used in the study of human cultures and the comparative method for looking at human biology worldwide. One of Boas’s significant contributions to biological anthropology was the study of skull dimensions with respect to race. After a long-term research project, he demonstrated how cranial form was highly dependent on cultural and environmental factors and that human behaviors were influenced primarily not by genes but by social learning. He wrote in one essay for the journal Science: “While individuals differ, biological differences between races are small. There is no reason to believe that one race is by nature so much more intelligent, endowed with great willpower, or emotionally more stable than another, that the difference would materially influence its culture” (Boas 1931:6). This conclusion directly contrasted with the theories of the past that relied on biological determinism. Biological anthropologists today have found evidence that corroborates Boas’s explanations: societies do not exist on a hierarchy or gradation of “civilizedness” but instead are shaped by the world around them, their demographic histories, and the interactions they have with other groups.
The first half of the 1900s still involved some research that was essentialist and focused on proving racial determinism. Anthropologists like Francis Galton (1822‒1911) and Earnest A. Hooton (1887‒1954) created the field of eugenics as an attempt to formalize the social scientific study of “fitness” and “superiority” among members of 19th-century Europe. As a way of “dealing with” criminals, diseased individuals, and “uncivilized” people, eugenicists recommended prohibiting parts of the population from being married or sterilizing these members of society so they could no longer procreate (Figure 13.9). They instead encouraged “reproduction in individual families with sound physiques, good mental endowments, and demonstrable social and economic capability” (Hooton 1936). In the 1930s, Nazi Germany used this false idea of there being “pure races” to highly destructive effect. The need to be protected against admixture from “unfit” groups was their justification for their blatant racism and purging of citizens that fell under their subjective criteria.
It should be noted that eugenicist ideas were popularly discussed and debated in many non-European contexts, as in the U.S., China, and South Africa, too. The Immigration Restriction Act of 1924 was passed in the United States, with the explicit aim of reducing the country’s “burden” of people considered inferior by restricting immigration of eastern European Jews, Italians, Africans, Arabs, and Asians. In the early 1900s, Chinese scientists and politicians showed great interest in eugenic ideologies, which came to dictate decisions in law-making, family life, and the medical field. Noted American anthropologist Ruth Benedict wrote extensively on Japanese culture and society during and after World War II. Her essentialist portrayals of Japanese people were heavily cited in popular discourse at the time. In 1950s South Africa, interracial marriages and sexual relations were banned by law; antimiscegenation became one of the huge focuses of apartheid resistance activists in later years. We still see the continuation of eugenics-based logic today around the world—in exclusionary immigration laws, cases of incarcerated prison inmates being forcibly sterilized, and the persistence of intelligence testing as a form of measuring people’s “fitness” in a society.
Shortly after World War II and the Nazi Holocaust, the full extent of essentialist, eugenicist thinking became clear. Social constructions of race, and the notion that one can predict psychological or behavioral traits based on external appearance, had become unpopular both within and outside the discipline. It was up to those in the field of physical anthropology at the time to separate physical anthropology from race concepts that supported unscientific and socially damaging agendas. This does not mean that there are no physiological or behavioral differences between different members of the human species. However, going forward, a number of physical anthropologists saw human biological variation as more complicated than simple typologies could describe.
“The New Physical Anthropology”

After 1950, focus steered away from the concept of “race” as a unit of variation and toward understanding why variation exists in populations from an evolutionary perspective. This was outlined by those pioneering the “new physical anthropology,” such as Sherwood Washburn, Theodosius Dobzhansky (Figure 13.10), and Julian Huxley, who borrowed this approach from contemporary population geneticists. Whether using genetic or phenotypic markers as the units of study, “groups” or “clusters” of humans differentiated by these became defined as populations that differ in the frequency of some gene or genes. Anthropologists consider what “makes” a population—a group of individuals potentially capable of or actually interbreeding due to shared geographic proximity, language, ethnicity, culture, and/or values. Put another way, a population is a local interbreeding group with reduced gene flow between themselves and other groups of humans. Members of the same population may be expected to share many genetic traits (and, as a result, many phenotypic traits that may or may not be visible outwardly).

Thinking of humans in terms of populations was part of Julian Huxley’s (1942) “Modern Synthesis”—so named because it helped to reconcile two fundamental principles about evolution that had not been made sense of together before (Figure 13.11). As discussed in Chapter 3, Gregor Mendel (1822‒1884) was able to show that inheritance was mediated by discrete particles (or genes) and not blended in the offspring. However, it was difficult for some 19th-century scientists to accept this model of genetic inheritance at the time because much of biological variation appeared to be continuous and not particulate (take skin color or height as examples). In the 1930s, it was demonstrated that traits could be polygenic and that multiple alleles could be responsible for any one phenotypic trait, thus producing the continuous variation in traits such as eye color that we see today. Thus, Huxley’s “Modern Synthesis” outlines not only how human populations are capable of exchanging genes at the microevolutionary level but also how multiple alleles for one trait (polygenic exchanges) can cause gradual macroevolutionary changes.
Human Variation in Biological Anthropology Today
Many Human Traits Are Nonconcordant
In our studies of human (genetic) variation today, we understand most human traits to be nonconcordant (Figure 13.12). “Nonconcordance” is a term used to describe how biological traits vary independent of each other—that is, they do not get inherited in a correlative manner with other genetically controlled traits. For example, if you knew an individual had genes that coded for tall height, you would not be able to predict if they are lighter-skinned or have red hair. This is different from earlier essentialist views of human variation: the idea that skin color could predict one’s brain function or even “temperament” and tendencies toward criminal behavior.
Human Variation Is Clinal/Continuous (Not Discrete)
Frank B. Livingstone (1928‒2005) wrote: “There are no races, only clines” (1962: 279). A cline is a gradation in the frequency of an allele/trait between populations living in different geographic regions. Human variation cannot be broken into discrete “races,” because most physical traits vary on a continuous or “clinal” basis. One obvious example of this is how human height does not only come in three values (“short,” “medium,” and “tall”) but instead varies across a spectrum of vertical heights achievable by humans all over the world. On the one hand, we can describe human height as exhibiting continuous variation, forming a continuous pattern, but height does not vary according to where people live across the globe and does not exhibit a clinal pattern. On the other hand, skin color variation between populations does show patterning that fits quite well on to how near or far they are from each other on a world map. This makes a trait like skin color clinally distributed worldwide. When large numbers of genetic loci for large numbers of samples were sampled from human populations distributed worldwide during the 1960s and 1970s, the view that certain facets of human diversity were clinally distributed was further supported by genetic data.
To study human traits that are clinally distributed, genetic tests must be performed to uncover the true frequencies of an allele or trait across a certain geographic space. One easily visible example of a clinal distribution seen worldwide is the patterning of human variation in skin color. Whether in southern Asia, sub-Saharan Africa, or Australia, dark brown skin is found. Paler skin tones are found in higher-latitude populations such as those who have lived in areas like Europe, Siberia, and Alaska for millennia. Skin color is easily observable as a phenotypic trait exhibiting continuous variation.
A clinal distribution still derives from genetic inheritance; however, clines often correspond to some gradually changing environmental factor. Clinal patterns arise when selective pressures in one geographic area differ from those in another as well as when people procreate and pass on genes together with their most immediate neighbors. There are several mechanisms, selective and neutral, that can lead to the clinal distribution of an allele or a biological trait. Natural selection is the mechanism that produced a global cline of skin color, whereby darker skin color protects equatorial populations from high amounts of UV radiation; there is a transition of lessening pigmentation in individuals that reside further and further away from the tropics (Jablonski 2004; Jablonski and Chaplin 2000; see Figure 13.13). The ability and inability to digest lactose (milk sugar) among different world communities varies according to differential practices and histories of milk and dairy-product consumption (Gerbault et al. 2011; Ingram et al. 2009). Where malaria seems to be most prevalent as a disease stressor on human populations, a clinal gradient of increasing sickle cell anemia experience toward these regions has been studied extensively by genetic anthropologists (Luzzatto 2012). Sometimes culturally defined mate selection based on some observable trait can lead to clinal variation between populations as well.
Two neutral microevolutionary processes that may produce a cline in a human allele or trait are gene flow and genetic drift (see Chapter 4). The ways in which neutral processes can produce clinal distributions is seen clearly when looking at clinal maps for different blood groups in the human ABO blood group system (Figure 13.14). For instance, scientists have identified an East-to-West cline in the distribution of the blood type B allele across Eurasia. The frequency of B allele carriers decreases gradually westward when we compare the blood groups of East and Southeast Asian populations with those in Europe. This shows how populations residing nearer to one another are more likely to interbreed and share genetic material (i.e., undergo gene flow). We also see 90%‒100% of native South American individuals, as well as between 70%‒90% of Aboriginal Australian groups, carrying the O allele (Mourant, Kopeć, and Domaniewska-Sobczak 1976). These high frequencies are likely due to random genetic drift and founder effects, in which population sizes were severely reduced by the earliest O allele-carrying individuals migrating into those areas. Over time, the O blood type has remained predominant.
Genetic Variation Is Greater Within Group than Between Groups
One problem with race-based classifications is they relied on an erroneous idea that individuals with particular characteristics would share more similar genes with each other within a particular “race” and share less with individuals of other “races” possessing different traits and genetic makeups. However, since around 50 years ago, scientific studies have shown that the majority of human genetic differences worldwide exist within groups (or “races”) individually rather than between groups. Indeed, most genetic variation we see occurs in Africa, and many variants are shared among individuals on all continents (Figure 13.15).
In 2002, a landmark article by Noah Rosenberg and colleagues explored worldwide human genetic variation using an even-greater genetic data set. They used 377 highly variable markers in the human genome and sampled from 1,056 individuals representative of 52 populations. The markers chosen for study were not ones that code for any expressed genes. Because these regions of the human genome were made of unexpressed genes, we may understand these markers as neutrally derived (as opposed to selectively derived) because they do not code for functional advantages or disadvantages. These neutral genetic markers likely reflect an intricate combination of regional founder effects and population histories. Analyses of these neutral markers allowed scientists to identify that 93%‒95% of global genetic differences, referred to as variance, can be accounted for by within-population differences, while only a small proportion of genetic variance (3%‒5%) can be attributed to differences among major groups (Rosenberg et al. 2002). This research supports the theory that distinct biological races do not exist, even though misguided concepts of race may still have real social and political consequences.
Biological Data Fit Isolation-By-Distance and Out-of-Africa Models
One further note is that the world’s population may be genetically divided into “groups,” “subsets,” “clumps,” or “clusters” that reflect some degree of genetic similarity. These identifiable clusters reflect genetic or geographic distances—either with gene flow facilitated by proximity between populations or impeded by obstacles like oceans or environmentally challenging habitats (Rosenberg et al. 2005). Sometimes, inferred clusters using multiple genetic loci are interpreted by nongeneticists literally as “ancestral populations.” However, it would be wrong to assume from these genetic results that highly differentiated and “pure” ancestral groups ever existed. These groupings reflect differences that have arisen over time due to clinal patterning, genetic drift, and/or restricted or unrestricted gene flow (Weiss and Long 2009). The clusters identified by scientists are arbitrary and the parameters used to split up the global population into groups is subjective and dependent on the particular questions or distinctions being brought into focus (Relethford 2009).
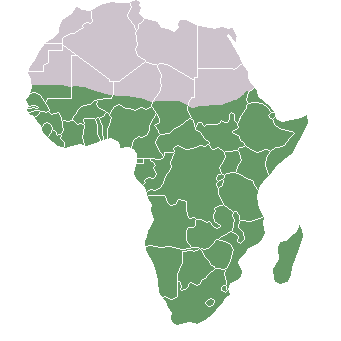
Additionally, research on worldwide genetic variation has shown that human variation decreases with increasing distance from sub-Saharan Africa, where there is evidence for this vast region being the geographical origin of anatomically modern humans (Liu et al. 2006; Prugnolle, Manica, and Balloux 2005; see Figures 13.16 and 13.17). Genetic differentiation decreases in human groups the further you sample data from relative to sub-Saharan Africa because of serial founder effects (Relethford 2004). Over the course of human colonization of the rest of the world outside Africa, populations broke away in expanding waves across continents into western Asia, then Europe and eastern Asia, followed by Oceania and the Americas. As a result, founder events occurred whereby genetic variation was lost, as the colonization of each new geographical region involved a smaller number of individuals moving from the original larger population to establish a new one (Relethford 2004). The most genetic variation is found across populations residing in different parts of sub-Saharan Africa, while other current populations in places like northern Europe and the southern tip of South America exhibit some of the least genetic differentiation relative to all global populations (Campbell and Tishkoff 2008).
Besides fitting nicely into the Out-of-Africa model, worldwide human genetic variation conforms to an isolation-by-distance model, which predicts that genetic similarity between groups will decrease exponentially as the geographic distance between them increases (Kanitz et al. 2018). This is because of the greater and greater restrictions to gene flow presented by geographic distance, as well as cultural and linguistic differences that occur as a result of certain degrees of isolation. Since genetic data conform to isolation-by-distance and Out-of-Africa models, these findings support the abolishment of “race” groupings. This research demonstrates that human variation is continuous and cannot be differentiated into geographically discrete categories. There are no “inherent” or “innate” differences between human groups; instead, variation derives from some degree of natural selection, as well as neutral processes like population bottle-necking (Figure 13.18), random mutations in the DNA, genetic drift, and gene flow through between-mate interbreeding.
Humans Have Higher Homogeneity Compared to Many Other Species
An important fact to bear in mind is that humans are 99.9% identical to one another. This means that the apportionments of human variation discussed above only concern that tiny 0.1% of difference that exists between all humans globally. Compared to other mammalian species, including the other great apes, human variation is remarkably lower. This may be surprising given that the worldwide human population has already exceeded seven billion, and, at least on the surface level, we appear to be quite phenotypically diverse. Molecular approaches to human and primate genetics tells us that external differences are merely superficial. For a proper appreciation of human variation, we have to look at our closest relatives in the primate order and mammalian class. Compared to chimpanzees, bonobos, gorillas and other primates, humans have remarkably low average genome-wide heterogeneity (Osada 2005).
When we look at chimpanzee genetic variation, it is fascinating that western, central, eastern, and Cameroonian chimpanzee groups have substantially more genetic variation between them than large global samples of human DNA (Bowden et al. 2012; Figure 13.19). This is surprising given that all of these chimpanzee groups live relatively near one another in Africa, while measurements of human genetic variation have been conducted using samples from entirely different continents. First, geneticists suppose that this could reflect differential experiences of the founder effect between humans and chimpanzees. Because all non-African human populations descended from a small number of anatomically modern humans who left Africa, it would be expected that all groups descended from that smaller ancestral group would be similar genetically. Second, our species is really young, given that we have only existed on the planet for around 150,000 to 300,000 years. This gave humans little time for random genetic mutations to occur as genes get passed down through genetic interbreeding and meiosis. Chimpanzees, however, have inhabited different ecological niches, and less interbreeding has occurred between the four chimpanzee groups over the past six to eight million years compared to the amount of gene flow that occurred between worldwide human populations (Bowden et al. 2012).
Recent advances have now enabled the attainment of genetic samples from the larger family of great apes and the evaluation of genetic variation among bonobos, orangutans, and gorillas alongside that of chimpanzees and humans (Prado-Martinez et al. 2013). Collecting such data and analyzing primate genetic variation has been important not only to elucidate how different ecological, demographic, and climatic factors have shaped our evolution but also to inform upon conservation efforts and medical research. Genes that may code for genetic susceptibilities to tropical diseases that affect multiple primates can be studied through genome-wide methods. Species differences in the genomes associated with speech, behavior, and cognition could tell us more about how human individuals may be affected by genetically derived neurological or speech-related disorders and conditions (Prado-Martinez et al. 2013; Staes et al. 2017). In 2018, a great ape genomic study also reported genetic differences between chimpanzees and humans related to brain cell divisions (Kronenberg et al. 2018). From these results, it may be inferred that cognitive or behavioral variation between humans and the great apes might relate to an increased number of cortical neurons being formed during human brain development (Kronenberg et al. 2018). Comparative studies of human and nonhuman great ape genetic variation highlight the complex interactions of population histories, environmental changes, and natural selection between and within species. When viewed in the context of overall great ape variation, we may reconsider how variable the human species is relatively and how unjustified previous “race” concepts really were.
Phenotypic Traits That Reflect Neutral Evolution
Depending on the trait being observed, different patterns of phenotypic variation may be found within and among groups worldwide. In this subsection, some phenotypic traits that reflect the aforementioned patterns of genetic variation will be discussed.

Looking beyond genetic variation briefly, recent studies have revisited biological anthropology’s earlier themes of externally observable traits, such as skull shape. In the last 20 or so years, anthropologists have evaluated the level to which human cranial shape variation reflects the results from genetic markers, such as those used previously to fit against Out-of-Africa models (Relethford 2004) or those used in the apportionment of human variation between and within groups (Lewontin 1972; Rosenberg et al. 2002). Using larger sample sizes of cranial data collected from thousands of skulls worldwide and a long list of cranial measurements, studies demonstrate a similar decrease in variation with distance from Africa and show that a majority of cranial variation occurs within populations rather than between populations (Betti et al. 2009; Betti et al. 2010; Manica et al. 2007; Relethford 2001; von Cramon-Taubadel and Lycett 2008; see Figure 13.20). The greatest cranial variation is found among skulls of sub-Saharan African origin, while the least variation is found among populations inhabiting places like Tierra del Fuego at the southern tip of Argentina and Chile. While ancient and historical thinkers previously thought “race” categories could reasonably be determined based on skull dimensions, modern-day analyses using more informative sets of cranial traits simply show that migrations out of Africa and the relative distances between populations can explain a majority of worldwide cranial variation (Betti et al. 2009).
This same patterning in phenotypic variation has even been found in studies examining shape variation of the pelvis (Betti et al. 2013; Betti et al. 2014), the teeth (Rathmann et al. 2017), and the human bony labyrinth of the ear (Ponce de León et al. 2018;Figure 13.21). The skeletal morphology of these bones still varies worldwide, but a greater proportion of that variation can still be attributed to the ways in which human populations migrated across the world and exchanged genes with those closer to them rather than those further away. Human skeletal variation in these parts of the body is continuous and nondiscrete. Given the important functions of the cranium and these other skeletal parts, we may infer that the genes that underpin their development have been relatively conserved by neutral evolutionary processes such as genetic drift and gene flow. It is also important to note that while some traits such as height, weight, cranial dimensions, and body composition are determined, in part, by genes, the underlying developmental processes behind these traits are underpinned by complex polygenic mechanisms that have led to the continuous spectrum of variation in such variables among modern-day human populations.
Phenotypic Traits That Reflect Natural Selection
Even though 99.9% of our DNA is the same across all humans worldwide, and many traits reflect neutral processes, there are parts of that remaining 0.1% of the human genome that code for individual and regional differences. Similarly to craniometric analyses that have been conducted in recent decades, human variation in skin color has also been reassessed using new methods and in light of greater knowledge of biological evolution.
New technologies allow scientists to use color photometry to sample and quantify the visible wavelength of skin color, in a way 19th- and 20th-century readers could not. In one report, it was found that 87.9% of global skin color variation can be attributed to genetic differences between groups, 3.2% to those among local populations within regions, and 8.9% within local populations (Relethford 2002). This apportionment differs significantly and is the reverse situation found in the distribution of genetic differences we see when we examine genetic markers such as blood type–related alleles. However, this pattern of human skin color worldwide is not surprising, given that we now understand that past selection has occurred for darker skin near the equator and lighter skin at higher latitudes (Jablonski 2004; Jablonski and Chaplin 2000). While most genetic variation reflects neutral variation due to population migrations, geographic isolation, and restricted gene flow dynamics, some human genetic/phenotypic variation is best explained as local adaptation to environmental conditions (i.e., selection). Given that skin color variation is atypical compared to other genetic markers and biological traits, this, in fact, goes against earlier “race” typologies. This is because recent studies ironically show how so much of genetic variation relates to neutral processes, while skin color does not. It follows that skin color cannot be viewed as useful in making inferences about other human traits.

It is also true that some populations have not been studied extensively in skin pigmentation genetics (e.g., African, Austronesian, Melanesian, Southeast Asian, Indigenous American, and Pacific Islander populations, according to Lasisi and Shriver 2018). Earlier dispersals of these populations, and their local genetic varition, will have contributed to worldwide genetic variation, inclusive of skin pigmentation variation. Gene loci we did not previously appreciate as being linked to pigmentation are now being recognized thanks to better tools, more diverse genetic samples, and more accessible datasets (Quillen et al. 2018). Biological anthropologists look forward to further discoveries elucidating the different selective pressures and population dynamics that influence skin pigmentation evolution.
Social Implications
To finish this chapter, we will consider the social, economic, political, and biological implications of poor understandings of race and the deliberate perpetuation of social and medical racism.
The Black Lives Matter movement (BLM) of 2013 began with the work of racial justice activists and community organizers Alicia Garza, Opal Tometi, and Patrissa Cullors. First incited by the murder of Trayvon Martin, a 17-year-old African American, and the acquittal of the man who shot him, BLM went on to protest against the deaths of numerous Black individuals, most of whom were killed by police officers (for example, Ahmaud Arbery was killed in February of 2020 by two white non-police officers). Some key characteristics of BLM from the start were its decentralized grassroots structure, the role of university students and social media in spreading awareness of the movement, and its embrace of other movements (e.g., climate justice, ending police brutality, feminist campaigns, queer activism, immigration reform, etc.). When George Floyd was murdered by a white police officer on May 25, 2020, the BLM gained new momentum, across 2,000-plus cities in the United States, and among many protesting against historic racism and police brutality in other contexts around the globe. Many in the biological anthropology community have responded to these events with a great dedication to working against systemic racism in society and institutions (American Association of Biological Anthropologists 2020).
BLM continues to be an important movement, as is evidenced in the degree of community organizing, mutual aid efforts, calls for political reform, progress toward curriculum reform and equality, inclusion and diversity (EDI) work in businesses and universities, the removal of monuments honoring historical figures associated with slavery and racism, and many other important actions. Garza (2016) writes: “The reality is that race in the United States operates on a spectrum from black to white … the closer you are to white on that spectrum, the better off you are.” Tometi (2016) has stated: “We need [a human rights movement that challenges systemic racism] because the global reality is that Black people are subject to all sorts of disparities in most of our challenging issues of our day. I think about climate change, and how six of the ten worst impacted nations by climate change are actually on the continent of Africa.” In the words of Cullors (2016), “Black Lives Matter is our call to action. It is a tool to reimagine a world where Black people are free to exist, free to live. It is a tool for our allies to show up differently for us.” We gather from their words the importance of learning from the egregious role that anthropologists have played in the past, recognizing the legacies of “scientific” justifications for eugenics and racism in our society today, and proactively working toward environmental and social equity.
Another major industry that engages in the quantification and interpretation of human variation is medical and clinical work (National Research Council [U.S.] Committee on Human Genome Diversity 1997). Large-scale genomic studies sampling from human populations distributed worldwide have produced detailed knowledge on variation in disease resistance or susceptibility between and within populations. Let’s think about drug companies who develop medicines for Black patients particularly. The predispositions to particular diseases are higher among people of African descent than some pharmaceutical businesses have taken into account. Through targeted sampling of various world groups, clinical geneticists may also identify genetic risk factors of certain common disorders such as chronic heart disease, asthma, diabetes, autoimmune diseases, and behavioral disorders. Having an understanding of population-specific biology is crucial in the development of therapies, medicines, and vaccinations, as not all treatments may be suitable for every human, depending on their genotype. During diagnosis and treatment, it is important to have an evolutionary perspective on gene-environment relationships in patients. Typological concepts of “race” are not useful, given that most racial groups (whether self-identified or not) popularly recognized lack homogeneity and are, in fact, variable. Cystic fibrosis, for instance, occurs in all world populations but can often be underdiagnosed in populations with African ancestry because it is thought of as a “white” disease (Yudell et al. 2016).
Sociologists, law scholars, and professors of race studies have written extensively on how genetic/technological/medical revolutions impact people of color. In her book, Fatal Invention: How Science, Politics, and Big Business Re-create Race in the Twenty-First Century (2013), Professor Dorothy E. Roberts writes about how technological advances have been used in resuscitating race as a biological category for dividing humans in essentialist ways (Figure 13.23). She notes how members of law enforcement have engaged in racial profiling, sometimes with the use of machine-learning and facial-recognition technologies. Ancestry-testing services also purport to tell us “what” we are and to insist that this information is “written” in our genes. Such advertising campaigns obscure the nuances of genetic variation with the primary motive of tapping into people’s desire to “know themselves” and driving up profits for their businesses. Commercial genetic testing reinforces the idea that genes map neatly onto race, all while generating massive stores of data in DNA databases. In Roberts’s view, the myth of the biological concept of race being perpetuated in these ways undermines a just society and reproduces racial inequalities.

The COVID-19 pandemic has had a significant impact on the world’s population, particularly people living in the economic Global South and many Black, Indigenous and communities of color residing in the Global North. We have witnessed disproportionately high numbers of COVID-related deaths and infection cases among marginalized groups. Many immigrants and ethnic minorities in various societies have also experienced scapegoating and blame directed at them for being the source of COVID-19 spread.
To inform us on how to interpret this current worldwide pandemic, historians and anthropologists are looking back at the lessons learned from past instances of racist medicine (discriminatory practices based on broader social discrimination) and medical racism (application of discriminatory practices justified on medical grounds). Historically, who could become doctors and medical professionals was often racialized, gendered, and class specific. This made it difficult for many to overcome prejudices against women, Black people, Indigenous individuals, or other people of color from becoming doctors and clinical researchers in places such as South Africa and the United States. This, in turn, affects the sorts of information we know about health levels and health outcomes among these very groups. In the past decade, long-overdue attention is finally being paid to how race affects biological outcomes. For instance, researchers have focused on the negative legacies of racial discrimination and racism-induced stress on hormone (im)balances, mental health disorders, cardiovascular disease prevalence, and other health outcomes (Kuzawa and Sweet 2009; Shonkoff, Slopen, and WIlliams 2021; Williams 2018). The technology and standards of protocol in medical testing have been scrutinized (for more on how pulse oximeters were not designed with nonwhite patients in mind, for example, see Sjoding et al. 2020). Scholars of race and medicine have also written on how illness and disease spread have often been used to perpetuate societal prejudices. This manifests as xenophobic tendencies at a societal level, such as the blaming of “outgroups” and increased “in-group” protectiveness. Overreliance on the idea that people are “inherently” disease carriers due to genetic or biological reasons leads to improper accounting for socioeconomic or infrastructural issues that lead to differential disease prevalence amongst minority communities. (For more on race and COVID, see Tsai 2021 as well as this textbook’s Chapter 16: Contemporary Topics: Human Biology and Health.)
Lastly, consider the changing field of forensic anthropology. In the past, forensic anthropologists ascribed ancestry or racial categories to sets of skeletons, reliant on the belief that different human groups will exhibit biologically “discrete” assortments so as to divide along culturally constructed categories (Sauer 1992). Now, a number of forensic anthropologists have argued that we should abandon these methods, both because it is unscientific and because it further validates and perpetuates this idea that race is biologically meaningful. As scientists, whether we affirm biological race as real has huge influence on the beliefs of members of the public, the judicolegal system, and law enforcement. Not all forensic experts agree with abandoning ancestry estimation. Some prefer to refocus on the neutral or selective causes of human biological variation, and assess how probabilistic it may be to assign bones of certain dimensions to one of several identified racial categories. These debates continue today as this textbook chapter is being written. More details on population affinity may be found in Chapter 15: Bioarchaeology and Forensic Anthropology.
It is important to remember that while it is possible to look for clues about one’s ancestry or geographic origin based on skull morphology, again, the amount of distinctiveness in any given sample makes it impossible to distinguish whether a cranium belongs to one group (Relethford 2009). Individuals can vary in their skeletal dimensions by continental origin, country origin, regional origin, sex, age, environmental factors, and the time period in which they lived, making it difficult to assign individuals to particular categories in a completely meaningful way (Ousley, Jantz, and Freid 2009). When forensic reports and scientific journal articles give an estimation of ancestry, it is crucial to keep in mind that responsible assignments of ancestry will be done through robust statistical testing and stated as a probability estimate. Today, we also live in a more globalized world where a skeletal individual may have been born originally to parents of two separate traditional racial categories. In contexts of great heterogeneity within populations, this definitely adds difficulty to the work of forensic scientists and anthropologists preparing results for the courtroom (genetic testing may be comparatively more helpful in such situations).
Did Deeper: Measuring FST
Richard Lewontin (1929‒) is a biologist and evolutionary geneticist who authored an article evaluating where the total genetic variation in humans lies. Titled “The Apportionment of Human Diversity” (Lewontin 1972), the article addressed the following question: On average, how genetically similar are two randomly chosen people from the same group when compared to two randomly chosen people from different groups?
Lewontin studied this problem by using genetic data. He obtained data for a large number of different human populations worldwide using 17 genetic markers (including alleles that code for various important enzymes and proteins, such as blood-group proteins). The statistical analysis he ran used a measure of human genetic differences in and among populations known as the fixation index (FST).
Technically, FST can be defined as the proportion of total genetic variance within a subpopulation relative to the total genetic variance from an entire population. Therefore, FST values range from 0 to 1 (or, sometimes you will see this stated as a percentage between 0% and 100%). The closer the FST value of a population (e.g., the world’s population) approaches 1, the higher the degree of genetic differentiation among subpopulations relative to the overall population (see Figure 13.24 for a detailed illustration).
In his article, Lewontin (1972) identified that most of human genetic differences (85.4%) were found within local subpopulations (e.g., the Germans or Easter Islanders), whereas 8.3% were found between populations within continental human groups, and 6.3% were attributable to traditional “race” groups (e.g., “Caucasian” or “Amerind”). These findings have been important for scientifically rejecting the existence of biological races (Long and Kittles 2003).
Talking About Human Biological Variation Going Forward
To conclude, utilizing the term races to describe human biological variation is not accurate or productive. Using a select few hundred genetic loci, or perhaps a number of phenotypic traits, it may be possible to assign individuals to a geographic ancestry, but what constitutes a bounded genetic or geographical grouping is both arbitrary and potentially harmful owing to ethical and historical reasons. The discipline of biological anthropology has moved past typological frameworks that shoehorn continuously variable human populations into discrete and socially constructed subsets. Improvements in the number of markers, the genetic technologies used to study variation, and the number of worldwide populations sampled have led to more nuanced understandings of human variation. It is of utmost importance that scientists make the following points clear to the public:
- Today, we refer to different local human groups as “populations.” What constitutes a population should be carefully defined in scientific reports based on some geographical, linguistic, or cultural criteria and some degree of relativity to other closely or distantly related human groups.
- Humans have significantly less genetic variation than other primates and mammals, and all human beings on Earth share 99.9% of their overall DNA. Some of the remaining 0.1% of human variation varies on a clinal or continuous basis, such as can be seen when looking at ABO blood-type polymorphisms worldwide.
- Many biological characteristics in humans are actually determined nonconcordantly and/or polygenically. Therefore, superiority or inferiority in human behavior or body form cannot justifiably be linked to fixed and innate differences between groups.
- Genetic distances are correlated with geographic distances among the global human population. This is especially apparent when we consider that genetic variation is highest in sub-Saharan Africa, and average genetic heterogeneity decreases in populations further away from the African continent in accordance with the migratory history of anatomically modern Homo sapiens.
- The effects of gene flow, genetic drift, and population bottlenecking are reflected in some phenotypic traits, such as cranial shape.
- We recognize other traits, like skin color and lactase persistence, to be the products of many millennia of natural selective pressures influencing human biology from the external environment.
Taken together, genetic analyses of human variation do not support 20th-century (or even-earlier) concepts of race. In discussions about human variation, these genomic results help clarify how biological variation is distributed across the human population today. Taking care to think about and debate the nature of human variation is important, because although the effects and events that produced genetic differences among groups occurred in the ancient past, sociocultural concepts about race and ethnicity continue to have real social, economic, and political consequences.
Beyond talking about variation in the university setting, it is important that teachers, researchers, and students of anthropology recognize and assume the responsibility of influencing public perspectives of human variation. Race-based classification systems were developed during the colonial era, the transatlantic trafficking of kidnapped Africans and the so-called “Scientific Revolution” by the first “anthropologists” and scholars of humankind’s variation. Unfortunately, some of their early ideas have persisted and evolved into present-day lived realities. Some of today’s politicians and socioeconomic bodies have racially charged agendas that promote racism or certain kinds of economic or racial inequalities. As anthropologists, we must acknowledge that while human “races” are not a biological reality, their status as a (misguided) social construction does have real consequences for many people (Antrosio 2011).
In other words, while “race” is a sociocultural invention, the treatment different individuals receive due to their perceived “race” can have significant financial, emotional, sociopolitical, and physiological costs. However—and importantly assuming a “color-blind” position when it comes to the topics of “race” and ethnicity (especially in political discussions) is actually counterproductive, because the negative social consequences of modern “race” ideas could be ignored, making it harder to examine and address instances of discrimination properly (Wise 2010). Rather than shy away from these topics, we can use our scientific findings to establish socially relevant and biologically accurate ideas concerning human diversity. Today, research into genetic and phenotypic differentiation among and within various human populations continues to expand in its scope, its technological capabilities, its sample sizes, and its ethical concerns. It is thanks to such scientific work done in the past few decades that we now have a deeper understanding not only of how humans vary but also of how we are biologically a rather homogenous, intermixing world population.
Review Questions
- How is the genetic variation of the human species distributed worldwide?
- What evolutionary processes are responsible for producing genotypic/phenotypic variation within and between human populations?
- Should we continue to attribute any value to “race” concepts older than 1950, based on our current understandings of human biological variation?
- How should we communicate scientific findings about human biological variation more accurately and responsibly to those outside the anthropological discipline?
Key Terms
Age of Discovery: A period between the late 1400s and late 1700s when European explorers and ships sailed extensively across the globe in pursuit of new trading routes and territorial conquest.
Ancestry: Biogeographical information about an individual, traced either through the study of an individual’s genome, skeletal characteristics, or some other form of forensic/archaeological evidence. Anthropologists carry out probabilistic estimates of ancestry. They attribute sets of human remains to distinctive “ancestral” groups using careful statistical testing and should report ancestry estimations with statistical probability values.
Binomial nomenclature: A system of naming living things developed by Linnaeus in the 1700s. It employs a scientific name made up of two italicized Latin or Greek words, with the first word capitalized and representative of an organism’s genus and the second word indicating an organism’s species (e.g., Homo sapiens, Australopithecus afarensis, Pongo tapanuliensis, etc.).
Biological anthropology: A branch of study under anthropology (the study of humankind) that focuses on when and where humans and our human ancestors first originated, how we have evolved and adapted globally over time, and the reasons why we see biological variation among humans worldwide today.
Biological determinism: The erroneous concept that an individual’s behavioral characteristics are innate and determined by genes, brain size, or other physiological attributes—and, notably, without the influence of social learning or the environment around the individual during development.
Bony labyrinth: A system of interconnected canals within the auditory (ear- or hearing-related) apparatus, located in the inner ear and responsible for balance and the reception of sound waves.
Cline: A gradient of physiological or morphological change in a single character or allele frequency among a group of species across environmental or geographical lines (e.g., skin color varies clinally, as, over many generations, human groups living nearer the equator have adapted to have more skin pigmentation).
Continuous variation: This term refers to variation that exists between individuals and cannot be measured using distinct categories. Instead, differences between individuals within a population in relation to one particular trait are measurable along a smooth, continuous gradient.
Cystic fibrosis: A genetic disorder in which one defective gene causes overproduction and buildup of mucus in the lungs and other bodily organs. It is most common in northern Europeans (but also occurs in other world populations).
Ecological niche: The position or status of an organism within its community and/or ecosystem, resulting from the organism’s structural and functional adaptations (e.g., bipedalism, omnivorous diets, lactose digestion, etc.).
Essentialism: A belief or view that an entity, organism, or human grouping has a specific set of characteristics that are fundamentally necessary to its being and classification into definitive categories.
Ethnicity: A term used commonly in an interchangeable way with the term race, complicated because how different people define this term depends on the qualities and characteristics they use to assign a label or identity to themselves and/or others (which may include aspects of family background, skin color, language(s) spoken, religion, physical proportions, behavior and temperament, etc.).
Eugenics: A set of beliefs and practices that involves the controlled selective breeding of human populations with the hope of improving their heritable qualities, especially through surgical procedures like sterilization and legal rulings that affect marriage rights for interracial couples.
Gene flow: A neutral (or nonselective) evolutionary process that occurs when genes get shared between populations.
Genetic drift: A neutral evolutionary process in which allele frequencies change from generation to generation due to random chance.
Heterogeneity: The quality of being diverse genetically.
Homogenous: The quality of being uniform genetically.
Human diversity: Human diversity is a measure of variation that may describe how many different forms of human there are, separated or clustered into groups according to some genetic, phenotypic, or cultural trait(s). The term can be applied to culture (in which case humans can be described as significantly diverse) or genetics (in which case humans are not diverse because all humans on Earth share a majority of their genes).
Human variation: Differences in biology, physiology, body chemistry, behavior, and culture. By measuring these differences, we understand the degrees of variation between individuals, groups, populations, or species.
Isolation-by-distance model: A model that predicts a positive relationship between genetic distances and geographical distances between pairs of populations.
Monogenetic: Pertaining to the idea that the origin of a species is situated in one geographic region or time (as opposed to polygenetic).
Mutation: A gene alteration in the DNA sequence of an organism. As a random, neutral evolutionary process that occurs over the course of meiosis and early cell development, gene mutations are possible sources of variation in any given human gene pool. Genetic mutations that occur in more than 1% of a population are termed polymorphisms.
Natural selection: An evolutionary process whereby certain traits are perpetuated through successive generations, likely owing to the advantages they give organisms in terms of chances of survival and/or reproduction.
Nonconcordance: The fact of genes or traits not varying with one another and instead being inherited independently.
Otherness: In postcolonial anthropology, we now understand “othering” to mean any action by someone or some group that establishes a division between “us” and “them” in relation to other individuals or populations. This could be based on linguistic or cultural differences, and it has largely been based on external characteristics throughout history.
Out-of-Africa model: A model that suggests that all humans originate from one single group of Homo sapiens in (sub-Saharan) Africa who lived between 100,000 and 315,000 years ago and who subsequently diverged and migrated to other regions across the globe.
Physical anthropology: This used to be the more common name given to the subdiscipline of anthropology centered upon the study of human origins, evolution and variation (also see biological anthropology above). This name for the field has gradually become less popular due to two reasons: first, it may not reflect our interests in other aspects of humankind that are not physical (such as those behavioral, cultural and spiritual), and second, using this term popular in the early decades of our field may be viewed by some as harkening back to a time when biological anthropologists conducted their work in unethical ways.
Polygenetic: Having many different ancestries, as in older theories about human origins that involved multiple traditional groupings of humans evolving concurrently in different parts of the world before they merged into one species through interbreeding and/or intergroup warfare. These earlier suggestions have now been overwhelmed by insurmountable evidence for a single origin of the human species in Africa (see the “Out-of-Africa model”).
Polymorphism: A genetic variant within a population (caused either by a single gene or multiple genes) that occurs at a rate of over 1% among the population. Polymorphisms are responsible for variation in phenotypic traits such as blood type and skin color.
Population: A group of humans living in a particular geographical area, with more local interbreeding within-group than interbreeding with other groups. A limited or restricted amount of gene flow between populations can occur due to geographical, cultural, linguistic, or environmental factors.
Population bottlenecking: An event in which genetic variation is significantly reduced owing to a sharp reduction in population size. This can occur when environmental disaster strikes or as a result of human activities (e.g., genocides or group migrations). An important example of this loss in genetic variation occurred over the first human migrations out of Africa and into other continental regions.
Prejudice: An unjustified attitude toward an individual or group that is not based on reason, whether positive (and showing preference for one group of people over another) or negative (and resulting in harm or injury to others).
Race: The identification of a group based on a perceived distinctiveness that makes that group more similar to each other than they are to others outside the group. This may be based on cultural differences, genetic parentage, physical characteristics, behavioral attributes, or something arbitrarily and socially constructed. As a social or demographic category, perceptions of “race” can have real and serious consequences for different groups of people. This is despite the fact that biological anthropologists and geneticists have demonstrated that all humans are genetically homogenous and that more differences can be found within populations than between them in the overall apportionment of human biological variation. This term is sometimes used interchangeably with ethnicity.
Racism: Any action or belief that discriminates against someone based on perceived differences in race or ethnicity.
Scientific Revolution: A period between the 1400s and 1600s when substantial shifts occurred in the social, technological, and philosophical sense, when a scientific method based on the collection of empirical evidence through experimentation was emphasized and inductive reasoning was used to test hypotheses and interpret their results.
Typological: Of or describing an assortment system that relies on the interpretation of qualitative similarities or differences in the study of variation among objects or people. The categorization of cultures or human groups according to “race” was performed with a typological approach in the earliest practice of anthropology, but this practice has since been discredited and abandoned.
Variance: In statistics, variance measures the dispersal of a set of data around the mean or average value.
About the Author

Michael B. C. Rivera, Ph.D.
University of Hong Kong, mrivera@hku.hk
Michael B. C. Rivera is a biological anthropologist and human bioarchaeologist who studies human evolution and history and works to develop these disciplines in Hong Kong, East/Southeast Asia, and the “Global South.” His doctoral thesis focused on the transition into agriculture in coastal environments and adaptations of ancient people along the beach. He is the only biological anthropologist working at the University of Hong Kong and the lead archaeologist managing the excavation of a WWII military aircraft that crashed in Hong Kong in 1945. Michael is also an advocate for greater inclusion, diversity, equality, and access to learning in academia. Much of his work also includes science communication and public engagement activities online, in schools, and in collaboration with museums.
For Further Exploration
Videos
American Medical Association (AMA). 2020. “Examining Race-Based Medicine.” YouTube, October 29. Accessed June 4, 2023.
Crenshaw, Kimberlé. 2016. “The Urgency of Intersectionality.” YouTube, December 7. Accessed June 4, 2023.
Golash-Boza, Tanya. 2018. “What Is Race? What Is Ethnicity? Is There a Difference?.” YouTube, October 28. Accessed June 4, 2023.
Lasisi, Tina. 2020. “How Hair Reveals the Futility of Race Categories.” National Museum of Natural History webinar, October 21.
Lasisi, Tina. 2022. “Where Does My Skin Color Come From?.” PBS Terra, August 18. Accessed June 4, 2023.
PBS Origins. 2018. “The Origin of Race in the USA.” YouTube, April 3. Accessed June 4, 2023.
Roberts, Dorothy. 2016. “The Problem with Race-Based Medicine.” YouTube, March 4. Accessed June 4, 2023.
Vox. 2015. “The Myth of Race, Debunked in 3 Minutes.” YouTube, January 13. Accessed June 4, 2023.
Podcast Episodes
Kwong, Emily, and Rebecca Ramirez. 2021. “Here’s a Better Way to Talk about Hair: A 16 Minute Listen with Tina, Lasisi” NPR Short Wave, October 6. Accessed June 4, 2023.
Speaking of Race. 2020. “Race and Health series.” Speaking of Race, April 10. Accessed June 4, 2023.
Websites
Choices Program. 2023. “An Interactive Timeline: Black Activism and the Long Fight for Racial Justice.” Choices Program, Brown University [Interactive Timeline], Updated February, 2023.
References
American Association of Biological Anthropologists. 2020. “An Open Letter to Our Community in Response to Police Brutality against African-Americans and a Call to Antiracist Action”. American Association of Biological Anthropologists, June 10, 2020. Accessed June 4, 2023.
Antrosio, Jason. 2011. “‘Race Reconciled’: Race Isn’t Skin Color, Biology, or Genetics.” Living Anthropologically (website), June 5, 2011; updated May 20, 2020. Accessed June 4, 2023.
Beals, Kenneth L., Courtland L. Smith, Stephen M. Dodd, J. Lawrence Angel, Este Armstrong, Bennett Blumenberg, Fakhry G. Girgis, et al. 1984. “Brain Size, Cranial Morphology, Climate, and Time Machines [and Comments and Reply].” Current Anthropology 25 (3): 301‒330.
Betti, Lia, François Balloux, Tsunehiko Hanihara, and Andrea Manica. 2010. “The Relative Role of Drift and Selection in Shaping the Human Skull.” American Journal of Physical Anthropology 141 (1): 76‒82. https://doi.org/10.1002/ajpa.21115.
Betti, Lia, François Balloux, William Amos, Tsunehiko Hanihara, and Andrea Manica. 2009. “Distance from Africa, Not Climate, Explains Within-Population Phenotypic Diversity in Humans.” Proceedings: Biological Sciences 276 (1658): 809‒814. https://doi.org/10.1098/rspb.2008.1563.
Betti, Lia, Noreen von Cramon-Taubadel, Andrea Manica, and Stephen J. Lycett. 2013. “Global Geometric Morphometric Analyses of the Human Pelvis Reveal Substantial Neutral Population History Effects, Even across Sexes.” PloS ONE 8 (2): e55909. https://doi.org/10.1371/journal.pone.0055909.
Betti, Lia, Noreen von Cramon-Taubadel, Andrea Manica, and Stephen J. Lycett. 2014. “The Interaction of Neutral Evolutionary Processes with Climatically Driven Adaptive Changes in the 3D Shape of the Human Os Coxae.” Journal of Human Evolution 73 (August): 64‒74. https://doi.org/10.1016/j.jhevol.2014.02.021.
Boas, Franz. 1931. “Race and Progress.” Science 74 1905): 1‒8.
Bowden, Rory, Tammie S. MacFie, Simon Myers, Garrett Hellenthal, Eric Nerrienet, Ronald E. Bontrop, Colin Freeman, Peter Donnelly, and Nicholas I. Mundy. 2012. “Genomic Tools for Evolution and Conservation in the Chimpanzee: Pan troglodytes ellioti Is a Genetically Distinct Population.” PLoS Genetics 8 (3): e1002504. https://doi.org/10.1371/journal.pgen.1002504.
Campbell, Michael C., and Sarah A. Tishkoff. 2008. “African Genetic Diversity: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping.” Annual Review of Genomics and Human Genetics 9: 403‒433.
Clee, Paul R. Sesink, Ekwoge E. Abwe, Ruffin D. Ambahe, Nicola M. Anthony, Roger Forso, Sabrina Locatelli, Fiona Maisels, et al. 2015. “Chimpanzee Population Structure in Cameroon and Nigeria Is Associated with Habitat Variation That May Be Lost Under Climate Change.” BMC Evolutionary Biology 15: 2. https://doi.org/10.1186/s12862-014-0275-z.
Cullors, Patrisse. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Fuentes, Agustín, Rebecca Rogers Ackermann, Sheela Athreya, Deborah Bolnick, Tina Lasisi, Sang-Hee Lee, Shay-Akil McLean, and Robin Nelson. 2019. “AAPA Statement on Race and Racism.” American Journal of Physical Anthropology 169 (3): 400‒402.
Garza, Alicia. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Gerbault, Pascale, Anke Liebert, Yuval Itan, Adam Powell, Mathias Currat, Joachim Burger, Dallas M. Swallow, and Mark G. Thomas. 2011. “Evolution of Lactase Persistence: An Example of Human Niche Construction.” Philosophical Transactions of the Royal Society B 366 (1566): 863‒877. https://doi.org/10.1098/rstb.2010.0268.
Hooton, Earnest A. 1936. “Plain Statements about Race.” Science 83 (2161): 511‒513.
Hrdlička, Aleš. 1918. “Physical Anthropology: Its Scope and Aims; Its History and Present Status in America. A: Physical Anthropology; Its Scopes and Aims.” American Journal of Physical Anthropology 1 (1): 3‒23.
Huxley, Julian. 1942. Evolution: The Modern Synthesis. London: Allen and Unwin.
Ingram, Catherine J. E., Charlotte A. Mulcare, Yuval Itan, Mark G. Thomas, and Dallas M. Swallow. 2009. “Lactose Digestion and the Evolutionary Genetics of Lactase Persistence.” Human Genetics 124 (6): 579‒591. https://doi.org/10.1007/s00439-008-0593-6.
Jablonski, Nina G. 2004. “The Evolution of Human Skin and Skin Color.” Annual Review of Anthropology 33: 585‒623. https://doi.org/10.1146/annurev.anthro.33.070203.143955.
Jablonski, Nina G., and George Chaplin. 2000. “The Evolution of Human Skin Coloration.” Journal of Human Evolution 39 (1): 57‒106. https://doi.org/10.1006/jhev.2000.0403.
Kanitz, Ricardo, Elsa G. Guillot, Sylvain Antoniazza, Samuel Neuenschwander, and Jérôme Gedout. 2018. “Complex Genetic Patterns in Human Arise from a Simple Range-Expansion Model over Continental Landmasses.” PLoS ONE 13 (2): e0192460.
Kronenberg, Zev N., Ian T. Fiddes, David Gordon, Shwetha Murali, Stuart Cantsilieris, Olivia S. Meyerson, Jason G. Underwood, et al. 2018. “High-Resolution Comparative Analysis of Great Ape Genomes.” Science 360 (6393): eaar6343. https://doi.org/10.1126/science.aar6343.
Kuzawa, Christopher W., and Elizabeth Sweet. 2009. “Epigenetics and the Embodiment of Race: Development Origins of US Racial Disparities in Cardiovascular Health.” American Journal of Human Biology 21 (1) : 2‒15.
Lasisi, Tina, and Mark D. Shriver. 2018. “Focus on African Diversity Confirms Complexity of Skin Pigmentation Genetics.” Genomic Biology 19: 13.
Lewontin, Richard. 1972. “The Apportionment of Human Diversity.” In Evolutionary Biology, vol. 6, edited by Theodosius Dobzhansky, Max K. Hecht, and William C. Steere, 381‒398. New York: Springer.
Linnaeus, Carl. 1758. Systema Naturae. Stockholm: Laurentius Salvius. http://www.cabdirect.org/abstracts/20057000018.html.
Liu, Hua, Franck Prugnolle, Andrea Manica, and François Balloux. 2006. “A Geographically Explicit Genetic Model of Worldwide Human-Settlement History.” American Journal of Human Genetics 79 (2): 230‒237.
Livingstone, Frank B. 1962. “On the Nonexistence of Human Races.” Current Anthropology 3 (3): 279‒281.
Long, Jeffery C., and Rick A. Kittles. 2003. “Human Genetic Diversity and the Nonexistence of Biological Races.” Human Biology 75 (4): 449‒471.
Luzzatto, Lucio. 2012. “Sickle Cell Anaemia and Malaria.” Mediterranean Journal of Hematology and Infectious Diseases 4 (1). https://doi.org/10.4084/MJHID.2012.065.
Manica, Andrea, William Amos, François Balloux, and Tsunehiko Hanihara. 2007. “The Effect of Ancient Population Bottlenecks on Human Phenotypic Variation.” Nature 448 (7151): 346‒348. https://doi.org/10.1038/nature05951.
McLean, Shay-Akil. 2014. “‘Race, Ethnicity, & Racism.” Decolonize ALL The Things Website, Accessed January 10, 2023. https://decolonizeallthethings.com/learning-tools/race-ethnicity-racism/.
Morton, Samuel George. 1839. Crania Americana, or, A Comparative View of the Skulls of Various Aboriginal Nations of North and South America. Philadelphia: J. Dobson.
Mourant, A. E., Ada C. Kopeć, and Kazimiera Domaniewska-Sobczak. 1976. The Distribution of the Human Blood Groups and Other Polymorphisms, 2nd edition. Oxford: Oxford University Press.
National Research Council (U.S.) Committee on Human Genome Diversity. 1997. Evaluating Human Genetic Diversity. Washington, D.C.: National Academies Press.
Omi, Michael, and Howard Winant. 2014. “The Theory of Racial Formation.” In Racial Formation in the United States,3rd edition, edited by Michael Omi and Howard Winant, 105‒126. Routledge: New York.
Osada, Naoki. 2015. “Genetic Diversity in Humans and Non-Human Primates and Its Evolutionary Consequences.” Genes and Genetic Systems 90 (3): 133‒145.
Ousley, Stephen D., Richard L. Jantz, and Donna Freid. 2009. “Understanding Race and Human Variation: Why Forensic Anthropologists Are Good at Identifying Race.” American Journal of Physical Anthropology 139 (1): 68‒76. https://doi.org/10.1002/ajpa.21006.
Ponce de León, Marcia S., Toetik Koesbardiati, John David Weissmann, Marco Millela, Carlos S. Reyna-Blanco, Gen Suwa, Osamu Kondo, Anna-Sapfo Malaspinas, Tim D. White, and Christoph P. E. Zollikofer. 2018. “Human Bony Labyrinth Is an Indicator of Population History and Dispersal from Africa.” Proceedings of the National Academy of Sciences 115 (16): 4128‒4133. https://doi.org/10.1073/pnas.1808125115.
Prado-Martinez, Javier, Peter H. Sudmant, Jeffrey M. Kidd, Heng Li, Joanna L. Kelley, Belen Lorente-Galdos, Krishna R. Veeramah, et al. 2013. “Great Ape Genetic Diversity and Population History.” Nature 499 (7459): 471–475. https://doi.org/10.1038/nature12228.
Prugnolle, Franck, Andrea Manica, and François Balloux. 2005. “Geography Predicts Neutral Genetic Diversity of Human Populations.” Current Biology 15 (5): 159‒160.
Quillen, Ellen E., Heather L. Norton, Esteban J. Parra, Frida Lona-Durazo, Khai C. Ang, Florin Mircea Illiescu, Laurel N. Pearson, et al. 2018. “Shades of Complexity: New Perspectives on the Evolution and Genetic Architecture of Human Skin.” American Journal of Physical Anthropology 168 (S67): 4–26.
Rathmann, Hannes, Hugo Reyes-Centeno, Silvia Ghirotto, Nicole Creanza, Tsunehiko Hanihara, and Katerina Harvati. 2017. “Reconstructing Human Population History from Dental Phenotypes.” Scientific Reports 7: 12495. https://doi.org/10.1038/s41598-017-12621-y.
Relethford, John H. 2001. “Global Analysis of Regional Differences in Craniometric Diversity and Population Substructure.” Human Biology 73 (5): 629‒636. https://doi.org/10.1353/hub.2001.0073.
Relethford, John H. 2002. “Apportionment of Global Human Genetic Diversity Based on Craniometrics and Skin Color.” American Journal of Physical Anthropology 118 (4): 393‒398. https://doi.org/10.1002/ajpa.10079.
Relethford, John H. 2004. “Global Patterns of Isolation by Distance Based on Genetic and Morphological Data.” Human Biology 76 (4): 499‒513. https://doi.org/10.1353/hub.2004.0060.
Relethford, John H. 2009. “Race and Global Patterns of Phenotypic Variation.” American Journal of Physical Anthropology 139 (1): 16‒22. https://doi.org/10.1002/ajpa.20900.
Roberts, Dorothy. 2013. Fatal Invention: How Science, Politics, and Big Business Re-Create Race in the Twenty-First Century. New York: The New Press.
Rosenberg, Noah A., Saurabh Mahajan, Sohini Ramachandran, Chengfeng Zhao, Jonathan K. Pritchard, and Marcus W. Feldman. 2005. “Clines, Clusters, and the Effect of Study Design on the Inference of Human Population Structure.” PLoS Genetics 1 (6): e70. https://doi.org/10.1371 /journal.pgen.0010070.
Rosenberg, Noah A., Jonathan K. Pritchard, James L. Weber, Howard M. Cann, Kenneth K. Kidd, Lev A. Zhivotovsky, and Marcus W. Feldman. 2002. “Genetic Structure of Human Populations.” Science 298 (5602): 2381‒2385.
Sauer, Norman J. 1992. “Forensic Anthropology and the Concept of Race: If Races Don’t Exist, Why Are Forensic Anthropologists So Good at Identifying Them?” Social Science and Medicine 34 (2): 107‒111. https://doi.org/10.1016/0277-9536(92)90086-6.
Shonkoff, Jack P., Natalie Slopen, and David R. Williams. 2021. “Early Childhood Adversity, Toxic Stress, and the Impacts of Racism on the Foundations of Health.” Annual Review of Public Health 42: 115‒134.
Sjoding, Michael W., Robert P. Dickson, Theodore J. Iwashyna, Steven E. Gay, and Thomas S. Valley. 2020. “Racial Bias in Pulse Oximetry Measurement.” The New England Journal of Medicine 383: 2477-2478.
Staes, Nicky, Chet C. Sherwood, Katharine Wright, Marc de Manuel, Elaine E. Guevara, Tomas Marques-Bonet, Michael Krützen, et al. 2017. “FOXP2 Variation in Great Ape Populations Offers Insight into the Evolution of Communication Skills.” Scientific Reports 7 (1): 1‒10. https://doi.org/10.1038/s41598-017-16844-x.
Tomati, Opal. 2016. “An Interview with the Founders of Black Lives Matter.” TED Talks 2016, October 26‒28. Accessed June 15, 2023. https://www.ted.com/talks/alicia_garza_patrisse_cullors_and_opal_tometi_an_interview_with_the_founders_of_black_lives_matter/up-next.
Tsai, Jennifer. 2021. “COVID-19 Is Not a Story of Race, but a Record of Racism—Our Scholarship Should Reflect That Reality.” The American Journal of Bioethics 21 (2): 43‒47. https://doi.org/10.1080/15265161.2020.1861377.
von Cramon-Taubadel, Noreen, and Stephen J. Lycett. 2008. “Brief Communication: Human Cranial Variation Fits Iterative Founder Effect Model with African Origin.” American Journal of Physical Anthropology 136 (1): 108‒113. https://doi.org/10.1002/ajpa.20775.
Weiss, Kenneth M., and Jeffrey C. Long. 2009. “Non-Darwinian Estimation: My Ancestors, My Genes’ Ancestors.” Genome Research 19: 703‒710. https://doi.org/10.1101/gr.076539.108.19.
Williams, David W. 2018. “Stress and the Mental Health of Populations of Color: Advancing Our Understanding of Race-related Stressors.” Journal of Health and Social Behavior 59 (4): 466‒485.
Wise, Tim. 2010. Colorblind: The Rise of Post-Racial Politics and the Retreat from Racial Equity. San Francisco: City Lights.
Yudell, Michael, Dorothy Roberts, Rob DeSalle, and Sarah Tishkoff. 2016. “Taking Race out of Human Genetics.” Science 351 (6273): 564‒565. https://doi.org/10.1126/science.aac4951.
Jonathan Marks, Ph.D., University of North Carolina at Charlotte
Adam P. Johnson, M.A., University of North Carolina at Charlotte/University of Texas at San Antonio
This chapter is an adaptation of "Chapter 2: Evolution” by Jonathan Marks. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Explain the relationship among genes, bodies, and organismal change.
- Discuss the shortcomings of simplistic understandings of genetics.
- Describe what is meant by the "biopolitics of heredity."
- Discuss issues caused by misuse of ideas about adaptations and natural selection.
- Examine and correct myths about evolution.
The Human Genome Project, an international initiative launched in 1990, sought to identify the entire genetic makeup of our species. For many scientists, it meant trying to understand the genetic underpinnings of what made humans uniquely human. James Watson, a codiscoverer of the helical shape of DNA, wrote that “when finally interpreted, the genetic messages encoded within our DNA molecules will provide the ultimate answers to the chemical underpinnings of human existence” (Watson 1990, 248). The underlying message is that what makes humans unique can be found in our genes. The Human Genome Project hoped to find the core of who we are and where we come from.
Despite its lofty goal, the Human Genome Project—even after publishing the entire human genome in January 2022—could not fully account for the many factors that contribute to what it is to be human. Richard Lewontin, Steven Rose, and Leon Kamin (2017) argue that genetic determinism of the sort assumed by the Human Genome Project neglects other essential dimensions that contribute to the development and evolution of human bodies, not to mention the role that culture plays. They use an apt metaphor of a cake to illustrate the incompleteness of reductive models. Consider the flavor of a cake and think of the ingredients listed in the recipe. The recipe includes ingredients such as flour, sugar, shortening, vanilla extract, eggs, and milk. Does raw flour taste like cake? Does sugar, vanilla extract, or any of the other ingredients taste like cake? They do not, and knowing the individual flavors of each ingredient does not tell us much about what cake tastes like. Even mixing all of the ingredients in the correct proportions does not get us cake. Instead, external factors such as baking at the right temperature, for the right amount of time, and even the particularities of our evolved sense of taste and smell are all necessary components of experiencing the cake.
Lewontin, Rose, and Kamin (2017) argue that the same is true for humans and other organisms.
Knowing everything about cake ingredients does not allow us to fully know cake. Equally so, knowing everything about the genes found in our DNA does not allow us to fully know humans. Different, interacting levels are implicated in the development and evolution of all organisms, including humans. Genes, the structure of chromosomes, developmental processes, epigenetic tags, environmental factors, and still-other components all play key roles such that genetically reductive models of human development and evolution are woefully inadequate.
The complex interactions across many levels—genetic, developmental, and environmental—explain why we still do not know how our one-dimensional DNA nucleotide sequence results in a four-dimensional organism. This was the unfulfilled promise of the inception of the Human Genome Project in the 1980s and 1990s: the project produced the complete DNA sequence of a human cell in the hopes that it would reveal how human bodies are built and how to cure them when they are built poorly. Yet, that information has remained elusive. Presumably, the knowledge of how organisms are produced from DNA sequences will one day permit us to reconcile the discrepancies between patterns in anatomical evolution and molecular evolution.
In this chapter, we will consider multilevel evolution and explore evolution as a complex interaction between genetic and epigenetic factors as well as the environments in which organisms live. Next, we will examine the biopolitical nature of human evolution. We will then investigate problems that arise from attributing all traits to an adaptive function. Finally, we will address common misconceptions about evolution. The goal of this chapter is to provide you with the necessary toolkit for understanding the molecular, anatomical, and political dimensions of evolution.
Evolution Happens at Multiple Levels
Following Richard Dawkins’s publication of The Selfish Gene in 1976, the scientific imagination was captured by the potential of genomics to reveal how genes are copied by Darwinian selection. Dawkins argues that the genes in individuals that contribute to greater reproductive success are the units of selection. His conception of evolution at the molecular level undercuts the complex interactions between organisms and their environments, which are not expressed genomically but are nevertheless key drivers in evolution.
By the 1980s, the acknowledgment among most biologists that even though genes construct bodies, genes and bodies evolve at different rates and with distinct patterns. This realization led to a renewed focus on how bodies change. The Evolutionary Synthesis of the 1930s–1970s had reduced organisms to their genotypes and species to their gene pools, which provided valuable insights about the processes of biological change, but it was only a first approximation. Animals are in fact reactive and adaptable beings, not passive and inert genotypes. Species are clusters of socially interacting and reproductively compatible organisms.

Once we accept that evolutionary change is fundamentally genetic change, we can ask: How do bodies function and evolve? How do groups of animals come to see one another as potential mates or competitors for mates, as opposed to just other creatures in the environment? Are there evolutionary processes that are not explicable by population genetics? These questions—which lead us beyond reductive assumptions—were raised in the 1980s by Stephen Jay Gould, the leading evolutionary biologist of the late 20th century (see: Gould 2003; 1996).
Gould spearheaded a movement to identify and examine higher-order processes and features of evolution that were not adequately explained by population genetics. For example, extinction, which was such a problem for biologists of the 1600s, could now be seen as playing a more complex role in the history of life than population genetics had been able to model. Gould recognized that there are two kinds of extinctions, each with different consequences: background extinctions and mass extinctions. Background extinctions are those that reflect the balance of nature, because in a competitive Darwinian world, some things go extinct and other things take their place. Ecologically, your species may be adapted to its niche, but if another species comes along that’s better adapted to the same niche, eventually your species will go extinct. It sucks, but it is the way of all life: you come into existence, you endure, and you pass out of existence. But mass extinctions are quite different. They reflect not so much the balance of nature as the wholesale disruption of nature: many species from many different lineages dying off at roughly the same time—presumably as the result of some kind of rare ecological disaster. The situation may not be survival of the fittest as much as survival of the luckiest. The result, then, would be an ecological scramble among the survivors. Having made it through the worst, the survivors could now simply divide up the new ecosystem amongst themselves, since their competitors were gone. Something like this may well have happened about 65 million years ago, when a huge asteroid hit the Yucatan Peninsula, which mammals survived but dinosaurs did not (Figure 17.1). Something like this may be happening now, due to human expansion and environmental degradation. Note, though, that there is only a limited descriptive role here for population genetics: the phenomena we are describing are about organisms and species in ecosystems.
Another question involved the disconnect between properties of species and the properties of gene pools. For example, there are upwards of 15 species of gibbons but only two species of chimpanzees. Why? There are upwards of 20 species of guenons but fewer than ten of baboons. Why? Are there genes for that? It seems unlikely. Gould suggested that species, as units of nature, might have properties that are not reducible to the genes in their cells. For example, rates of speciation and extinction might be properties of their ecologies and histories rather than their genes. Thus, relationships between environmental contexts and variability within a species result in degrees of resistance to extinction and affect the frequency and rates at which clades diversify (Lloyd and Gould 1993). Consistent biases of speciation rates might well produce patterns of macroevolutionary diversity that are difficult to explain genetically and better understood ecologically. Gould called such biases in speciation rates species selection—a higher-order process that invokes competition between species, in addition to the classic Darwinian competition between individuals.
One of Gould’s most important studies involved the very nature of species. In the classical view, a species is continually adapting to its environment until it changes so much that it is a different species than it was at the beginning of this sentence (Eldredge and Gould 1972). That implies that the species is a fundamentally unstable entity through time, continuously changing to fit in. But suppose, argued Gould along with paleontologist Niles Eldredge, a species is more stable through time and only really adapts during periods of ecological instability and change. Then we might expect to find in the fossil record long equilibrium periods—a few million years or so—in which species don’t seem to change much, punctuated by relatively brief periods in which they change a bit and then stabilize again as new species. They called this idea punctuated equilibria. The idea helps to explain certain features of the fossil record, notably the existence of small anatomical “gaps” between closely related fossil forms (Figure 17.2). Its significance lies in the fact that although it incorporates genetics, punctuated equilibria is not really a theory of genetics but one of types bodies in deep time.
Punctuated equilibria is seen across taxa, with long periods in the fossil record representing little phenotypic change. These periods of stability are disrupted by shorter periods of rapid adaptation, the process through which populations of organisms become suited to living in their environments. Phenotypic changes are often coupled with drastic climatic or ecological changes that affect the milieu in which organisms live. For example, throughout much of hominin evolutionary history, brain size was closely associated with body size and thus remained mostly stable. However, changes occurred in average hominin brain size at around 100 thousand years ago, 1 million years ago, and 1.8 million years ago. Several hypotheses have been put forth to explain these changes, including unpredictability in climate and environment (Potts 1998), social development (Barton 1996), and the evolution of language (Deacon 1998). Evidence from the fossil record, paleoclimate models, and comparative anatomy suggests that the changes observed in hominin lineage result from biocultural processes—that is, the coalescence of environmental and cultural factors that selected for larger brains (Marks 2015; Shultz, Nelson, and Dunbar 2012).
In response to the call for a theory of the evolution of form, the field of evo-devo—the intersection of evolutionary and developmental biology—arose. The central focus here is on how changes in form and shape arise. An embryo matures by the stimulation of certain cells to divide, forming growth fields. The interactions and relationships among these growth fields generate the structures of the body. The hox genes that regulate these growth fields turn out to be highly conserved across the animal kingdom. This is because they repeatedly turn on and off the most basic genes guiding the animal’s development, and thus any changes to them would be catastrophic. Indeed, these genes were first identified by manipulating them in fruit flies, such that one could produce a bizarre mutant fruit fly that grew a pair of legs where its antennae were supposed to be (Kaufman, Seeger, and Olsen 1990).
Certain genetic changes can alter the fates of cells and the body parts, while other genetic changes can simply affect the rates at which neighboring groups of cells grow and divide, thus producing physical bumps or dents in the developing body. The result of altering the relationships among these fields of cellular proliferation in the growing embryo is allometry, or the differential growth of body parts. As an animal gets larger—either over the course of its life or over the course of macroevolution—it often has to change shape in order to live at a different size. Many important physiological functions depend on properties of geometric area: the strength of a bone, for example, is proportional to its cross-sectional area. But area is a two-dimensional quality, while growing takes place in three dimensions—as an increase in mass or volume. As an animal expands, its bones necessarily weaken, because volume expands faster than area does. Consequently a bigger animal has more stress on its bones than a smaller animal does and must evolve bones even thicker than they would be by simply scaling the animal up proportionally. In other words, if you expand a mouse to the size of an elephant, it will nevertheless still have much thinner bones than the elephant does. But those giant mouse bones will unfortunately not be adequate to the task. Thus, a giant mouse would have to change aspects of its form to maintain function at a larger size (see Figure 17.3).
Physiologically, we would like to know how the body “knows” when to turn on and off the genes that regulate growth to produce a normal animal. Evolutionarily, we would like to know how the body “learns” to alter the genetic on/off switch (or the genetic “slow down/speed up” switch) to produce an animal that looks different. Moreover, since organisms differ from one another, we would like to know how the developing body distinguishes a range of normal variation from abnormal variation. And, finally, how does abnormal variation eventually become normal in a descendant species?
Taking up these questions, Gould invoked the work of a British geneticist named Conrad H. Waddington, who thought about genetics in less reductive ways than his colleagues. Rather than isolate specific DNA sites to analyze their function, Waddington instead studied the inheritance of an organism’s reactivity—its ability to adapt to the circumstances of its life. In a famous experiment, he grew fruit fly eggs in an atmosphere containing ether. Most died, but a few survived somehow by developing a weird physical feature: a second thorax with a second pair of wings. Waddington bred these flies and soon developed a stable line of flies who would reliably develop a second thorax when grown in ether. Then he began to lower the concentration of ether, while continuing to selectively breed the flies that developed the strange appearance. Eventually he had a line of flies that would stably develop the “bithorax” phenotype–the suite of traits of an organism–even when there was no ether; it had become the “new normal.” The flies had genetically assimilated the bithorax condition.
Waddington was thus able to mimic the inheritance of acquired characteristics: what had been a trait stimulated by ether a few generations ago was now a normal part of the development of the descendants. Waddington recognized that while he had performed a selection experiment on genetic variants, he had not selected for particular traits. Rather, he helped produce the physiological tendency to develop particular traits when appropriately stimulated. He called that tendency plasticity and its converse, the tendency to stay the same even under weird environmental circumstances, canalization. Waddington had initially selected for plasticity, the tendency to develop the bithorax phenotype under weird conditions, and then, later, for canalization, the developmental normalization of that weird physical trait. Although Waddington had high stature in the community of geneticists, evolutionary biologists of the 1950s and 1960s regarded him with suspicion because he was not working within the standard mindset of reductionism, which saw evolution as the spread of genetic variants that coded for favorable traits. Both Waddington and Gould resisted contemporary intellectual paradigms that favored reductive accounts of evolutionary processes. They conceived of evolution as an emergent process in which many external factors (e.g. climate, environment, predation) and internal factors (e.g., genotypes, plasticity, canalization) coalesce to produce the evolutionary trends that we observe in the fossil record and our genome.
While Gould and Waddington both looked beyond the genome to understand evolution, the Human Genome Project—an international project with the goal of identifying each base pair in the human genome in the 1990s—generated a great deal of public interest in analyzing the human DNA sequence from the standpoint of medical genetics. Some of the rhetoric aimed to sell the public on investing a lot of money and resources in sequencing the human genome in order to show the genetic basis of heritable traits, cure genetic diseases, and learn what it means ultimately to be biologically human. However, the Human Genome Project was not actually able to answer those questions through the use of genetics alone, and thus a broader, more holistic account was required.
This holistic account came from decades of research in human biology and anthropology, which understood the human body as highly adaptable, dynamic, and emergent. For example, in the early 20th century, anthropologist Franz Boas measured the skulls of immigrants to the U.S., revealing that environmental, not merely genetic, factors affected skull shape. The growing human body adjusts itself to the conditions of life, such as diet, sunshine, high altitude, hard labor, population density, how babies are carried—any and all of which can have subtle but consistent effects upon its development. There can thus be no normal human form, only a context-specific range of human forms.
However, what the human biologists called human adaptability, evolutionary biologists called developmental plasticity, and evidence quickly began to mount for its cause being epigenetic modifications to DNA. Epigenetic modifications are changes to how genes are used by the body (as opposed to changes in the DNA sequences; see Chapter 3). Scientific interest shifted from the focus of the Human Genome Project to the ways that bodies are made by evolutionary-developmental processes, including epigenetics. What is meant by “epigenetic modification”? Evolution is about how descendants diverge from their ancestors. Inheritance from parent to offspring is still critical to this process, which occurs through genetic recombination: the pairing of homologous chromosomes and sharing of genetic material during meiosis (see Chapter 3). However, in the 21st century, the link between evolution and inheritance has broadened with a clearer understanding of how environmental and developmental factors shape bodies and the expression of genes, including epigenetic inheritance patterns. While offspring inherit their genes through random assortment during meiosis, environmental factors also shape how genes are used. When these epigenetic modifications occur in germ cells, they can be passed onto offspring. In these cases, there is no change in the DNA sequence but rather in how genes are used by the body due to DNA methylation and the structure of chromosomes due to histone acetylation (see Chapter 3).
In addition, we now recognize that evolution is affected by two other forms of intergenerational transmission and inheritance (in addition to genetics and epigenetics). These forms include behavioral variation and culture. That is, behavioral information can be transmitted horizontally (intragenerationally), permitting more rapid ways for organisms to adjust to the environment. And, then there is the fourth mode of transmission: the cultural or symbolic mode. Humans are the only species that horizontally transmits an arbitrary set of rules to govern communication, social interaction, and thought. This shared information is symbolic and has resulted in what we recognize as “culture”: locally emergent worlds of names, words, pictures, classifications, revered pasts, possible futures, spirits, dead ancestors, unborn descendants, in-laws, politeness, taboo, justice, beauty, and story, all accompanied by practices and a material world of tools.
Consequently our contemporary ideas about evolution see the evolutionary processes as hierarchically organized and not restricted to the differential transmission of DNA sequences into the next generation. While that is indeed a significant part of evolution, the organism and species are nevertheless crucial to understanding how those DNA sequences get transmitted. Further, the transmission of epigenetic, behavioral, and symbolic information play a complex role in perpetuating our genes, bodies, and species. In the case of human evolution, one can readily see that symbolic information and cultural adaptation are far more central to our lives and our survival today than DNA and genetic adaptation. It is thus misleading to think of humans passively occupying an environmental niche. Rather, humans are actively engaged in constructing our own niches, as well as adapting to them and using them to adapt. The complex interplay between a species and its active engagement in creating its own ecology is known as niche construction. If we understand natural selection–the process by which populations adapt to their specific environments–as the effects that environmental context has on the reproductive success of organisms, then niche construction is the process through which organisms shape their own selective pressures.
The Biopolitics of Heredity
“Science isn’t political” is a sentiment that you have likely heard before. Science is supposed to be about facts and objectivity. It exists, or at least ought to, outside of petty human concerns. However, the sorts of questions we ask as scientists, the problems we choose to study, the categories and concepts we use, who gets to do science, and whose work gets cited are all shaped by culture. Doing science is a political act. This fact is markedly true for human evolution. While it is easier to create intellectual distance between us and fruit flies and viruses, there is no distance when we are studying ourselves. The hardest lesson to learn about human evolution is that it is intensely political. Indeed, to see it from the opposite side, as it were, the history of creationism—the belief that the universe was divinely created around 6,000 years ago—is essentially a history of legal decisions. For instance, in Tennessee v. John T. Scopes (1925), a schoolteacher was prosecuted for violating a law in Tennessee that prohibited the teaching of human evolution in public schools, where teachers were required by law to teach creationism.
More recently, legal decisions aimed at legislating science education have shaped how students are exposed to evolutionary theory. For instance, McLean v. Arkansas (1982) dispatched “scientific creationism” by arguing that the imposition of balanced teaching of evolution and creationism in science classes violates the Establishment Clause, separating church and state. Additionally, Kitzmiller v. Dover (Pennsylvania) Area School District (2005) dispatched the teaching of “intelligent design” in public school classrooms as it was deemed to not be science. In some cases, people see unbiblical things in evolution, although most Christian theologians are easily able to reconcile science to the Bible. In other cases, people see immoral things in evolution, although there is morality and immorality everywhere. And some people see evolution as an aspect of alt-religion, usurping the authority of science in schools to teach the rejection of the Christian faith, which would be unconstitutional due to the protected separation of church and state.
Clearly, the position that politics has nothing to do with science is untenable. But is the politics in evolution an aberration or is it somehow embedded in science? In the early 20th century, scientists commonly promoted the view that science and politics were separate: science was seen as a pure activity, only rarely corrupted by politics. And yet as early as World War I, the politics of nationalism made a hero of the German chemist Fritz Haber for inventing poison gas. And during World War II, both German doctors and American physicists, recruited to the war effort, helped to end many civilian lives. Therefore, we can think of the apolitical scientist as a self-serving myth that functions to absolve scientists of responsibility for their politics. The history of science shows how every generation of scientists has used evolutionary theory to rationalize political and moral positions. In the very first generation of evolutionary science, Darwin’s Origin of Species (1859) is today far more readable than his Descent of Man (1871). The reason is that Darwin consciously purged The Origin of Species of any discussion of people. And when he finally got around to talking about people, in The Descent of Man, he simply imbued them with the quaint Victorian prejudices of his age, and the result makes you cringe every few pages. There is plenty of politics in there—sexism, racism, and colonialism—because you cannot talk about people apolitically.
One immediate faddish deduction from Darwinism, popularized by Herbert Spencer (1864) as “survival of the fittest,” held that unfettered competition led to advancement in nature and to human history. Since the poor were purported losers in that struggle, anything that made their lives easier would go against the natural order. This position later came to be known ironically as “Social Darwinism.” Spencer was challenged by fellow Darwinian Thomas Huxley (1863), who agreed that struggle was the law of the jungle but observed that we don’t live in jungles anymore. The obligation to make lives better for others is a moral, not a natural, fact. We simultaneously inhabit a natural universe of descent from apes and a moral universe of injustice and inequality, and science is not well served by ignoring the latter.
Concurrently, the German biologist Ernst Haeckel’s 1868 popularization of Darwinism was translated into English a few years later as The History of Creation. As we saw earlier, Haeckel was determined to convince his readers that they were descended from apes, even in the absence of fossil evidence attesting to it. When he made non-Europeans into the missing links that connected his readers to the apes, and depicted them as ugly caricatures, he knew precisely what he was doing. Indeed, even when the degrading racial drawings were deleted from the English translation of his book, the text nevertheless made his arguments quite clear. And a generation later, when the Americans had not yet entered the Great War in 1916, a biologist named Vernon Kellogg visited the German High Command as a neutral observer and found that the officers knew a lot about evolutionary biology, which they had gotten from Haeckel and which rationalized their military aggressions. Kellogg went home and wrote a bestseller about it, called Headquarters Nights (1917). World War I would have been fought with or without evolutionary theory, but as a source of scientific authority, evolution—even if a perversion of the Darwinian theory—had very quickly attained global geopolitical relevance.
Oftentimes, politics in evolutionary science is subtle, due to the pervasive belief in the advancement of science. We recognize the biases of our academic ancestors and modify our scientific stories accordingly. But we can never be free of our own cultural biases, which are invisible to us, as much as our predecessors’ biases were invisible to them. In some cases, the most important cultural issues resurface in different guises each generation, like scientific racism. Scientific racism is the recruitment of science for the evil political ends of racism, and it has proved remarkably impervious to evolution. Before Darwin, there was creationist scientific racism, and after Darwin, there was evolutionist scientific racism. And there is still scientific racism today, self-justified by recourse to evolution, which means that scientists have to be politically astute and sensitive to the uses of their work to counter these social tendencies.
Consider this: Are you just your ancestry, or can you transcend it? If that sounds like a weird question, it was actually quite important to a turn-of-the-20th-century European society in which an old hereditary aristocracy was under increasing threat from a rising middle class. And that is why the very first English textbook of Mendelian genetics concluded with the thought that “permanent progress is a question of breeding rather than of pedagogics; a matter of gametes, not of training … the creature is not made but born” (Punnett 1905, 60). Translation: Not only do we now know a bit about how heredity works, but it’s also the most important thing about you. Trust me, I’m a scientist.
Yet evolution is about how descendants come to differ from ancestors. Do we really know that your heredity, your DNA, your ancestry, is the most important thing about you? That you were born, not made? After all, we do know that you could be born into slavery or as a peasant, and come from a long line of enslaved people or peasants, and yet not have slavery or peasantry be the most important thing about you. Whatever your ancestors were may unfortunately constrain what you can become, but as a moral precept, it should not. But just as science is not purely “facts and objectivity,” ancestry is not a strictly biological concept. Human ancestry is biopolitics, not biology.
Evolution is fundamentally a theory about ancestry, and yet ancestors are, in the broad anthropological sense, sacred: ancestors are often more meaningful symbolically than biologically. Just a few years after The Origin of Species (Darwin 1859), the British politician and writer Benjamin Disraeli declared himself to be on the side of the angels, not the apes, and to “repudiate with indignation and abhorrence those new-fangled theories” (Monypenny, Flavelle, and Buckle 1920, 105). He turned his back on an ape ancestry and looked to the angel; yet, he did so as a prominent Jew-turned-Anglican, who had personally transcended his humble roots and risen to the pinnacle of the Empire. Ancestry was certainly important, and Disraeli was famously proud of his, but it was also certainly not the most important thing, not the primary determinant of his place in the world. Indeed, quite the opposite: Disraeli’s life was built on the transcendence of many centuries of Jewish poverty and oppression in Europe. Humble ancestry was there to be superseded and nobility was there to be earned; Disraeli would later become the Earl of Beaconsfield. Clearly, “are you just your ancestry” is not a value-neutral question, and “the creature is not made, but born” is not a value-neutral answer.
Ancestry being the most important thing about a person became a popular idea twice in 20th century science. First, at the beginning of the century, when the eugenics movement in America called attention to “feeble-minded stocks,” which usually referred to the poor or to immigrants (see Figure 17.4; and see Chapter 2). This movement culminated in Congress restricting the immigration of “feeble-minded races” (said to include Jews and Italians) in 1924, and the Supreme Court declaring it acceptable for states to sterilize their “feeble-minded” citizens involuntarily in 1927. After the Nazis picked up and embellished these ideas during World War II, Americans moved swiftly away from them in some contexts (e.g., for most people of European descent) while still strictly adhering in other contexts (e.g., Japanese internment camps and immigration restrictions).

Ancestry again became paramount in the drumming up of public support for the Human Genome Project in the 1990s. Public support for sequencing the human genome was encouraged by a popular science campaign that featured books titled The Book of Man (Bodmer and McKie 1997), The Human Blueprint (Shapiro 1991), and The Code of Codes (Kevles and Hood 1993). These books generally promised cures for genetic diseases and a deeper understanding of the human condition. We can certainly identify progress in molecular genetics over the last couple of decades since the human genome was sequenced, but that progress has notably not been accompanied by cures for genetic diseases, nor by deeper understandings of the human condition.
Even at the most detailed and refined levels of genetic analysis, we still don’t have much of an understanding of the actual basis by which things seem to “run in families.” While the genetic basis of simple, if tragic, genetic diseases have become well-known—such as sickle-cell anemia, cystic fibrosis, and Tay-Sachs’ Disease—we still haven’t found the ostensible genetic basis for traits that are thought to have a strong genetic component. For example, a recent genetic summary found over 12,000 genetic sites that contributed to height yet still explained only about 40-50 percent of the variation in height among European ancestry but no more than 10-20 percent of variation of other ancestries, which we know strongly runs in families (Yengo et al. 2022).
Partly in reaction to the reductionistic hype of the Human Genome Project, the study of epigenetics has become the subject of great interest. One famous natural experiment involves a Nazi-imposed famine in Holland over the winter of 1944–1945. Children born during and shortly after the famine experienced a higher incidence of certain health problems as adults, many decades later. Apparently, certain genes had been down-regulated early in development and remained that way throughout the course of life. Indeed, this modified regulation of the genes in response to the severe environmental conditions may have been passed on to their children.
Obviously one’s particular genetic constitution may play an important role in one’s life trajectory. But overvaluing that role may have important social and political consequences. In the first place, genotypes are rendered meaningful in a cultural universe. Thus, if you live in a strongly patriarchal society and are born without a Y chromosome (since human males are chromosomally XY and females XX), your genotype will indeed have a strong effect upon your life course. So even though the variation is natural, the consequences are political. The mediating factors are the cultural ideas about how people of different sexes ought to be treated, and the role of the state in permitting certain people to develop and thrive. More broadly, there are implications for public education if variation in intelligence is genetic. There are implications for the legal system if criminality is genetic. There are implications for the justice system if sexual preference, or sexual identity, is genetic. There are implications for the development of sports talent if that is genetic. And yet, even for the human traits that are more straightforward to measure and known to be strongly heritable, the DNA base sequence variation seems to explain little.
Genetic determinism or hereditarianism is the idea that “the creature is made, not born”—or, in a more recent formulation by James Watson, that “our fate is in our genes.” One of the major implications drawn from genetic determinism is that the feature in question must inevitably express itself; therefore, we can’t do anything about it. Therefore, we might as well not fund the social programs designed to ameliorate economic inequality and improve people’s lives, because their courses are fated genetically. And therefore, they don’t deserve better lives.
All of the “therefores” in the preceding paragraph are open to debate. What is important is that the argument relies on a very narrow understanding of the role of genetics in human life, and it misdirects the causes of inequality from cultural to natural processes. By contrast, instead of focusing on genes and imagining them to place an invisible limit upon social progress, we can study the ways in which your DNA sequence does not limit your capability for self-improvement or fix your place in a social hierarchy. In general, two such avenues exist. First, we can examine the ways in which the human body responds and reacts to environmental variation: human adaptability and plasticity. This line of research began with the anthropometric studies of immigrants by Franz Boas in the early 20th century and has now expanded to incorporate the epigenetic inheritance of modified human DNA. And second, we can consider how human lives are shaped by social histories—especially the structural inequalities within the societies in which they grow up.
Although it arises and is refuted every generation, the radical hereditarian position (genetic determinism) perennially claims to speak for both science and evolution. It does not. It is the voice of a radical fringe—perhaps naive, perhaps evil. It is not the authentic voice of science or of evolution. Indeed, keeping Charles Darwin’s name unsullied by protecting it from association with bad science often seems like a full-time job. Culture and epigenetics are very much a part of the human condition, and their roles are significant parts of the complete story of human evolution.
Adaptationism and the Panglossian Paradigm
The story of human evolution, and the evolution of all life for that matter, is never settled because evolution is ongoing. Additionally, because the conditions that shape evolutionary trajectories are not predetermined, evolution itself is emergent. Even during periods of ecological stability, when fewer macroevolutionary changes occur, populations of organisms continue to experience change. When ecological stability is disrupted, populations must adapt to the changes. Darwin explained in naturalistic terms how animals adapt to their environments: traits that contribute to an organism's ability to survive and reproduce in specific environments will become more common. The most “fit”—those organisms best suited to the current environmental conditions in which they live—have survived over eons of the history of life on earth to cocreate ecosystems full of animals and plants. Our own bodies are full of evident adaptations: eyes for seeing, ears for hearing, feet for walking on, and so forth.
But what about hands? Feet are adapted to be primarily weight-bearing structures (rather than grasping structures, as in the apes) and that is what we primarily use them for. But we use our hands in many ways: for fine-scale manipulation, greeting, pointing, stimulating a sexual partner, writing, throwing, and cooking, among other uses. So which of these uses express what hands are “for,” when all of them express what hands do?
Gould and Lewontin (1979) illustrate the problem with assuming that the function of a trait defines its evolutionary cause. Consider the case of Dr. Pangloss—the protagonistic of Voltaire’s Candide—who believed that we lived in the best of all possible worlds. Gould and Lewontin use his pronouncement that “noses were made for spectacles and so we have spectacles” to demonstrate the problem with assuming any trait has evolved for a specific purpose. Identifying a function of a trait does not necessitate that the function is the ultimate cause of the trait. Individual traits are not under selection pressures in isolation; in fact, an entire organism must be able to survive and reproduce in their environment. When natural selection results in adaptations, changes that occur in some traits can have cascading effects throughout the phenotype and features that are not under selection pressure can also change.
There is an important lesson in recognizing that what things do in the present is not a good guide to understanding why they came to exist. Gunpowder was invented for entertainment—only later was it adopted for killing people. The Internet was invented to decentralize computers in case of a nuclear attack—and only later adopted for social media. Apes have short thumbs and use their hands in locomotion; our ancestors stopped using their hands in locomotion by about six million years ago and had fairly modern-looking hands by about two million years ago. We can speculate that a combination of selection for abstract thought and dexterity led to evolution of the human hand, with its capability for toolmaking that exceeds what apes can do (see Figure 17.5). But let’s face it—how many tools have you made today?
Consequently, we are obliged to see the human foot as having a purpose to which it is adapted and the human hand as having multiple purposes, most of which are different from what it originally evolved for. Paleontologists Gould and Elisabeth Vrba suggested that an original use be regarded as an adaptation and any additional uses be called “exaptations.” Thus, we would consider the human hand to be an adaptation for toolmaking and an exaptation for writing. So how do we know whether any particular feature is an adaptation, like the walking foot, rather than an exaptation, like the writing hand? Or more broadly, how can we reason rigorously from what a feature does to what it evolved for?
The answer to the question “what did this feature evolve for?” creates an origin myth. This origin myth contains three assumptions: (1) features can be isolated as evolutionary units; (2) there is a specific reason for the existence of any particular feature; and (3) there is a clear and simplistic explanation for why the feature evolved.
The first assumption was appreciated a century ago as the “unit-character problem.” Are the units by which the body grows and evolves the same as units we name? This is clearly not the case: we have genes and we have noses, and we have genes that affect noses, but we don’t have “nose genes.” What is the relationship between the evolving elements that we see, identify, and name, and the elements that biologically exist and evolve? It is hard to know, but we can use the history of science as a guide to see how that fallacy has been used by earlier generations. Back in the 19th century, the early anatomists argued that since the brain contained the mind, they could map different mental states (acquisitiveness, punctuality, sensitivity) onto parts of the brain. Someone who was very introspective, say, would have an enlarged introspection part of the brain, a cranial bulge to represent the hyperactivity of this mental state. The anatomical science was known as phrenology, and it was predicated on the false assumption that units of thought or personality or behavior could be mapped to distinct parts of the brain and physically observed (see Figure17.6). This is the fallacy of reification, imagining that something named is something real.
Long alt text: Side view of human head. At the top are the words “Know Thyself.” On the upper head are small illustrations and word qualities such as “friendship,” “self-esteem,” and “secretiveness.” On the lower part of the man’s man’s face are the words The Phrenological Journal and Science of Health, A First Class Monthly. The caption at the bottom reads: “Specially devoted to the ‘.’ Contains PHRENOLOGY and PHYSIOGNOMY, with all the SIGNS OF CHARACTER, and how to read them; ETHNOLOGY, or the Natural History of Man in all his relations.” (All emphases in original.)
The second assumption, that everything has a reason, has long been recognized as a core belief of religion. Our desire to impose order and simplicity on the workings of the universe, however, does not constrain it to obey simple and orderly causes. Magic, witchcraft, spirits, and divine agency are all powerful explanations for why things happen. Consequently, it is probably not a good idea to lump natural selection in with those. Sometimes things do happen for a reason, of course, but other times things happen as byproducts of other things, or for very complicated and entangled reasons, or for no reason at all. What phenomena have reasons and thereby merit explanation? Chimpanzees have very large testicles, and we think we know why: their promiscuous sexual behavior triggers intense competition for high sperm count. But chimpanzees also have very large ears, but much less scientific attention has been paid to this trait (see Figure 17.7). Why not? Why should there be a reason for chimp testicles but not for chimp ears? What determines the kinds of features that we try to explain, as opposed to the ones that we do not? Again, the assumption that any specific feature has a reason is metaphysical; that is to say, it may be true in any particular case, but to assume it in all cases is gratuitous.
And third, the possibility of knowing what the reason for any particular feature is, assuming that it has one, is a challenge for evolutionary epistemology (the theory of how we know things). Consider the big adaptations of our lineage: bipedalism and language. Nobody doubts that they are good, and they evolved by natural selection, and we know how they work. But why did they evolve? If talking and walking are simply better than not talking and not walking, then why did they evolve in just a single branch of the ape lineage in the primate family tree? We don’t know what bipedalism evolved for, although there are plenty of speculations: walking long distances, running long distances, cooling the head, seeing over tall grass, carrying babies, carrying food, wading, threatening, counting calories, sexual display, and so on. Neither do we know what language evolved for, although there are speculations: coordinating hunting, gossiping, manipulating others. But it is also possible that bipedality is simply the way that a small arboreal ape travels on the ground, if it isn’t in the treetops. Or that language is simply the way that a primate with small canine teeth and certain mental propensities comes to communicate. If that were true, then there might be no reason for bipedality or language: having the unique suite of preconditions and a fortuitous set of circumstances simply set them in motion, and natural selection elaborated and explored their potentials. It is possible that walking and talking simply solved problems that no other lineage had ever solved; but even if so, the fact remains that the rest of the species in the history of life have done pretty well without having solved them.
It is certainly very optimistic to think that all three assumptions (that organisms can be meaningfully atomized, that everything has a reason, and that we can know the reason) would be simultaneously in effect. Indeed, just as there are many ways of adapting (genetically, epigenetically, behaviorally, culturally), there are also many ways of being nonadaptive, which would imply that there is no reason at all for the feature in question.
First, there is the element of randomness of population histories. There are more cases of sickle-cell anemia among sub-Saharan Africans than other peoples, and there is a reason for it: carriers of sickle-cell anemia have a resistance to malaria, which is more frequent in parts of Africa (as discussed in Chapters 4 and 14). But there are more cases of a blood disease called variegated porphyria, a rare genetic metabolic disorder, in the Afrikaners of South Africa (descendants of mostly Dutch settlers in the 17th century) than in other peoples, and there is no reason for it. Yet we know the cause: One of the founding Dutch colonial settlers had the allele–a variant of a gene–and everyone in South Africa with it today is her descendant. But that is not a reason—that is simply an accident of history.
Second, there is the potential mismatch between the past and the present. The value of a particular feature in the past may be changed as the environmental circumstances change. Our species is diurnal, and our ancestors were diurnal. But beginning around a few hundred thousand years ago, our ancestors could build fires, which extended the light period, which was subsequently further amplified by lamps and candles. And over the course of the 20th century, electrical power has made it possible for people to stay up very late when it is dark—working, partying, worrying—to a greater extent than any other closely related species. In other words, we evolved to be diurnal, yet we are now far more nocturnal than any of our recent ancestors or close relatives. Are we adapting to nocturnality? If so, why? Does it even make any sense to speak of the human occupation of a nocturnal ape niche, despite the fact that we empirically seem to be doing just that? And if so, does it make sense to ask what the reason for it is?
Third, there is a genetic phenomenon known as a selective sweep, or the hitchhiker effect. Imagine three genes—A, B, and C—located very closely together on a chromosome. They each have several variants, or alleles, in the population. Now, for whatever reason, it becomes beneficial to have one of the B alleles, say B4; this B4 allele is now under strong positive selection. Obviously, we will expect future generations to be characterized by mostly B4. But what was B4 attached to? Because whatever A and C alleles were adjacent to it will also be quickly spread, simply by virtue of the selection for B4. Even if the A and C alleles are not very good, they will spread because of the good B4 allele between them. Eventually the linkage groups will break up because of genetic crossing-over in future generations. But in the meantime, some random version of genes A and C are proliferating in the species simply because they are joined to superior allele B4. And clearly, the A and C alleles are there because of selection—but not because of selection for them!
Fourth, some features are simply consequences of other properties rather than adaptations to external conditions. We already noted the phenomenon of allometric growth, in which some physical features have to outgrow others to maintain function at an increased size. Can we ask the reason for the massive brow ridges of Homo erectus, or are brow ridges simply what you get when you have a conjunction of thick skull bones, a large face, and a sloping forehead—and, thus, again would have a cause but no reason?
Fifth, some features may be underutilized and on the way out. What is the reason for our two outer toes? They aren’t propulsive, they don’t do anything, and sometimes they’re just in the way. Obviously they are there because we are descended from ancestors with five digits on their hands and feet. Is it possible that a million years from now, we will just have our three largest toes, just as the ancestors of the horse lost their digits in favor of a single hoof per limb? Or will our outer toes find another use, such as stabilizing the landings in our personal jet-packs? For the time being, we can just recognize vestigiality as another nonadaptive explanation for the presence of a given feature.
Finally, Darwin himself recognized that many obvious features do not help an animal survive. Some things may instead help an animal breed. The peacock’s tail feathers do not help it eat, but they do help it mate. There is competition, but only against half of the species. Darwin called this sexual selection. Its result is not a fit to the environment but, rather, a fit to the opposite sex. In some species, that is literally the case, as the male and female genitalia have specific ways of anatomically fitting together. The specific form is less important than the specific match, so inquiring about the reason for a particular form of the reproductive anatomy may be misleading. The specific form may be effectively random, as long as it fits the opposite sex and is different from the anatomies of other species. Nor is sexual selection the only form of selection that can affect the body differently from natural selection. Competition might also take place between biological units other than organisms—perhaps genes, perhaps cells, or populations, or species. The spread of cultural things, such as head-binding or cheap refined fructose or forced labor, can have significant effects upon bodies, which are also not adaptations produced by natural selection. They are often adaptive physiological responses to stresses but not the products of natural selection.
With so many paths available by which a physical feature might have organically arisen without having been the object of natural selection, it is unwise to assume that any individual trait is an adaptation. And that generalization applies to the best-known, best-studied, and most materially based evolutionary adaptations of our lineage. But our cultural behaviors are also highly adaptive, so what about our most familiar social behaviors? Patriarchy, hierarchy, warfare—are these adaptations? Do they have reasons? Are they good for something?
This is where some sloppy thinking has been troublesome. What would it mean to say that patriarchy evolved by natural selection in the human species? If, on the one hand, it means that the human mind evolved by natural selection to be able to create and survive in many different kinds of social and political regimes, of which patriarchy is one, then biological anthropologists will readily agree. If, on the other hand, it means that patriarchy evolved by natural selection, that implies that patriarchy is genetically determined (since natural selection is a genetic process) and out-reproduced the alleles for other, more egalitarian, social forms. This in turn would imply that patriarchy is an adaptation and therefore of some beneficial value in the past and has become an ingrained part of human nature today. This would be bad news, say, if you harbored ambitions of dismantling it. Dismantling patriarchy in that case would be to go against nature, a futile gesture. In other words, this latter interpretation would be a naturalistic manifesto for a conservative political platform: don’t try to dismantle the patriarchy, because it is within us, the product of evolution—suck it up and live with it.
Here, evolution is being used as a political instrument for transforming the human genome into an imaginary glass ceiling against equality. There is thus a convergence between the pseudo-biology of crude adaptationism (the idea that everything is the product of natural selection) and the pseudo-biology of hereditarianism. Naturalizing inequality is not the business of evolutionary theory, and it represents a difficult moral position for a scientist to adopt, as well as a poor scientific position.
Concluding Thoughts
Now that you have finished reading this chapter, you are equipped to understand the historical and political dimensions of evolution. Evolution is an ongoing process of change and diversification. Evolutionary theory is a tool that we use to understand this process. The development of evolutionary theory is shaped both by scientific innovation and political engagement. Since Darwin first articulated natural selection as an observable mechanism by which species adapt to their environments, our understanding of evolution has grown. Initially, scientists focused on the adaptive aspects of evolution. However, with the emergence of genetics, our understanding of heredity and the level at which evolution acts has changed. Genetics led to a focus on the molecular dimensions of evolution. For some, this focus resulted in reductive accounts of evolution. Further developments in our understanding of evolution shifted our view to epigenetic processes and how organisms shape their own evolutionary pressures (e.g., niche construction).
Evolutionary theory will continue to develop in the future as we invent new technologies, describe new dimensions of biology, and experience cultural changes. Current innovations in evolutionary theory are asking us to consider evolutionary forces beyond natural selection and genetics to include the ways organisms shape their environments (niche construction), inheritances beyond genetics (inclusive inheritance), constraints on evolutionary change (developmental bias), and the ability of bodies to change in response to external factors (plasticity). The future of evolutionary theory looks bright as we continue to explore these and other dimensions. Biological anthropology is well-positioned to be a lively part of this conversation, as it extends standard evolutionary theory by considering the role of culture, social learning, and human intentionality in shaping the evolutionary trajectories of humans (Zeder 2018). Remember, at root, human evolutionary theory consists of two propositions: (1) the human species is descended from other similar species and (2) natural selection has been the primary agent of biological adaptation. Pretty much everything else is subject to some degree of contestation.
Review Questions
- How is the study of your ancestors biopolitical, not just biological? Does that make it less scientific or differently scientific?
- What was gained by reducing organisms to genotypes and species to gene pools? What is gained by reintroducing bodies and species into evolutionary studies?
- How do genetic or molecular studies complement anatomical studies of evolution?
- How are you reducible to your ancestry? If you could meet your ancestors from the year 1700 (and you would have well over a thousand of them!), would their lives be meaningfully similar to yours? Would you even be able to communicate with them?
- The molecular biologist François Jacob argued that evolution is more like a tinkerer than an engineer. In what ways do we seem like precisely engineered machinery, and in what ways do we seem like jerry-rigged or improvised contraptions?
- How might biological anthropology contribute to future developments in evolutionary theory?
Key Terms
Adaptation: A fit between the organism and environment.
Adaptationism: The idea that everything is the product of natural selection.
Allele: A genetic variant.
Allometry: The differential growth of body parts.
Canalization: The tendency of a growing organism to be buffered toward normal development.
Epigenetics: The study of how genetically identical cells and organisms (with the same DNA base sequence) can nevertheless differ in stably inherited ways.
Eugenics: An idea that was popular in the 1920s that society should be improved by breeding “better” kinds of people.
Evo-devo: The study of the origin of form; a contraction of “evolutionary developmental biology.”
Exaptation: An additional beneficial use for a biological feature.
Extinction: The loss of a species from the face of the earth.
Gene: A stretch of DNA with an identifiable function (sometimes broadened to include any DNA with recognizable structural features as well).
Gene pool: Hypothetical summation of the entire genetic composition of population or species.
Genotype: Genetic constitution of an individual organism.
Hereditarianism: The idea that genes or ancestry is the most crucial or salient element in a human life. Generally associated with an argument for natural inequality on pseudo-genetic grounds.
Hox genes: A group of related genes that control for the body plan of an embryo along the head-tail axis.
Inheritance of acquired characteristics: The idea that you pass on the features that developed during your lifetime, not just your genes; also known as Lamarckian inheritance.
Natural selection: A consistent bias in survival and fertility, leading to the overrepresentation of certain features in future generations and an improved fit between an average member of the population and the environment.
Niche construction: The active engagement by which species transform their surroundings in favorable ways, rather than just passively inhabiting them.
Phenotype: Observable manifestation of a genetic constitution, expressed in a particular set of circumstances. The suite of traits of an organism.
Phrenology: The 19th-century anatomical study of bumps on the head as an indication of personality and mental abilities.
Plasticity: The tendency of a growing organism to react developmentally to its particular conditions of life.
Punctuated equilibria: The idea that species are stable through time and are formed very rapidly relative to their duration. (The opposite theory, that species are unstable and constantly changing through time, is called phyletic gradualism.)
Scientific racism: The use of pseudoscientific evidence to support or legitimize racial hierarchy and inequality.
Sexual selection: Natural selection arising through preference by one sex for certain characteristics in individuals of the other sex.
Species selection: A postulated evolutionary process in which selection acts on an entire species population, rather than individuals.
About the Authors

Jonathan Marks, Ph.D.
University of North Carolina at Charlotte, jmarks@uncc.edu
Jonathan Marks is Professor of Anthropology at the University of North Carolina at Charlotte. He has published many books and articles on broad aspects of biological anthropology. In 2006 he was elected a Fellow of the American Association for the Advancement of Science. In 2012 he was awarded the First Citizen’s Bank Scholar’s Medal from UNC Charlotte. In recent years he has been a Visiting Research Fellow at the ESRC Genomics Forum in Edinburgh, a Visiting Research Fellow at the Max Planck Institute for the History of Science in Berlin, and a Templeton Fellow at the Institute for Advanced Study at Notre Dame. His work has received the W. W. Howells Book Prize and the General Anthropology Division Prize for Exemplary Cross-Field Scholarship from the American Anthropological Association as well as the J. I. Staley Prize from the School for Advanced Research. Two of his books are titled What It Means to Be 98% Chimpanzee and Why I Am Not a Scientist, but actually he is about 98 percent scientist and not a chimpanzee.

Adam P. Johnson, M.A.
University of North Carolina at Charlotte/University of Texas at San Antonio, ajohn344@uncc.edu
Adam Johnson is a doctoral candidate at the University of Texas at San Antonio and part-time lecturer at the University of North Carolina at Charlotte. He earned his M.A. in anthropology at UNC-Charlotte in 2017 and will complete his Ph.D. in anthropology at UTSA by 2024. His interests include human-animal relations, science studies, primate behavior, ecology, and the history of anthropology. His recent research project analyzes the social, historical, political, and evolutionary dimensions that shape human-javelina encounters. His goal is to understand how humans and animals find ways to get along in a precarious world.
For Further Exploration
Ackermann, Rebecca Rogers, Alex Mackay, and Michael L. Arnold. 2016. “The Hybrid Origin of ‘Modern’ Humans.” Evolutionary Biology 43 (1): 1–11.
Bateson, Patrick, and Peter Gluckman. 2011. Plasticity, Robustness, Development and Evolution. New York: Cambridge University Press.
Cosans, Christopher E. 2009. Owen's Ape and Darwin's Bulldog: Beyond Darwinism and Creationism. Bloomington, IN: Indiana University Press.
Desmond, Adrian, and James Moore. 2009. Darwin's Sacred Cause: How a Hatred of Slavery Shaped Darwin's Views on Human Evolution. New York: Houghton Mifflin Harcourt.
Dobzhansky, Theodosius, Francisco J. Ayala, G. Ledyard Stebbins, and James W. Valentine. 1977. Evolution. San Francisco: W.H. Freeman and Company.
Fuentes, Agustín. 2017. The Creative Spark: How Imagination Made Humans Exceptional. New York: Dutton.
Gould, Stephen J. 2003. The Structure of Evolutionary Theory. Cambridge, MA: Harvard University Press.
Haraway, Donna J. 1989. Primate Visions: Gender, Race, and Nature in the World of Modern Science. New York: Routledge.
Huxley, Thomas. 1863. Evidence as to Man's Place in Nature. London: Williams & Norgate.
Jablonka, Eva, and Marion J. Lamb. 2005. Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. Cambridge, MA: The MIT Press.
Kuklick, Henrika, ed. 2008. A New History of Anthropology. New York: Blackwell.
Laland, Kevin N., Tobias Uller, Marcus W. Feldman, Kim Sterelny, Gerd B. Muller, Armin Moczek, Eva Jablonka, and John Odling-Smee. 2015. “The Extended Evolutionary Synthesis: Its Structure, Assumptions and Predictions.” Proceedings of the Royal Society, Series B 282 (1813): 20151019.
Lamarck, Jean Baptiste. 1809. Philosophie Zoologique. Paris: Dentu.
Landau, Misia. 1991. Narratives of Human Evolution. New Haven: Yale University Press.
Lee, Sang-Hee. 2017. Close Encounters with Humankind: A Paleoanthropologist Investigates Our Evolving Species. New York: W. W. Norton.
Livingstone, David N. 2008. Adam's Ancestors: Race, Religion, and the Politics of Human Origins. Baltimore: Johns Hopkins University Press.
Marks, Jonathan. 2015. Tales of the Ex-Apes: How We Think about Human Evolution. Berkeley, CA: University of California Press.
Pigliucci, Massimo. 2009. “The Year in Evolutionary Biology 2009: An Extended Synthesis for Evolutionary Biology.” Annals of the New York Academy of Sciences 1168: 218–228.
Simpson, George Gaylord. 1949. The Meaning of Evolution: A Study of the History of Life and of Its Significance for Man. New Haven: Yale University Press.
Sommer, Marianne. 2016. History Within: The Science, Culture, and Politics of Bones, Organisms, and Molecules. Chicago: University of Chicago Press.
Stoczkowski, Wiktor. 2002. Explaining Human Origins: Myth, Imagination and Conjecture. New York: Cambridge University Press.
Tattersall, Ian, and Rob DeSalle. 2019. The Accidental Homo sapiens: Genetics, Behavior, and Free Will. New York: Pegasus.
References
Barton, Robert A. 1996. "Neocortex Size and Behavioural Ecology in Primates." Proceedings of the Royal Society of London. Series B: Biological Sciences 263 (1367): 173–177.
Bodmer, Walter, and Robin McKie. 1997. The Book of Man: The Hman Genome Project and the Quest to Discover our Genetic Heritage. Oxford University Press.
Darwin, Charles. 1859. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life. London: J. Murray.
Darwin, Charles. 1871. The Descent of Man, and Selection in Relation to Sex. London: J. Murray.
Dawkins, Richard. 1976. The Selfish Gene. Oxford University Press.
Deacon, T. W. 1998. The Symbolic Species: The Co-evolution of Language and the Brain. W. W. Norton & Company.
Eldredge, N., and S. J. Gould. 1972. "Punctuated Equilibria: An Alternative to Phyletic Gradualism." In Models in Paleobiology, edited by T. J. Schopf, 82–115. San Francisco: W. H. Freeman.
Gould, Stephen J. 2003. The Structure of Evolutionary Theory. Cambridge, MA: Harvard University Press.
Gould, Stephen J. 1996. Mismeasure of Man. New York: WW Norton & Company.
Gould, Stephen Jay, and Richard C. Lewontin. 1979. "The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme." Proceedings of the Royal Society of London. Series B: Biological Sciences 205 (1151): 581–598.
Haeckel, Ernst. 1868. Natürliche Schöpfungsgeschichte. Berlin: Reimer.
Huxley, Thomas Henry. 1863. Evidence as to Man’s Place in Nature. London: Williams and Norgate.
Kaufman, Thomas C., Mark A. Seeger, and Gary Olsen. 1990. "Molecular and Genetic Organization of the Antennapedia Gene Complex of Drosophila melanogaster." Advances in Genetics 27: 309–362.
Kellogg, Vernon. 1917. Headquarters Nights. Boston: The Atlantic Monthly Press.
Kevles, Daniel J., and Leroy Hood. 1993. The Code of Codes: Scientific and Social Issues in the Human Genome Project. Cambridge, MA: Harvard University Press.
Lewontin, Richard, Steven Rose, and Leon Kamin. 2017. Not in Our Genes : Biology, Ideology, and Human Nature, 2nd ed. Chicago: Haymarket Books.
Lloyd, Elisabeth A., and Stephen J. Gould. 1993. "Species Selection on Variability." Proceedings of the National Academy of Sciences 90 (2): 595–599.
Marks, Jonathan. 2015. “The Biological Myth of Human Evolution.” In Biologising the Social Sciences: Challenging Darwinian and Neuroscience Explanations, edited by David Canter and David A. Turner, 59–78. London: Routledge.
Monypenny, William Flavelle, and George Earle Buckle. 1929. The Life of Benjamin Disraeli, Earl of Beaconsfield, Volume II: 1860–1881. London: John Murray.
Potts, Rick. 1998. “Variability Selection in Hominid Evolution.” Evolutionary Anthropology 7: 81–96.
Punnett, R. C. 1905. Mendelism. Cambridge: Macmillan and Bowes.
Shapiro, Robert. 1991. The Human Blueprint: The Race to Unlock the Secrets of Our Genetic Script. New York: St. Martin’s Press.
Shultz, Susanne, Emma Nelson, and Robin Dunbar. 2012. "Hominin Cognitive Evolution: Identifying Patterns and Processes in the Fossil and Archaeological Record." Philosophical Transactions of the Royal Society B: Biological Sciences 367 (1599): 2130–2140.
Spencer, Herbert. 1864. Principles of Biology. London: Williams and Norgate.
Watson, James D. 1990. "The Human Genome Project: Past, Present, and Future." Science 248 (4951): 44–49.
Yengo, L., Vedantam, S., Marouli, E., Sidorenko, J., Bartell, E., Sakaue, S., Graff, M., Eliasen, A.U., Jiang, Y., Raghavan, S. and Miao, J., 2022. A saturated map of common genetic variants associated with human height. Nature, 610 (7933): 704-712.
Zeder, Melinda A. 2018. "Why Evolutionary Biology Needs Anthropology: Evaluating Core Assumptions of the Extended Evolutionary Synthesis." Evolutionary Anthropology: Issues, News, and Reviews 27 (6): 267–284.
Kristin Snopkowski, Ph.D., Boise State University
This chapter is a revision from "Appendix C: Human Behavioral Ecology” by Kristin Snopkowski. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Define human behavioral ecology.
- Describe the types of behaviors that human behavioral ecologists study.
- Explain why humans share food.
- Identify how human behavioral ecology contributes to contemporary world issues.

On December 26, 2004, an earthquake in the Indian Ocean resulted in a tsunami that killed over 200,000 people in at least a dozen different countries (Figure C.1; Editors of Encyclopedia Britannica 2018). In the aftermath, 30% of households in the United States donated an estimated $2.78 billion to help the victims (Center on Philanthropy at Indiana University 2008). At the same time, despite being one of the wealthiest countries in the world, the United States has over a million children who experience homelessness each year (National Center for Homeless Education 2017). Why is it that sometimes humans work together to help those in need, but at other times, humans struggle to solve basic problems? The field of Human Behavioral Ecology seeks to understand this and many other questions to learn why humans behave the way they do. Human Behavioral Ecology is the field of anthropology that explores how evolutionary history and ecological factors combine to influence human behavior.
Human Behavioral Ecology
Evolutionary History
Natural selection is the force of evolution by which individuals with heritable traits that result in greater survival and reproduction have more offspring than individuals without those traits. By having more offspring (specifically, offspring who themselves survive and reproduce), these heritable traits become more common in future generations. As an example, hominin brain size has increased dramatically over the past two million years. Our ancestors with larger brains were better able to survive and reproduce than those with smaller brains, possibly because they were better able to acquire food or navigate the social complexities of living in a large group (Dunbar 1998; Parker and Gibson 1979).

Human behavioral ecology uses the theory of evolution by natural selection to understand how modern behaviors were advantageous in our evolutionary history. For most of human history, humans lived as hunter-gatherers, meaning they collected or hunted food; they typically resided in small communities with individuals related through blood or marriage; and they had no access to modern medicines or other modern conveniences. It is useful to think about this environment—which is much different than how humans live today—to help us understand how current behaviors may have evolved. For example, humans today enjoy consuming food high in fats and sugars (Figure C.2; see Chapter 16). In the past, eating fatty and sugary food was a good survival strategy since food was limited in this environment and these foods contained a lot of calories. Over time, those individuals who sought out these foods were probably better able to survive and reproduce, resulting in a population of people today who have preferences for these foods. In modern environments, where food is abundant, this preference has likely contributed to the obesity epidemic, which increases people’s risk of cardiovascular diseases and no longer improves people’s ability to survive and reproduce.
Ecology
In addition to evolutionary history, the field of human behavioral ecology also focuses on the influence of ecology. Ecology is defined as one’s physical environment, including types of resources, predators, terrain, and weather, as well as one’s social environment, including the behaviors of other individuals and cultural rules. For example, if one lives in an environment where there are abundant fruit trees, then the diet likely includes fruit. Since fruits are easy to acquire, children can engage in food gathering at young ages. In contrast, in environments like the Arctic, where there are fewer plant resources, the diet focuses more on hunting and fishing. Since these skills take longer to acquire, children may only be able to contribute to their own subsistence at older ages. One’s environment influences the behaviors in which individuals engage, such as children’s foraging.
Another component of ecology is one’s social environment, including cultural rules. Throughout the world, different cultures have quite different norms of behavior. For instance, in some societies marriages are required to be monogamous, meaning that a marriage is between just two individuals. This is a cultural norm in the United States, and it is illegal to violate this rule. In other societies, marriages can occur between one man and several women or one woman and several men, referred to as polygyny and polyandry respectively. Across the world, polyandry tends to be quite rare, and in cultures with polyandrous marriage, polygynous and monogamous marriages also occur. The age difference of married people frequently depends on the type of marriages allowed in their culture. In cultures with polygynous marriage, the age difference between husbands and wives tends to be larger than it is in monogamous cultures, as the men who are able to attract additional wives tend to have high status or wealth and are typically older than the women who are available for marriage. In cultures with fraternal polyandry, defined as the marriage of one woman to a set of brothers, marriage typically occurs when the eldest brother is ready to marry and he typically marries a woman close in age. This results in the wife being older than some of her husbands, with the exception of the eldest one. The environment (both physical and social) influences one’s behavioral options, and human behavioral ecologists examine how one’s ecology influences people’s behavior. In Figure C.3, we see a visual depiction of the field of human behavioral ecology, using evolutionary history and ecology (physical environment plus culture) to explain modern human behavior.
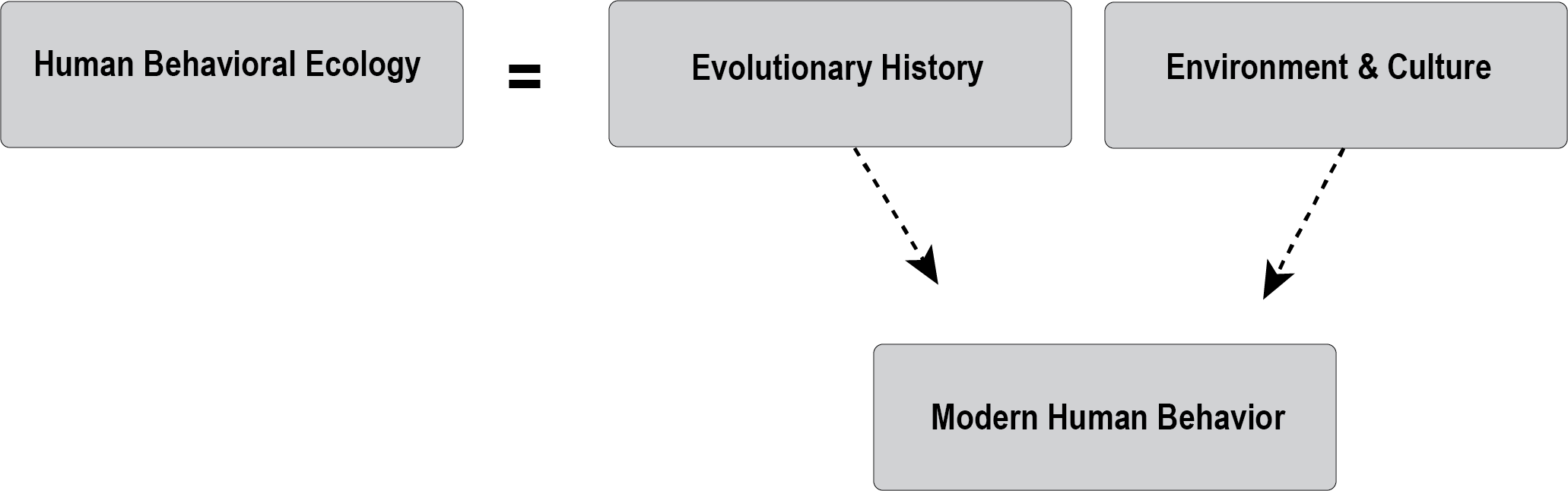
Both Genes and Environment Influence Behavior
While physical characteristics (like height) are clearly heritable, we also know that they depend on the environment. When children grow up with poor nutrition and do not ingest enough calories, their growth is stunted. At the same time, if parents are both tall, then their child is more likely to be tall as well. Physical traits are the result of both genes and environment. Behavior is the same—dependent on both genes and environment. While there are no genes for specific behaviors, behavioral tendencies do show some level of heritability. Personality disorders, for instance, may be partially heritable, but it also depends on the environment in which a child is raised, where child neglect or sexual abuse may increase the risk of personality disorders (Johnson et al. 1999).
Human behavioral ecologists assume that even though there are not genes for specific behaviors, genes may influence behavioral tendencies. Additionally, behaviors are flexible and people use information from the environment to determine under which conditions they should behave in particular ways. For example, the ability to cooperate has evolved over evolutionary time, but whether or not an individual cooperates in a particular instance likely depends on the situation. Research shows that people are more likely to cooperate if: (1) their behavior is known to others (that is to say their identity is not anonymous); (2) it will improve their reputation; or (3) they will be punished for not cooperating (Andreoni and Petrie 2004; Fehr and Fischbacher 2003; Milinski, Semmann, and Krambeck 2002).
How Can Human Behavioral Ecology Help Us Understand Altruism?
Altruism is defined as providing a benefit to someone without expecting anything in return. A perfect example is donating money to tsunami victims. From an evolutionary perspective, it seems that providing benefits to others would be disadvantageous for one’s own survival and reproduction, as resources given to others are resources that cannot be used for oneself. But people do engage in altruistic behaviors, so how can the field of human behavioral ecology help us understand this behavior? We will use the example of food sharing to think about different ways that human behavioral ecologists have examined this question. In many small-scale hunter-gatherer societies, people share food extensively with other people living in their communities. This sharing is most widespread when the item is a hunted animal, which can typically feed many people. Just as giving away money seems counterintuitive, so does giving away food. So, why do people in these foraging communities share so much food with each other?
Kin Selection

One of the first explanations for why humans share food is that they are sharing with their close family members. Kin selection proposes that individuals help kin, even at a cost to themselves, because this help is directed at individuals with whom they share genes. Genes that result in a person acting altruistically toward close kin would have become more frequent over time if individuals sharing that gene are more successful than those not sharing that gene (Hamilton 1964). Taking this perspective is described as a gene’s eye view. Since family members share genes, this may explain why kin help one another. Figure C.4 shows a Lao family eating together. It is very common around the world for families to share food with one another. In many small-scale societies, people share food with family members but also with those who are not family members. Kin selection helps explain some food sharing, but it doesn’t explain all food sharing.
Reciprocal Altruism
Another potential explanation for why humans share food is that they are engaging in reciprocal altruism, meaning that an individual shares food today with the expectation of repayment at some point in the future (Trivers 1971). This can work well, unless the person who receives the help chooses not to reciprocate in the future. In this case, the original sharer does not obtain anything in return. To maintain these relationships, it is important that individuals have the opportunity to share with one another repeatedly and that if one person chooses not to reciprocate, the original sharer terminates their sharing.
Reciprocal altruism is even more likely to occur if the value of the food is greater to the person receiving the food than the person sharing the food. For instance, imagine that you have an entire pizza. After you eat several slices, you are no longer hungry and the next piece of pizza has little value to you. In contrast, if you are hungry, receiving a slice of pizza from a friend would mean a lot to you. In this case, the person giving a piece of pizza after already eating their fill is giving away something of little value, but the person receiving a slice of pizza when they are hungry is receiving something with substantial value. If the following week the roles are reversed, then in both cases, the person receiving the food has received something of greater value than the person who gave it away.

This makes sense in the case of sharing hunted meat as well. In environments without refrigeration technology or in highly mobile groups where food storage is not feasible, the killing of a large animal will result in leftover meat. Sharing that meat with hungry community members has a lot of value to those receiving the meat. Then, at some point in the future, the person who received the meat may successfully hunt and share with others. Figure C.5 displays an Indigenous hunting party from Malaysia. Food is widely shared in small-scale societies, particularly when the item is large in size and when there is a lot of uncertainty around when the next successful hunt will occur (Gurven 2004). But, as with other skilled activities, some individuals are better hunters than others and acquire more meat than others consistently, so why would highly skilled hunters give more food to low-skilled hunters than will be reciprocated (e.g., Gurven et al. 2000)? Again, reciprocal altruism is one piece of the story but cannot explain all sharing behavior.
Costly Signaling
Another possible explanation for why people share food, particularly meat in small-scale societies, is because they want to signal their foraging abilities and generosity (Smith and Bliege Bird 2000). One way to communicate to others your inherent qualities is to do something that is hard to fake. For instance, telling someone that you are a good hunter is not as convincing as hunting a difficult-to-acquire animal and sharing it with them. If someone is a poor hunter, it will be difficult for them to successfully hunt, so sharing hunted meat demonstrates one’s abilities. The hunter who provides resources to the community is likely viewed as generous, allowing them to attract mates, friends, and allies. Costly signaling theory argues that a signaller produces a costly display (e.g., shares hunted meat) to communicate honest information about themselves to others (e.g., I am a generous, skilled hunter). Costly signals can occur across species for a variety of purposes, but this example may help us understand why people share food with unrelated others who are unlikely to reciprocate.

Among the Melanesian Meriam Islanders, turtles (Figure C.6) are hunted at two times of year; during the turtles’ feeding/mating season, which is risky and unpredictable, and during the turtles’ nesting season, which is low risk and relatively easier. Turtles hunted during the feeding/mating season are typically shared widely in the community, while turtles hunted during the nesting season are consumed by a small number of households. This suggests that more people know about high-risk hunts, which may result in hunters gaining more prestige for their successful hunts. Evidence also shows that hunters involved in high-risk hunting gain social and reproductive benefits, such as having children earlier and having more sexual (or reproductive) partners (Smith, Bliege Bird, and Bird 2003). While some sharing behavior may be best explained by a desire to display one’s skills to gain reputational benefits, it cannot explain all sharing behavior and likely works in conjunction with the other hypotheses described above.
What Does Food Sharing Tell Us about Altruism?
Examining these three explanations of sharing behavior—kin selection, reciprocal altruism, and costly signaling (Figure C.7)—helps explain a lot of sharing seen around the world, but donating money to tsunami victims is still hard to understand. Most donors from the United States were not related to the victims of the tsunami; donors probably did not expect reciprocation; and because the donors and receivers did not know each other, reputational benefits would have been limited to people who were made aware of the donation. While some charitable giving may be explained by the tax incentives, the donations to the tsunami victims were so extensive that it seems unlikely to be the main explanation. There are other hypotheses that have not been discussed here, but they also suffer from the inability to fully explain all examples of altruistic behavior. People commonly state that they donate because “it makes them feel good.” While helping others does make people feel good, this likely evolved because those that had the feel-good sensation helped others (like their family members) resulting in greater survival and reproduction. The “feel good” sensation is a proximate explanation, the immediate reason for the behavior, while human behavioral ecology seeks to understand the ultimate explanation, the deep evolutionary reason that this trait led to increased survival and reproduction. In the case of donating money to people living on the other side of the world, our modern environment (allowing us to help people living so far away) may lead us to act in ways that were adaptive in our evolutionary past but may not improve our survival or reproduction today.
Explanations of food sharing:
- Kin selection: Helping family members who share the same genes.
- Reciprocal altruism: Sharing food with someone with the expectation that they will reciprocate at some point in the future.
- Costly signaling: Providing food to others to display one's foraging skill and generosity to improve one's reputation or social standing.
At the same time, we struggle to solve the problem of homelessness across the United States. Using evolutionary theory may help us understand why people are unable to come together to eliminate this problem. Eradicating homelessness would be costly, would require the cooperation of lots of individuals (no single individual or small group can solve it on their own), and would be ongoing. This type of long-lasting commitment to help unrelated strangers may be difficult to acquire from large numbers of people.
How Can Human Behavioral Ecology Help Us Understand the World?
Throughout this appendix, I have been discussing one of the main research areas in Human Behavioral Ecology: cooperation and sharing. Two other prominent areas of research for Human Behavioral Ecologists include production and reproduction. Production research explores how people acquire the resources that they need. Some research in this area has examined which items people choose to include in their diets and how long people spend foraging. This research has shown that people do not simply acquire any food resource in their environment; instead they make strategic decisions based on the food options available and the possible nutrients gained. Research on reproduction includes an examination of how people choose mates, make reproductive choices, invest in children, and acquire help to raise offspring. This line of research has shown that human mothers need help from others to raise offspring, and this help can come from a variety of sources, including the child’s father, grandmothers, older siblings, grandfathers, or others (Hrdy 2009; Sear and Mace 2008). This is quite different from our nonhuman primate relatives, for whom almost all offspring care is given by mothers. These research areas capture many behaviors we faced in our evolutionary history: How did we obtain food, how did we distribute that food once we had it, and how did we make mating and reproductive decisions? All of the topics examined in the field of human behavioral ecology are closely linked to survival and reproduction and to understanding how the environment influences decision making.
Some common misperceptions about human behavioral ecology cause skepticism of this type of research. Some critiques have argued that studying the evolution of human behavior is problematic because of biological determinism, the idea that all behaviors are innate, determined by our genes. If behaviors are innate, then we cannot hold people accountable for their actions. But this is a misunderstanding. As mentioned previously, both genes and the environment influence behavior. Individuals may have a tendency to behave in a particular way, but behaviors are flexible. Also, there is no guarantee that everyone behaves in perfectly optimal ways. Over evolutionary time, those who behaved in ways that resulted in more successful offspring had a greater representation of genes in the next generation, but in each generation we have variation in environments, genotypes, phenotypes, and behaviors on which selection can act.
Another common misconception is that by studying human behavior, human behavioral ecologists are providing justifications for those behaviors. The naturalistic fallacy describes the incorrect belief that what occurs in nature is what ought to be. This is a fallacy because it is absolutely not the goal of researchers in this field. For instance, some researchers study human violence. It is wrong to assume that by studying violence, the researchers believe that violence is an acceptable behavior or is justifiable. It is easy to slip into this misconception.
Modern Applications
While it may seem that the field of human behavioral ecology is more concerned about our evolutionary past than our present, there are many contemporary issues that human behavioral ecology can help us solve. One area that human behavioral ecologists have focused on is climate change (Schradin 2021). In many ways, solving the climate crisis is similar to that of homelessness; it requires many people to come together and sacrifice for the benefit of all. Evidence has shown that people are more likely to sacrifice for others' benefit when their good deeds are known, their actions improve their reputation, or their failure to act produces negative consequences, like increased taxes (Milinski et al. 2002). By focusing on these motivators, policy makers may be able to leverage people to minimize their carbon usage, although current progress achieving targets has seen limited success. Researchers have also used evolutionary theory to improve handwashing rates around the world (Curtis 2013), reduce the obesity epidemic (Pepper and Nettle 2014), ease conflicts (de Waal 2000), and improve cooperation (Boyd and Richerson 1992).
Special Topic: Fertility Research in Human Behavioral Ecology
To understand how human behavior has evolved through time and responds to local environments, human behavioral ecologists collect data on populations across the world. Globally, people are choosing to have fewer children than in the past. Some countries are still dealing with overpopulation, but an even larger number are dealing with population aging and fear of depopulation. Understanding decisions about how many children to have is important in today’s world and is the focus of my research. To examine how family size is changing, researchers calculate total fertility rate, which is specific to a given year and is calculated as the total number of children that would be born to a female if she were to give birth at that particular year’s age-specific fertility rate for each age. This is a value that represents the fertility of females at all ages in a particular year but does not represent any particular person (since a real person experiences fertility across many years). I conducted fieldwork in rural Bolivia, a place where the total fertility rate was approximately 6 children per woman in 1970 but fell to only 3 children per woman by 2013 (World Bank 2022). By interviewing people who live in communities that are undergoing rapid changes in fertility rates, I attempt to understand how people make decisions about family size.

Figure C.8 shows me walking from house to house during my fieldwork in Bolivia. My interviews with over 500 Bolivian women found that those who had more education or those who expected their children to go further in school had fewer children and that family size was similar across groups of friends (Snopkowski and Kaplan 2014). While the conflict between work and childcare is particularly difficult for parents in postindustrialized contexts, in this rural Bolivian community, most women were able to integrate their daily work with childcare. For instance, a woman may own a shop where she could engage in childcare and run the shop simultaneously. To fully understand human behavior cross-culturally, we need to examine many different societies. Using large datasets collected in 45 different countries, my collaborator and I were able to examine how factors such as education and wealth may have different effects on fertility across the world (Colleran and Snopkowski 2018). Our results showed that in every country surveyed, more education for women was associated with having fewer children, but the effect of wealth varied. In countries with high fertility, more wealth typically associated with more children, but in countries with low fertility, more wealth was typically associated with fewer children. These results show that as people have access to more education and choose to educate themselves and their children, small families will become the norm everywhere in the world.
Review Questions
- In human behavioral ecology, human behavior is the result of the interaction among which two factors?
- What are the three main explanations for why people in small-scale societies share food extensively?
- Describe the difference between a proximate and an ultimate explanation and include an example of each.
- What are two misconceptions about human behavioral ecology?
- What contemporary world issues can human behavioral ecology help us solve?
Key Terms
Altruism: Providing a benefit to someone else at a cost to oneself, without expecting future reciprocation.
Biological determinism: The idea that behaviors are determined exclusively by genes.
Costly signaling theory: A theory by which individuals provide honest signals about personal attributes through costly displays.
Ecology: The physical and social environment, including food resources, predators, terrain, weather, social rules, behavior of other people, and cultural rules.
Evolutionary history: An understanding of how traits (including behaviors) may be the result of natural selection in our hominin past.
Human Behavioral Ecology: The field of anthropology that explores how ecological factors and evolutionary history combine to influence how humans behave.
Kin selection: A type of natural selection whereby people help relatives, which can evolve because people are helping other individuals with whom they share genes.
Naturalistic fallacy: The incorrect belief that what occurs is what ought to be.
Population aging: An increase in the number and proportion of people who are over the age of 60.
Proximate explanation: The mechanism that is immediately responsible for an event.
Reciprocal altruism: Helping behavior that occurs because individuals expect that any help they provide will be reciprocated in the future.
Total fertility rate: the number of children a hypothetical female would have at the end of their reproductive period if they experienced fertility rates of a given year for each year of their reproductive period and were not subject to mortality. It represents the fertility of all females in a given year. It is reported as children per woman.
Ultimate explanation: An explanation for an event that is further removed than a proximate explanation but provides a greater insight or understanding. In human behavioral ecology, ultimate explanations usually describe how a behavior is linked to reproduction and survival.
About the Author
Kristin Snopkowski, Ph.D.
Boise State University, kristinsnopkowski@boisestate.edu
Kristin Snopkowski is Associate Professor of Anthropology at Boise State University specializing in human behavioral ecology. Her research examines reproductive decisions, including how many children people have, how other family members influence fertility decisions, and the interaction between females and males in negotiating these decisions. She has conducted field work in Bolivia and Peru, interviewing women about their reproductive choices, and has been analyzing data sets from around the world to understand how environmental factors influence these decisions worldwide. She has published more than 15 peer-reviewed journal articles and co-edited the special issue The Behavioral Ecology of the Family.
For Further Exploration
Barrett, Louise, Robin Dunbar, and John Lycett. 2002. Human Evolutionary Psychology. Princeton: Princeton University Press.
Cronk, Lee, and Beth L. Leech. 2013. Meeting at Grand Central: Understanding the Social and Evolutionary Roots of Cooperation. Princeton: Princeton University Press.
Low, Bobbi S. 2015. Why Sex Matters: A Darwinian Look at Human Behavior. Princeton: Princeton University Press.
Raihani, Nichola. 2021. The Social Instinct: How Cooperation Shaped the World. New York: St. Martin’s Press.
REFERENCES
Andreoni, James, and Ragan Petrie. 2004. “Public Goods Experiments without Confidentiality: A Glimpse into Fund-Raising.” Journal of Public Economics 88 (7-8): 1605–1623. https://doi.org/10.1016/S0047-2727(03)00040-9.
Boyd, Robert, and Peter J. Richerson. 1992. “Punishment Allows the Evolution of Cooperation (or Anything Else) in Sizable Groups.” Ethology and Sociobiology 13 (3): 171–195. Center on Philanthropy at Indiana University. 2008. “Key Findings about Charitable Giving.” Accessed June 26, 2023. https://scholarworks.iupui.edu/bitstream/handle/1805/5775/copps_2005_key_findings.pdf?sequence=1&isAllowed=y.
Colleran, Heidi, and Kristin Snopkowski. 2018. “Variation in Wealth and Educational Drivers of Fertility Decline across 45 Countries.” Population Ecology 60: 155–169. https://doi.org/10.1007/s10144-018-0626-5.
Curtis, Valerie. 2013. Don’t Look, Don’t Touch, Don’t Eat. Chicago: University of Chicago Press.
de Waal, Frans B. M. 2000. “Primates—A Natural Heritage of Conflict Resolution.” Science 289 (5479): 586–590. https://doi.org/10.1126/science.289.5479.586.
Dunbar, Robin I. M. 1998. “The Social Brain Hypothesis.” Evolutionary Anthropology 6 (5): 178–190.
Editors of Encyclopaedia Britannica. 2018. “Indian Ocean Tsunami of 2004.” Encyclopaedia Britannica. Accessed June 26, 2023. https://www.britannica.com/event/Indian-Ocean-tsunami-of-2004.
Fehr, Ernst, and Urs Fischbacher. 2003. “The Nature of Human Altruism.” Nature 425: 785–791.
Gurven, Michael. 2004. “Reciprocal Altruism and Food-Sharing Decisions among Hiwi and Ache Hunter-Gatherers.” Behavioral Ecology and Sociobiology 56 (4): 366–380. https://doi.org/10.1007/s00265-004-0793-6.
Gurven, Michael, Wesley Allen-Arave, Kim Hill, and Magdalena Hurtado. 2000. “‘It’s a Wonderful Life’: Signaling Generosity among the Ache of Paraguay.” Evolution and Human Behavior 21 (4): 263–282.
Hamilton, W. D. 1964. “The Genetical Evolution of Social Behaviour I & II.” Journal of Theoretical Biology 7 (1): 1–52.
Hrdy, Sarah Blaffer. 2009. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: The Belknap Press of Harvard University Press.
Johnson, Jeffrey G., Patricia Cohen, Jocelyn Brown, Elizabeth M. Smailes, and David P. Bernstein. 1999. “Childhood Maltreatment Increases Risk for Personality Disorders during Early Adulthood.” Archives of General Psychiatry 56 (7): 600–606. https://doi.org/10.1001/archpsyc.56.7.600.
Milinski, Manfred, Dirk Semmann, and Hans-Jürgen Krambeck. 2002. “Reputation Helps Solve the ‘Tragedy of the Commons.’” Nature 415: 424–426.
National Center for Homeless Education. 2017. “Federal Data Summary: School Years 2013–2014 to 2015–2016.” Accessed June 26, 2023. https://nche.ed.gov/wp-content/uploads/2018/11/data-comp-1314-1516.pdf.
Parker, Sue Taylor, and Kathleen Rita Gibson. 1979. “A Developmental Model for the Evolution of Language and Intelligence in Early Hominids.” Behavioral and Brain Sciences 2 (3): 367–381. https://doi.org/10.1017/S0140525X0006307X.
Pepper, Gillian V., and Daniel Nettle. 2014. “Out-of-Control Mortality Matters: The Effect of Perceived Uncontrollable Mortality Risk on a Health-Related Decision.” PeerJ 2: e459. https://doi.org/10.7717/peerj.459.
Schradin, Carsten. 2021. “Corona, Climate Change, and Evolved Human Behavior” Trends in Ecology & Evolution 36 (7): 569-572.
Sear, Rebecca, and Ruth Mace. 2008. “Who Keeps Children Alive? A Review of the Effects of Kin on Child Survival.” Evolution and Human Behavior 29 (1): 1–18. https://doi.org/10.1016/j.evolhumbehav.2007.10.001.
Smith, Eric Alden, and Rebecca L. Bliege Bird. 2000. “Turtle Hunting and Tombstone Opening: Public Generosity as Costly Signaling.” Evolution and Human Behavior 21 (4): 245–261. https://doi.org/10.1016/S1090-5138(00)00031-3.
Smith, Eric Alden, Rebecca Bliege Bird, and Douglas W. Bird. 2003. “The Benefits of Costly Signaling: Meriam Turtle Hunters.” Behavioral Ecology 14 (1): 116–126. https://doi.org/10.1093/beheco/14.1.116.
Snopkowski, Kristin, and Hillard Kaplan. 2014. “A Synthetic Biosocial Model of Fertility Transition: Testing the Relative Contribution of Embodied Capital Theory, Changing Cultural Norms, and Women’s Labor Force Participation.” American Journal of Physical Anthropology 154 (3): 322–333. https://doi.org/10.1002/ajpa.22512.
Trivers, Robert L. 1971. “The Evolution of Reciprocal Altruism.” The Quarterly Review of Biology 46 (1): 35–57. https://doi.org/10.1086/406755.
World Bank. 2022. “Fertility Rate, Total (Births per Woman): Bolivia.” The World Bank Group. Accessed November 15, 2022. https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=BO.
Robyn Humphreys, MSc., University of Cape Town
This appendix is a revision of the “Chapter 11 Special Topics: Ancient DNA” by Robyn Humphreys. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Describe the challenges in recovering and sequencing ancient DNA.
- Explain how the Denisovans were discovered and what we have learned about them based on their aDNA.
- Describe the relationships between Neanderthals, Denisovans, and modern humans based on aDNA evidence.
- Explain how DNA can provide insights into the population structure of hominin groups of the past.
Ancient DNA (aDNA) has provided us with new insights into our evolutionary history that cannot be garnered from the fossil record alone. For example, it has assisted with the discovery of the Denisovans, for whom little fossil evidence is available. It has helped us better understand, and make inferences about, the evolution of and relationships among Neanderthals, Denisovans, and modern humans. It has also helped to answer some very important questions about what happened when modern humans migrated out of Africa and encountered these European/Asian hominins, as we will discuss in this appendix.
Sequencing Ancient Genomes
The first successful sequencing of aDNA from an archaic hominin took place in 1997 with the sequencing of mitochondrial DNA (mtDNA) from a Neanderthal-type specimen from Feldhofer Cave. mtDNA is ideal for aDNA studies because it is more abundant than nuclear DNA in our cells. This mitochondrial sequence provided evidence that Neanderthals belonged in a clade separate from modern humans and that their mtDNA was four times more different from modern humans than modern human mtDNA was from each other (Krings et al. 1997).
Sequencing of nuclear DNA would not occur until more than ten years later. The first nuclear genomic sequence representing Neanderthals was produced by sequencing three individuals and using their sequences to create a composite draft Neanderthal genome (Green et al. 2010). The first high-coverage sequence of a single Neanderthal was that of a female Neanderthal who lived in Siberia, followed by another high-coverage sequence from a female Neanderthal whose remains were found in the Vidja cave in Croatia (Prüfer et al. 2014). High-coverage sequences are produced when the genome has been sequenced multiple times, which ensures that the sequences are a true reflection of the genomic sequence and not due to errors that occur during the process of sequencing.
Collecting and Sequencing aDNA
While aDNA can be collected from many different sources (e.g., soft tissue, hair, paleo feces, soils, and sediments), when studying ancient hominins it is most often collected from bone and teeth. Because extraction of aDNA requires destruction of part of the tissue, and the morphology of the skeletal element might be informative, care needs to be taken when deciding what is sampled. Multiple samples are often taken to allow repeat sequencing and demonstrate reproducibility of results. All samples must be minimally handled to avoid contamination.
Endogenous aDNA, or DNA that was present in the tissue before the body decomposed, are usually in fragments 100 to 300 base pairs (bp) long due to degradation, and thus difficult to study. Sometimes DNA from other sources, known as exogenous DNA, are also found in samples. Some examples include DNA from microbes or modern human contamination (Figure D.1).
There are also modifications that occur to aDNA due to chemical reactions. For example, deamination results in Cytosine (C) to Thymine (T) conversions, which occur mostly at the 5’ end (5 prime end) of the DNA fragment. This in turn results in Guanine (G) to Adenine (A) substitutions on the 3’ end (3 prime end) of the DNA fragment. These sequence changes in aDNA might not reflect the original hominin sequence, yet these changes can be helpful when differentiating between aDNA and modern human DNA contamination. The environment plays a significant role, as DNA preserves well in cold conditions such as permafrost. aDNA has also been recovered from material found in drier environments under special conditions. Factors such as water percolation, salinity, pH, and microbial growth all affect the preservation of aDNA.
The bone that best preserves DNA after death is the petrous portion of the temporal bone. This forms part of the skull and protects the inner ear. Due to its high density, the petrous portion preserves DNA very well. Thus, it is possible to get DNA from older and less well-preserved individuals when using the petrous portion. Compared to other bones, the petrous portion not only preserves DNA better but also allows for the extraction of more DNA. The petrous portion can yield up to 100 times more DNA than other bones (Pinhasi et al. 2015)
Initially the short fragments and degraded nature of aDNA posed a big problem with the usual polymerase chain reaction (PCR) procedures used to sequence DNA. But, the advent of high-throughput sequencing has revolutionized sequencing the genomes of ancient hominins. High-throughput sequencing allows for the parallel sequencing of many fragments of DNA in one reaction, without prior knowledge of the target sequence. In this way, the maximum amount of available aDNA can be sequenced. Because the high-throughput sequencing method does not discriminate between endogenous aDNA and exogenous DNA, it is important to either ensure that there is as little contamination as possible and/or use methods that allow for differentiation of the target aDNA.
The Discovery of Denisovans
Denisovans, named after the Siberian cave in which they were discovered, are a distinct group of hominins that were identified through aDNA. Analysis of the ancient mtDNA from teeth and bone fragments revealed they had haplotypes outside the range of variation of modern humans and Neanderthals. The phalanx bone from which the DNA of the Denisovan was recovered did not have traits that indicated that it was another species. A haplotype is a set of genetic variants located on a single stretch of the genome. Shared or similar haplotypes can be used to identify ancestral relationships and to differentiate groups. Dubbed lineage X, the mtDNA sequence from these fossils suggested that Denisovans diverged from modern humans and Neanderthals.
Subsequent high-coverage sequence of a Denisovan (Denisovan 3) nuclear genome showed that Denisovans are a sister group to Neanderthals and thus more closely related to them than indicated by the mtDNA data (Brown et al. 2016). Because mtDNA and nuclear DNA have different patterns of inheritance, they can paint different pictures about the relationships between two groups. The Denisovans are now thought to have a mtDNA sequence derived from an ancient hominin group that hybridized with Denisovans and introduced the mtDNA sequence.
Sequences from three other Denisovans (Denisovan 2, 4, and 8) that provide insight into how old the specimens are, along with the usual dating methods (such as radio carbon dating and uranium dating), show that Denisovans occupied the Denisovan cave from around 195 kya to 52 kya to 76 kya. DNA can assist with dating because, compared to older sequences, younger sequences will have accumulated more mutations from the putative common ancestral sequence. Thus, it is possible to conclude from sequence data, that Denisovan 2 is 54.2 kya to 99.4 kya older than Denisovan 3, and 20.6 kya to 37.7 kya older than Denisovan 8. Molecular data indicates that Neanderthals and Denisovans separated between 381 kya and 473 kya and that the branch leading to Denisovans and modern humans diverged around 800 kya. Denisovans are also more closely related to another set of fossils found in the cave Sima de los Huesos dated to 430 kya. Thus, the split between Neanderthals and Denisovans must have occurred before 430 kya (Meyer et al. 2016).
What Can We Learn About Population Structure of the Neanderthals and Denisovans from aDNA?
Ancient DNA has helped us understand the demographics of Neanderthals and Denisovans and make inferences about population size and history. Three lines of evidence suggest that these groups had small populations toward the end of their existence.
The first line of evidence uses coalescent methods. This process is used to determine which population dynamics in the past are most likely to give rise to the genetic sequences we have, and it allows us to understand population changes of the past, including recombination, population subdivision, and variable population size.
The second indicator that Neanderthals and Denisovans had smaller population sizes is that these groups carried many deleterious (or harmful) genomic variants. Genomic variants are considered deleterious when the change in genomic sequence alters the amino acid sequence of a protein and affects the function of the protein; such variants are known as nonsynonymous mutations. By contrast, synonymous mutations that occur in protein-coding regions of the genome do not change the amino acid sequence nor affect the proteins produced. Denisovans and Neanderthals have a higher ratio of nonsynonymous to synonymous mutations when compared to contemporary modern human populations. This is indicative of a small population size, because if the population were larger, natural selection would have acted on these deleterious variants and weeded them out.
A third indicator of small population size is that the Neanderthals sequenced thus far have low levels of heterozygosity. Heterozygosity is measured by looking at how often two different alleles are found within a certain stretch of DNA. When you find many regions on the genome with different alleles, there is a high level of heterozygosity. When you find very few positions where there are two different alleles, this indicates a low level of heterozygosity. Both Neanderthals and Denisovans appear to have low levels of heterozygosity, indicating smaller population sizes. Ancient Neanderthal genomes also revealed that there were consanguineous relations (mating relationships between two closely related Neanderthals). This was determined by looking at the stretches of homozygosity in a female neanderthal’s genome that were longer than expected and could not be explained by small population size alone.
Sequencing Archaic Genomes to Understand Modern Humans
Not only did the sequencing of archaic genomes allow us to learn more about Neanderthals and Denisovans, it gave us important insights into our own evolution. Previously the human genome was only compared to our closest living relatives, the great apes, which helped us identify unique derived genomic changes that occurred since humans split from our last common ancestor with chimpanzees. Neanderthal and Denisovan genomes provide another set of comparative samples that might help us identify changes unique to modern humans that occurred after our split from the last common ancestor with Neanderthals/Denisovans. These changes may help account for our success as a species.
Hybridization Between Hominin Groups
aDNA also provides insight into interactions between modern humans migrating out of Africa and other hominins that evolved in Europe and Asia. One of the hypotheses tested was this: if hybridization between modern humans and Neanderthals occurred, Neanderthals would have shared more genomic variants with some modern human populations than with others. When this was tested, the data showed that Neanderthals shared more genomic variants with Europeans and Asians than with African individuals (Sankararaman 2016). This difference in relatedness was significant, indicating that there had been hybridization between Neanderthals and modern humans.
From the genetic data, we know that Europeans have a smaller proportion of Neanderthal-derived genes than East Asians do (Prüfer et al. 2017). Thus, there was more admixture into ancestral East Asian populations than into ancestral European populations. Oceanians (Melanesians, Australian aborigines, and other Southeast Asian islanders) have a higher proportion of their DNA derived from Denisovans and longer stretches of Denisovan DNA. DNA in chromosomes get exchanged and experience genetic recombination, whereby introgressed regions (inherited from different species or taxon) are broken down into smaller segments each generation. Thus, longer stretches of introgressed DNA indicate that hybridization occurred more recently. Genetic analysis shows that the admixture event between the Denisovan and human ancestors of these populations is more recent than the admixture events between Neanderthals and modern humans.
To determine whether shared sequences are a result of introgression or more ancient substructure, researchers use divergence time: a measure of how long two sequences have been changing independently. The longer the two sequences have been changing independently, the more differences they will accumulate, which will result in a longer divergence time. By measuring the divergence time between the introgressed regions in modern human genomes and the Neanderthal sequences, researchers can calculate that the shared sequences are recent as well as date to when the two taxa made secondary contact. This is well after the initial population split between modern humans and Neanderthals. There has been gene flow from Neanderthals and Denisovans into modern human populations, between Neanderthals and Denisovans, and from modern humans into Neanderthals.
There is variation in how much of the Neanderthal genome is represented in the modern human population. Individuals outside of Africa usually have 1% to 4% of their genome derived from Neanderthals. Approximately 30% of the Neanderthal genome is represented in modern human genomes, altogether.
Introgressed genes have signatures that allow us to identify them and differentiate them from parts of the genome that are not introgressed. This can be identified in at least three ways. First, in this case, if the sequence is more similar to the Neanderthal sequence (i.e.,fewer sequence differences from the Neanderthal than the African modern human), it is likely that it is derived from a Neanderthal. Second, what is the divergence time between the allele and the same allele in a Neanderthal? If it is shorter than the divergence time between humans and Neanderthal, then the gene is most likely introgressed. An example of this can be seen in Figure D.2. Third, consider whether the allele that meets the first two criteria and is identified as possibly being introgressed can be found at higher frequencies in populations outside of Africa.
Examining the genomes of modern humans, we can see that there are regions of the genome with no Neanderthal and Denisovan genomic variants. These are known as Neanderthal or Denisovan introgression deserts. There are also overlaps between regions in the human genome that are Neanderthal and Denisovan deserts, which might indicate genomic incompatibilities between modern humans and these groups, resulting in those genes being selected against in the modern human genome. We can also infer that hybridization may itself have been a barrier to gene flow because there is a significant reduction in introgression on the X chromosome and around genes that are disproportionately expressed in the testes compared to other tissue groups. This could indicate that hybridization between modern humans and Neanderthals may have resulted in male hybrid infertility.
Because of the climate in Africa, it has been difficult or impossible to extract aDNA from African fossil remains. However, analysis of genomes of modern African populations indicate that there was admixture between modern humans and other hominins within Africa (see Figure D.2).
Confirmed Fossil Hybrids
Another line of evidence concerns hybrids. A first-generation hybrid is called an F1 hybrid; it is the direct offspring of two lineages that have been evolving independently over an extended period. A second-generation hybrid (F2) would be the offspring of two F1 hybrids. A backcrossed individual is the result of an F1 or F2 hybrid mating with an individual from one of the parental populations. An example of a backcross would be when a Neanderthal-human hybrid produces offspring with a human; their offspring would be considered a first-generation backcrossed hybrid (B1). Sequencing of aDNA from fossil material has confirmed that hybridization between different hominins has occurred, supporting the introgression data from recent populations.
The sequencing of Oase 1, a suspected hybrid based on skeletal morphology, showed that it had a Neanderthal ancestor as recently as six to eight generations back. He would thus be considered a backcrossed individual. The recent sequencing of a 13-year-old Denisovan female showed that she was the F1 hybrid offspring of a Neanderthal mother (from whom she inherited Neanderthal mtDNA) and a Denisovan father. While these are only two examples of individuals who are confirmed hybrids, many other remains show some indication of gene flow between hominins.
The Future of Genetic Studies
We are continuing to learn how introgressed genes affect modern humans. Combining phenotypic and genetic information, Neanderthal-derived genes have been associated with diverse traits, ranging from thes skin's sun sensitivity to excessive blood clotting by certain individuals. Interesting research has also shown that introgressed alleles might produce different gene expression profiles when compared to non-introgressed alleles. However, there is a lot of research still to be done to fully understand the effects of introgression on modern populations and how it might have assisted modern humans who migrated out of Africa.
Review Questions
- What are three reasons that ancient DNA is so difficult to study?
- What are introgressed regions of DNA? What insights do studying introgression provide about early hominins?
- Diagram our current understanding of Denisovan, Neanderthal, and modern human lineages based on ancient DNA.
- How can ancient DNA help us understand Neanderthal demographics?
Key Terms
5 prime end: A nucleic acid strand that terminates at the chemical group attached to the fifth carbon in the sugar-ring.
3 prime end: A nucleic acid strand that terminates at the hydroxyl (-OH) chemical group attached to the third carbon in the sugar-ring.
Allele: Each of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome.
Coalescent methods: These are models which allow for inference of how genetic variants sampled from a population may have originated from a common ancestor
Deamination: The chemical process that results in the conversion of Cytosine to uracil, which results in Cytosine to Thymine conversions during sequencing.
Divergence time: A measure of how long two genomic sequences have been changing independently.
Endogenous aDNA: A form of ancient DNA in which DNA originates from the specimen being examined.
Exogenous DNA: DNA that originates from sources outside of the specimen you are trying to sequence.
Genetic recombination: This is the process of exchange of DNA between two strands to produce new sequence arrangements.
Haplotype: A set of genetic variants located on a single stretch of the genome. This unique combination of variants on a stretch of the genome can be used to differentiate groups that will have different combinations of variants.
Heterozygosity: A measure of how many genes within a diploid genome are made up of more than one variant for a gene.
High-coverage sequences: These are genomic sequences which have been sequenced multiple times to ensure that the sequence produced is a true reflection of the genomic sequence, and reduce the likelihood that the sequence has sequencing errors as a result of the sequencing process.
High-throughput sequencing: DNA sequencing technologies developed in the early 21st century that are capable of sequencing many DNA molecules at a time.
Homozygosity: A measure of how many genes within a diploid genome are made up of more than the same variant for a gene.
Hybridization: Mating between two genetically differentiated groups (or species).
Introgressed genes: This is the movement of genes from one species to the gene pool of another species through hybridization between the species and backcross into the parental population by hybrid offspring.
Nonsynonymous mutations: These are changes that occur in the protein-coding region of the genome and result in a change in amino acid sequence of the protein being produced.
Synonymous mutations: Mutations that occur in the protein-coding region of the genome but do not result in a change in amino acid sequence of the protein being produced.
About the Author
Robyn Humphreys, MSc.
University of the Western Cape, rhumphreys@uwc.ac.za
Robyn Humphreys is a biological anthropologist based in the archaeology department at the University of Cape Town. Her MSc focused on the role of hybridization in human evolution. She is now pursuing her Ph.D., which will involve looking at the relationship between archaeologists and communities in relation to research on human remains from historical sites in Cape Town.
For Further Exploration
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216.
Pääbo, Svante. 2011. “DNA Clues to Our Inner Neanderthal.,” TED Talk by Svante Pääbo, August 2011. Last accessed May 7, 2023. https://www.ted.com/talks/svante_paeaebo_dna_clues_to_our_inner_neanderthal?language=en.
References
Beyin, Amanuel. 2011. “Upper Pleistocene Human Dispersals out of Africa: A Review of the Current State of the Debate.” International Journal of Evolutionary Biology 2011: Article ID 615094. https://doi.org/10.4061/2011/615094.
Brown, Samantha, Thomas Higham, Viviane Slon, Svante Pääbo, Matthias Meyer, Katerina Douka, Fiona Brock, et al. 2016. “Identification of a New Hominin Bone from Denisova Cave, Siberia, Using Collagen Fingerprinting and Mitochondrial DNA Analysis.” Science Reports 6: 23559. https://doi.org/10.1038/srep23559.
Green, Richard E., Johannes Krause, Adrian W. Briggs, Tomislav Maricic, Udo Stenzel, Martin Kircher, Nick Patterson, et al. 2010. “A Draft Sequence of the Neanderthal Genome.” Science 328 (5979): 710–722.
Krings, Matthias, Anne Stone, Ralf W. Schmitz, Heike Krainitzki, Mark Stoneking, and Svante Pääbo. 1997. “Neanderthal DNA Sequences and the Origin of Modern Humans.” Cell 90 (1): 19–30.
Meyer, Matthias, Juan-Luis Arsuaga, Cesare de Filippo, Sarah Nagel, Ayinuer Aximu-Petri, Birgit Nickel, Ignacio Martínez, et al. 2016. “Nuclear DNA Sequences from the Middle Pleistocene Sima de los Huesos Hominins.” Nature 531: 504–507.
Pinhasi, Ron, Daniel Fernandes, Kendra Sirak, Mario Novak, Sarah Connell, Songül Alpaslan-Roodenberg, Fokke Gerritsen, et al. 2015. “Optimal Ancient DNA Yields from the Inner Ear Part of the Human Petrous Bone.” PLoS One 10 (6): e0129102. https://doi.org/10.1371/journal.pone.0129102.
Prüfer, Kay, Fernando Racimo, Nick Patterson, Flora Jay, Sriram Sankararaman, Susanna Sawyer, Anja Heinze, et al. 2014. “The Complete Genome Sequence of a Neanderthal from the Altai Mountains.” Nature 505 (7481): 43–49.
Prüfer, Kay, Cesare De Filippo, Steffi Grote, Fabrizio Mafessoni, Petra Korlević, Mateja Hajdinjak, Benjamin Vernot, et al. 2017. “A High-Coverage Neandertal Genome from Vindija Cave in Croatia.” Science 358 (6363): 655–658.
Sankararaman, Sriram, Swapan Mallick, Nick Patterson, and David Reich. 2016. "The Combined Landscape of Denisovan and Neanderthal Ancestry in Present-Day Humans." Current Biology 26 (9): 1241–1247.
Kerryn Warren, Ph.D., Grad Coach International
Lindsay Hunter, M.A., University of Iowa
Navashni Naidoo, M.Sc., University of Cape Town
Silindokuhle Mavuso, M.Sc., University of Witwatersrand
This chapter is a revision from "Chapter 9: Early Hominins" by Kerryn Warren, K. Lindsay Hunter, Navashni Naidoo, Silindokuhle Mavuso, Kimberleigh Tommy, Rosa Moll, and Nomawethu Hlazo. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Understand what is meant by “derived” and “ancestral” traits and why this is relevant for understanding early hominin evolution.
- Understand changing paleoclimates and paleoenvironments as potential factors influencing early hominin adaptations.
- Describe the anatomical changes associated with bipedalism and dentition in early hominins, as well as their implications..
- Describe early hominin genera and species, including their currently understood dates and geographic expanses.
- Describe the earliest stone tool techno-complexes and their impact on the transition from early hominins to our genus.
Defining Hominins
It is through our study of our hominin ancestors and relatives that we are exposed to a world of “might have beens”: of other paths not taken by our species, other ways of being human. But to better understand these different evolutionary trajectories, we must first define the terms we are using. If an imaginary line were drawn between ourselves and our closest relatives, the great apes, bipedalism (or habitually walking upright on two feet) is where that line would be. Hominin, then, means everyone on “our” side of the line: humans and all of our extinct bipedal ancestors and relatives since our divergence from the last common ancestor (LCA) we share with chimpanzees.
Historic interpretations of our evolution, prior to our finding of early hominin fossils, varied. Debates in the mid-1800s regarding hominin origins focused on two key issues:
- Where did we evolve?
- Which traits evolved first?
Charles Darwin hypothesized that we evolved in Africa, as he was convinced that we shared greater commonality with chimpanzees and gorillas on the continent (Darwin 1871). Others, such as Ernst Haeckel and Eugène Dubois, insisted that we were closer in affinity to orangutans and that we evolved in Eurasia where, until the discovery of the Taung Child in South Africa in 1924, all humanlike fossils (of Neanderthals and Homo erectus) had been found (Shipman 2002). and refer to chapter
Within this conversation, naturalists and early paleoanthropologists (people who study human evolution) speculated about which human traits came first. These included the evolution of a big brain (encephalization), the evolution of the way in which we move about on two legs (bipedalism), and the evolution of our flat faces and small teeth (indications of dietary change). Original hypotheses suggested that, in order to be motivated to change diet and move about in a bipedal fashion, the large brain needed to have evolved first, as is seen in the fossil species mentioned above.
However, we now know that bipedal locomotion is one of the first things that evolved in our lineage, with early relatives having more apelike dentition and small brain sizes. While brain size expansion is seen primarily in our genus, Homo, earlier hominin brain sizes were highly variable between and within taxa, from 300 cc (cranial capacity, cm3), estimated in Ardipithecus, to 550 cc, estimated in Paranthropus boisei. The lower estimates are well within the range of variation of nonhuman extant great apes. In addition, body size variability also plays a role in the interpretation of whether brain size could be considered large or small for a particular species or specimen. In this chapter, we will tease out the details of early hominin evolution in terms of morphology (i.e. the study of the form, size, or shape of things; in this case, skeletal parts).
We also know that early human evolution occurred in a very complicated fashion. There were multiple species (multiple genera) that featured diversity in their diets and locomotion. Specimens have been found all along the East African Rift System (EARS); that is, in Ethiopia, Kenya, Tanzania, and Malawi; see Figure 9.1), in limestone caves in South Africa, and in Chad. Dates of these early relatives range from around 7 million years ago (mya) to around 1 mya, overlapping temporally with members of our genus, Homo.

Yet there is still so much to understand. Modern debates now look at the relatedness of these species to us and to one another, and they consider which of these species were able to make and use tools. As a result, every site discovery in the patchy hominin fossil record tells us more about our evolution. In addition, recent scientific techniques (not available even ten years ago) provide new insights into the diets, environments, and lifestyles of these ancient relatives.
In the past, taxonomy was primarily based on morphology. Today it is tied to known relationships based on molecular phylogeny (e.g., based on DNA) or a combination of the two. This is complicated when applied to living taxa, but becomes much more difficult when we try to categorize ancestor-descendant relationships for long-extinct species whose molecular information is no longer preserved. We therefore find ourselves falling back on morphological comparisons, often of teeth and partially fossilized skeletal material.
It is here that we turn to the related concepts of cladistics and phylogenetics. Cladistics groups organisms according to their last common ancestors based on shared derived traits. In the case of early hominins, these are often morphological traits that differ from those seen in earlier populations. These new or modified traits provide evidence of evolutionary relationships, and organisms with the same derived traits are grouped in the same clade (Figure 9.2). For example, if we use feathers as a trait, we can group pigeons and ostriches into the clade of birds. In this chapter, we will examine the grouping of the Robust Australopithecines, whose cranial and dental features differ from those of earlier hominins, and therefore are considered derived.
Dig Deeper: Problems Defining Hominin Species
It is worth noting that species designations for early hominin specimens are often highly contested. This is due to the fragmentary nature of the fossil record, the large timescale (millions of years) with which paleoanthropologists need to work, and the difficulty in evaluating whether morphological differences and similarities are due to meaningful phylogenetic or biological differences or subtle differences/variation in niche occupation or time. In other words, do morphological differences really indicate different species? How would classifying species in the paleoanthropological record compare with classifying living species today, for whom we can sequence genomes and observe lifestyles?
There are also broader philosophical differences among researchers when it comes to paleo-species designations. Some scientists, known as “lumpers,” argue that large variability is expected among multiple populations in a given species over time. These researchers will therefore prefer to “lump” specimens of subtle differences into single taxa. Others, known as “splitters,” argue that species variability can be measured and that even subtle differences can imply differences in niche occupation that are extreme enough to mirror modern species differences. In general, splitters would consider geographic differences among populations as meaning that a species is polytypic (i.e., capable of interacting and breeding biologically but having morphological population differences). This is worth keeping in mind when learning about why species designations may be contested.
This further plays a role in evaluating ancestry. Debates over which species “gave rise” to which continue to this day. It is common to try to create “lineages” of species to determine when one species evolved into another over time. We refer to these as chronospecies (Figure 9.3). Constructed hominin phylogenetic trees are routinely variable, changing with new specimen discoveries, new techniques for evaluating and comparing species, and, some have argued, nationalist or biased interpretations of the record. More recently, some researchers have shifted away from “treelike” models of ancestry toward more nuanced metaphors such as the “braided stream,” where some levels of interbreeding among species and populations are seen as natural processes of evolution.
Finally, it is worth considering the process of fossil discovery and publication. Some fossils are easily diagnostic to a species level and allow for easy and accurate interpretation. Some, however, are more controversial. This could be because they do not easily preserve or are incomplete, making it difficult to compare and place within a specific species (e.g., a fossil of a patella or knee bone). Researchers often need to make several important claims when announcing or publishing a find: a secure date (if possible), clear association with other finds, and an adequate comparison among multiple species (both extant and fossil). Therefore, it is not uncommon that an important find was made years before it is scientifically published.
Paleoenvironment and Hominin Evolution
There is no doubt that one of the major selective pressures in hominin evolution is the environment. Large-scale changes in global and regional climate, as well as alterations to the environment, are (partially) all linked to hominin diversification, dispersal, and extinction (Maslin et al. 2014). Environmental reconstructions often use modern analogues. Let us take, for instance, the hippopotamus. It is an animal that thrives in environments that have abundant water to keep its skin cool and moist. If the environment for some reason becomes drier, it is expected that hippopotamus populations will reduce. If a drier environment becomes wetter, it is possible that hippopotamus populations may be attracted to the new environment and thrive. Such instances have occurred multiple times in the past, and the bones of some fauna (i.e., animals, like the hippopotamus) that are sensitive to these changes give us insights into these events.
Yet reconstructing a paleoenvironment relies on a range of techniques, which vary depending on whether research interests focus on local changes or more global environmental changes/reconstructions. For local environments (such as a single site or region), comparing the faunal assemblages (collections of fossils of animals found at a site) with animals found in certain modern environments allows us to determine if past environments mirror current ones in the region. Changes in the faunal assemblages, as well as when they occur and how they occur, tell us about past environmental changes. Other techniques are also useful in this regard. Chemical analyses, for instance, can reveal the diets of individual fauna, providing clues as to the relative wetness or dryness of their environment (e.g., nitrogen isotopes; Kingston and Harrison 2007).
Global climatic changes in the distant past, which fluctuated between being colder and drier and warmer and wetter on average, would have global implications for environmental change (Figure 9.4). These can be studied by comparing marine core and terrestrial soil data across multiple sites. These techniques are based on chemical analysis, such as examination of the nitrogen and oxygen isotopes in shells and sediments. Similarly, analyzing pollen grains shows which kinds of flora survived in an environment at a specific time period. There are multiple lines of evidence that allow us to visualize global climate trends over millions of years (although it should be noted that the direction and extent of these changes could differ by geographic region).
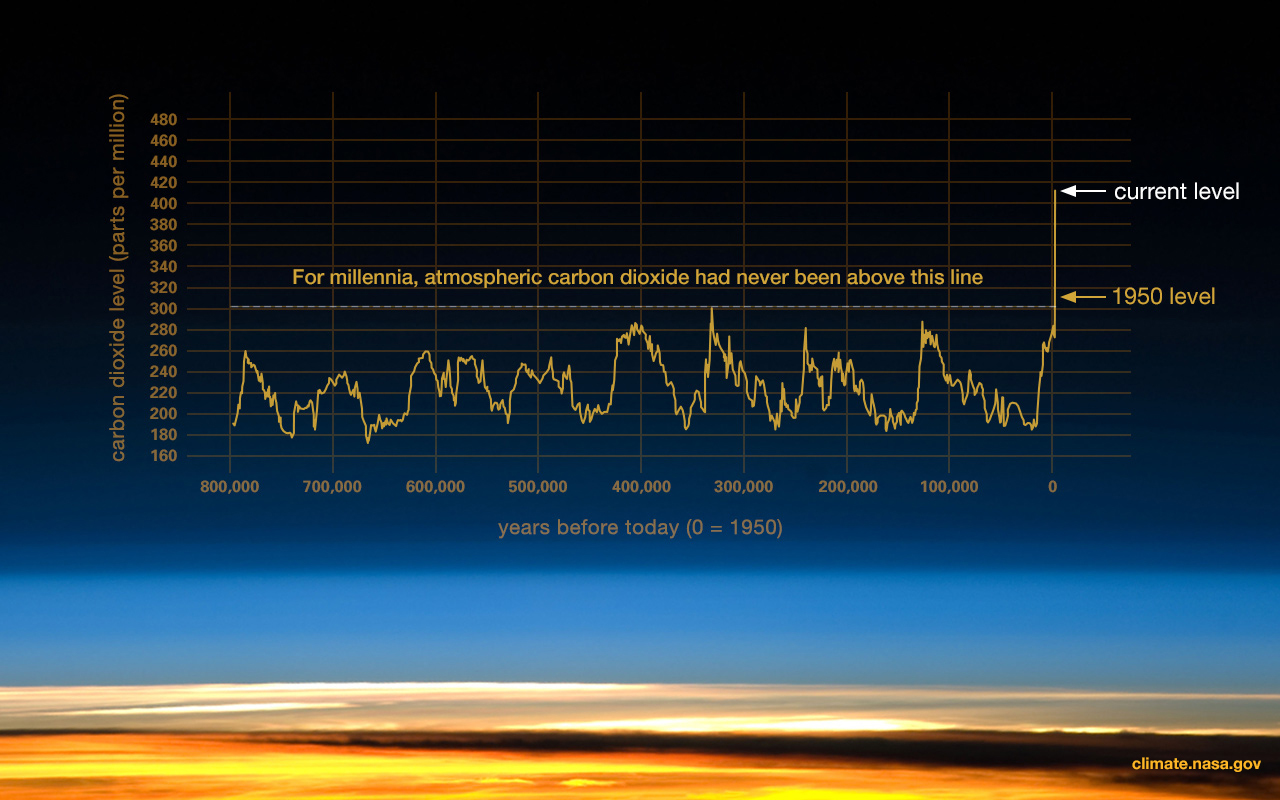
Both local and global climatic/environmental changes have been used to understand factors affecting our evolution (DeHeinzelin et al. 1999; Kingston 2007). Environmental change acts as an important factor regarding the onset of several important hominin traits seen in early hominins and discussed in this chapter. Namely, the environment has been interpreted as the following:
- the driving force behind the evolution of bipedalism,
- the reason for change and variation in early hominin diets, and
- the diversification of multiple early hominin species.
There are numerous hypotheses regarding how climate has driven and continues to drive human evolution. Here, we will focus on just three popular hypotheses.
Savannah Hypothesis (or Aridity Hypothesis)
The hypothesis: This popular theory suggests that the expansion of the savannah (or less densely forested, drier environments) forced early hominins from an arboreal lifestyle (one living in trees) to a terrestrial one where bipedalism was a more efficient form of locomotion (Figure 9.5). It was first proposed by Darwin (1871) and supported by anthropologists like Raymond Dart (1925). However, this idea was supported by little fossil or paleoenvironmental evidence and was later refined as the Aridity Hypothesis. This hypothesis states that the long-term aridification and, thereby, expansion of savannah biomes were drivers in diversification in early hominin evolution (deMenocal 2004; deMenocal and Bloemendal 1995). It advocates for periods of accelerated aridification leading to early hominin speciation events.

The evidence: While early bipedal hominins are often associated with wetter, more closed environments (i.e., not the Savannah Hypothesis), both marine and terrestrial records seem to support general cooling, drying conditions, with isotopic records indicating an increase in grasslands (i.e., colder and wetter climatic conditions) between 8 mya and 6 mya across the African continent (Cerling et al. 2011). This can be contrasted with later climatic changes derived from aeolian dust records (sediments transported to the site of interest by wind), which demonstrate increases in seasonal rainfall between 3 mya and 2.6 mya, 1.8 mya and 1.6 mya, and 1.2 mya and 0.8 mya (deMenocal 2004; deMenocal and Bloemendal 1995).
Interpretation(s): Despite a relatively scarce early hominin record, it is clear that two important factors occur around the time period in which we see increasing aridity. The first factor is the diversification of taxa, where high morphological variation between specimens has led to the naming of multiple hominin genera and species. The second factor is the observation that the earliest hominin fossils appear to have traits associated with bipedalism and are dated to around the drying period (as based on isotopic records). Some have argued that it is more accurately a combination of bipedalism and arboreal locomotion, which will be discussed later. However, the local environments in which these early specimens are found (as based on the faunal assemblages) do not appear to have been dry.
Turnover Pulse Hypothesis
The hypothesis: In 1985, paleontologist Elisabeth Vbra noticed that in periods of extreme and rapid climate change, ungulates (hoofed mammals of various kinds) that had generalized diets fared better than those with specialized diets (Vrba 1988, 1998). Specialist eaters (those who rely primarily on specific food types) faced extinction at greater rates than their generalist (those who can eat more varied and variable diets) counterparts because they were unable to adapt to new environments (Vrba 2000). Thus, periods with extreme climate change would be associated with high faunal turnover: that is, the extinction of many species and the speciation, diversification, and migration of many others to occupy various niches.
The evidence: The onset of the Quaternary Ice Age, between 2.5 mya and 3 mya, brought extreme global, cyclical interglacial and glacial periods (warmer, wetter periods with less ice at the poles, and colder, drier periods with more ice near the poles). Faunal evidence from the Turkana basin in East Africa indicates multiple instances of faunal turnover and extinction events, in which global climatic change resulted in changes from closed/forested to open/grassier habitats at single sites (Behrensmeyer et al. 1997; Bobe and Behrensmeyer 2004). Similarly, work in the Cape Floristic Belt of South Africa shows that extreme changes in climate play a role in extinction and migration in ungulates. While this theory was originally developed for ungulates, its proponents have argued that it can be applied to hominins as well. However, the link between climate and speciation is only vaguely understood (Faith and Behrensmeyer 2013).
Interpretation(s): While the evidence of rapid faunal turnover among ungulates during this time period appears clear, there is still some debate around its usefulness as applied to the paleoanthropological record. Specialist hominin species do appear to exist for long periods of time during this time period, yet it is also true that Homo, a generalist genus with a varied and adaptable diet, ultimately survives the majority of these fluctuations, and the specialists appear to go extinct.
Variability Selection Hypothesis
The hypothesis: This hypothesis was first articulated by paleoanthropologist Richard Potts (1998). It links the high amount of climatic variability over the last 7 million years to both behavioral and morphological changes. Unlike previous notions, this hypothesis states that hominin evolution does not respond to habitat-specific changes or to specific aridity or moisture trends. Instead, long-term environmental unpredictability over time and space influenced morphological and behavioral adaptations that would help hominins survive, regardless of environmental context (Potts 1998, 2013). The Variability Selection Hypothesis states that hominin groups would experience varying degrees of natural selection due to continually changing environments and potential group isolation. This would allow certain groups to develop genetic combinations that would increase their ability to survive in shifting environments. These populations would then have a genetic advantage over others that were forced into habitat-specific adaptations (Potts 2013).
The evidence: The evidence for this theory is similar to that for the Turnover Pulse Hypothesis: large climatic variability and higher survivability of generalists versus specialists. However, this hypothesis accommodates for larger time-scales of extinction and survival events.
Interpretation(s): In this way, the Variability Selection Hypothesis allows for a more flexible interpretation of the evolution of bipedalism in hominins and a more fluid interpretation of the Turnover Pulse Hypothesis, where species turnover is meant to be more rapid. In some ways, this hypothesis accommodates both environmental data and our interpretations of an evolution toward greater variability among species and the survivability of generalists.
Paleoenvironment Summary
Some hypotheses presented in this section pay specific attention to habitat (Savannah Hypothesis) while others point to large-scale climatic forces (Variability Selection Hypothesis). Some may be interpreted to describe the evolution of traits such as bipedalism (Savannah Hypothesis), and others generally explain the diversification of early hominins (Turnover Pulse and Variability Selection Hypotheses). While there is no consensus as to how the environment drove our evolution, it is clear that the environment shaped both habitat and resource availability in ways that would have influenced our early ancestors physically and behaviorally.
Derived Adaptations: Bipedalism
The unique form of locomotion exhibited by modern humans, called obligate bipedalism, is important in distinguishing our species from the extant (living) great apes. The ability to walk habitually upright is thus considered one of the defining attributes of the hominin lineage. We also differ from other animals that walk bipedally (such as kangaroos) in that we do not have a tail to balance us as we move.
The origin of bipedalism in hominins has been debated in paleoanthropology, but at present there are two main ideas: (theories)
- early hominins initially lived in trees, but increasingly started living on the ground, so we were a product of an arboreal last common ancestor (LCA) or,
- our LCA was a terrestrial quadrupedal knuckle-walking species, more similar to extant chimpanzees.
Most research supports the first theory of an arboreal LCA based on skeletal morphology of early hominin genera that demonstrate adaptations for climbing but not for knuckle-walking. This would mean that both humans and chimpanzees can be considered “derived” in terms of locomotion since chimpanzees would have independently evolved knuckle-walking.
There are many current ideas regarding selective pressures that would lead to early hominins adapting upright posture and locomotion. Many of these selective pressures, as we have seen in the previous section, coincide with a shift in environmental conditions, supported by paleoenvironmental data. In general, however, it appears that, like extant great apes, early hominins thrived in forested regions with dense tree coverage, which would indicate an arboreal lifestyle. As the environmental conditions changed and a savannah/grassland environment became more widespread, the tree cover would become less dense, scattered, and sparse such that bipedalism would become more important.
There are several proposed selective pressures for bipedalism:
- Energy conservation: Modern bipedal humans conserve more energy than extant chimpanzees, which are predominantly knuckle-walking quadrupeds when walking over land. While chimpanzees, for instance, are faster than humans terrestrially, they expend large amounts of energy being so. Adaptations to bipedalism include “stacking” the majority of the weight of the body over a small area around the center of gravity (i.e., the head is above the chest, which is above the pelvis, which is over the knees, which are above the feet). This reduces the amount of muscle needed to be engaged during locomotion to “pull us up” and allows us to travel longer distances expending far less energy.
- Thermoregulation: Less surface area (i.e., only the head and shoulders) is exposed to direct sunlight during the hottest parts of the day (i.e., midday). This means that the body has less need to employ additional “cooling” mechanisms such as sweating, which additionally means less water loss.
- Bipedalism (Freeing of Hands): This method of locomotion freed up our ancestors’ hands such that they could more easily gather food and carry tools or infants. This further enabled the use of hands for more specialized adaptations associated with the manufacturing and use of tools.
These selective pressures are not mutually exclusive. Bipedality could have evolved from a combination of these selective pressures, in ways that increased the chances of early hominin survival.
Skeletal Adaptations for Bipedalism
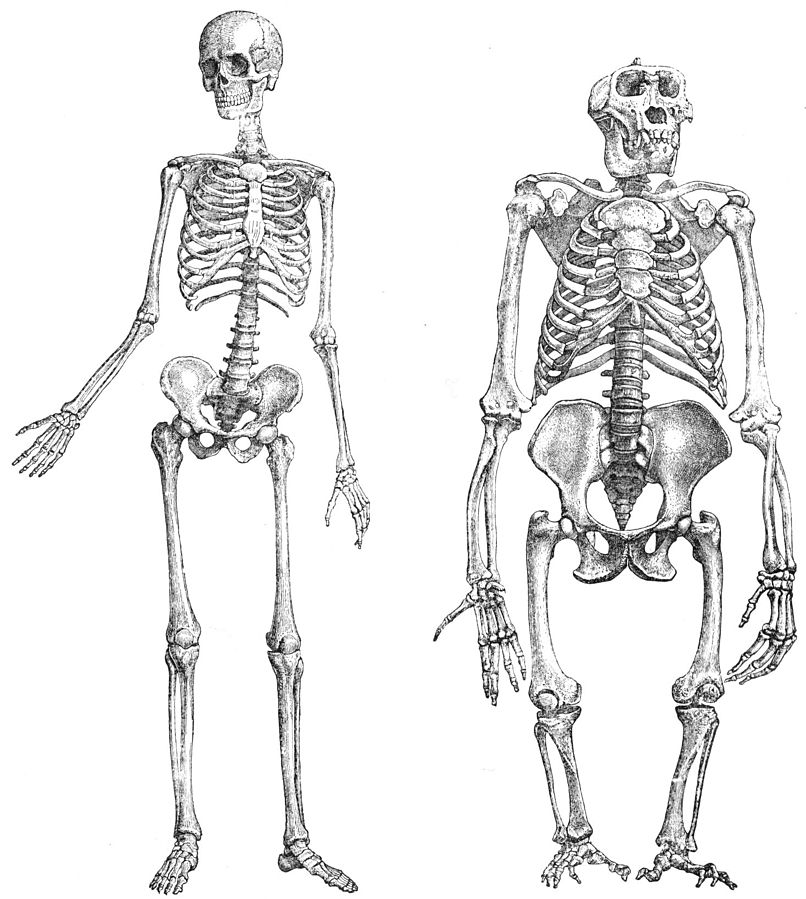
Humans have highly specialized adaptations to facilitate obligate bipedalism (Figure 9.6). Many of these adaptations occur within the soft tissue of the body (e.g., muscles and tendons). However, when analyzing the paleoanthropological record for evidence of the emergence of bipedalism, all that remains is the fossilized bone. Interpretations of locomotion are therefore often based on comparative analyses between fossil remains and the skeletons of extant primates with known locomotor behaviors. These adaptations occur throughout the skeleton and are summarized in Figure 9.7.
The majority of these adaptations occur in the postcranium (the skeleton from below the head) and are outlined in Figure 9.7. In general, these adaptations allow for greater stability and strength in the lower limb, by allowing for more shock absorption, for a larger surface area for muscle attachment, and for the “stacking” of the skeleton directly over the center of gravity to reduce energy needed to be kept upright. These adaptations often mean less flexibility in areas such as the knee and foot.
However, these adaptations come at a cost. Evolving from a nonobligate bipedal ancestor means that the adaptations we have are evolutionary compromises. For instance, the valgus knee (angle at the knee) is an essential adaptation to balance the body weight above the ankle during bipedal locomotion. However, the strain and shock absorption at an angled knee eventually takes its toll. For example, runners often experience joint pain. Similarly, the long neck of the femur absorbs stress and accommodates for a larger pelvis, but it is a weak point, resulting in hip replacements being commonplace among the elderly, especially in cases where the bone additionally weakens through osteoporosis. Finally, the S-shaped curve in our spine allows us to stand upright, relative to the more curved C-shaped spine of an LCA. Yet the weaknesses in the curves can lead to pinching of nerves and back pain. Since many of these problems primarily are only seen in old age, they can potentially be seen as an evolutionary compromise.
Despite relatively few postcranial fragments, the fossil record in early hominins indicates a complex pattern of emergence of bipedalism. Key features, such as a more anteriorly placed foramen magnum, are argued to be seen even in the earliest discovered hominins, indicating an upright posture (Dart 1925). Some early species appear to have a mix of ancestral (arboreal) and derived (bipedal) traits, which indicates a mixed locomotion and a more mosaic evolution of the trait. Some early hominins appear to, for instance, have bowl-shaped pelvises (hip bones) and angled femurs suitable for bipedalism but also have retained an opposable hallux (big toe) or curved fingers and longer arms (for arboreal locomotion). These mixed morphologies may indicate that earlier hominins were not fully obligate bipeds and thus thrived in mosaic environments.
Yet the associations between postcranial and the more diagnostic cranial fossils and bones are not always clear, muddying our understanding of the specific species to which fossils belong (Grine et al. 2022).
| Region | Feature | Obligate Biped (H. sapiens) | Nonobligate Biped |
| Cranium | Position of the foramen magnum | Positioned inferiorly (immediately under the cranium) so that the head rests on top of the vertebral column for balance and support (head is perpendicular to the ground). | Posteriorly positioned (to the back of the cranium). Head is positioned parallel to the ground. |
| Post
cranium |
Body proportions | Shorter upper limb (not used for locomotion). | Longer upper limbs (used for locomotion). |
| Post
cranium |
Spinal curvature | S-curve due to pressure exerted on the spine from bipedalism (lumbar lordosis). | C-curve. |
| Post
cranium |
Vertebrae | Robust lumbar (lower-back) vertebrae (for shock absorbance and weight bearing). Lower back is more flexible than that of apes as the hips and trunk swivel when walking (weight transmission). | Gracile lumbar vertebrae compared to those of modern humans. |
| Post
cranium |
Pelvis | Shorter, broader, bowl-shaped pelvis (for support); very robust. Broad sacrum with large sacroiliac joint surfaces. | Longer, flatter, elongated ilia; more narrow and gracile; narrower sacrum; relatively smaller sacroiliac joint surface. |
| Post
cranium |
Lower limb | In general, longer, more robust lower limbs and more stable, larger joints.
|
In general, smaller, more gracile limbs with more flexible joints.
|
| Post
cranium |
Foot | Rigid, robust foot, without a midtarsal break.
Nonopposable and large, robust big toe (for push off while walking) and large heel for shock absorbance. |
Flexible foot, midtarsal break present (which allows primates to lift their heels independently from their feet), opposable big toe for grasping. |
It is also worth noting that, while not directly related to bipedalism per se, other postcranial adaptations are evident in the hominin fossil record from some of the earlier hominins. For instance, the hand and finger morphologies of many of the earliest hominins indicate adaptations consistent with arboreality. These include longer hands, more curved metacarpals and phalanges (long bones in the hand and fingers, respectively), and a shorter, relatively weaker thumb. This allows for gripping onto curved surfaces during locomotion. The earliest hominins appear to have mixed morphologies for both bipedalism and arborealism. However, among Australopiths (members of the genus, Australopithecus), there are indications for greater reliance on bipedalism as the primary form of locomotion. Similarly, adaptations consistent with tool manufacture (shorter fingers and a longer, more robust thumb, in contrast to the features associated with arborealism) have been argued to appear before the genus Homo.
Early Hominins: Sahelanthropus and Orrorin
We see evidence for bipedalism in some of the earliest fossil hominins, dated from within our estimates of our divergence from chimpanzees. These hominins, however, also indicate evidence for arboreal locomotion.
The earliest dated hominin find (between 6 mya and 7 mya, based on radiometric dating of volcanic tufts) has been argued to come from Chad and is named Sahelanthropus tchadensis (Figure 9.8; Brunet et al. 1995). The initial discovery was made in 2001 by Ahounta Djimdoumalbaye and announced in Nature in 2002 by a team led by French paleontologist Michel Brunet. The find has a small cranial capacity (360 cc) and smaller canines than those in extant great apes, though they are larger and pointier than those in humans. This implies strongly that, over evolutionary time, the need for display and dominance among males has reduced, as has our sexual dimorphism. A short cranial base and a foramen magnum that is more humanlike in positioning have been argued to indicate upright walking.
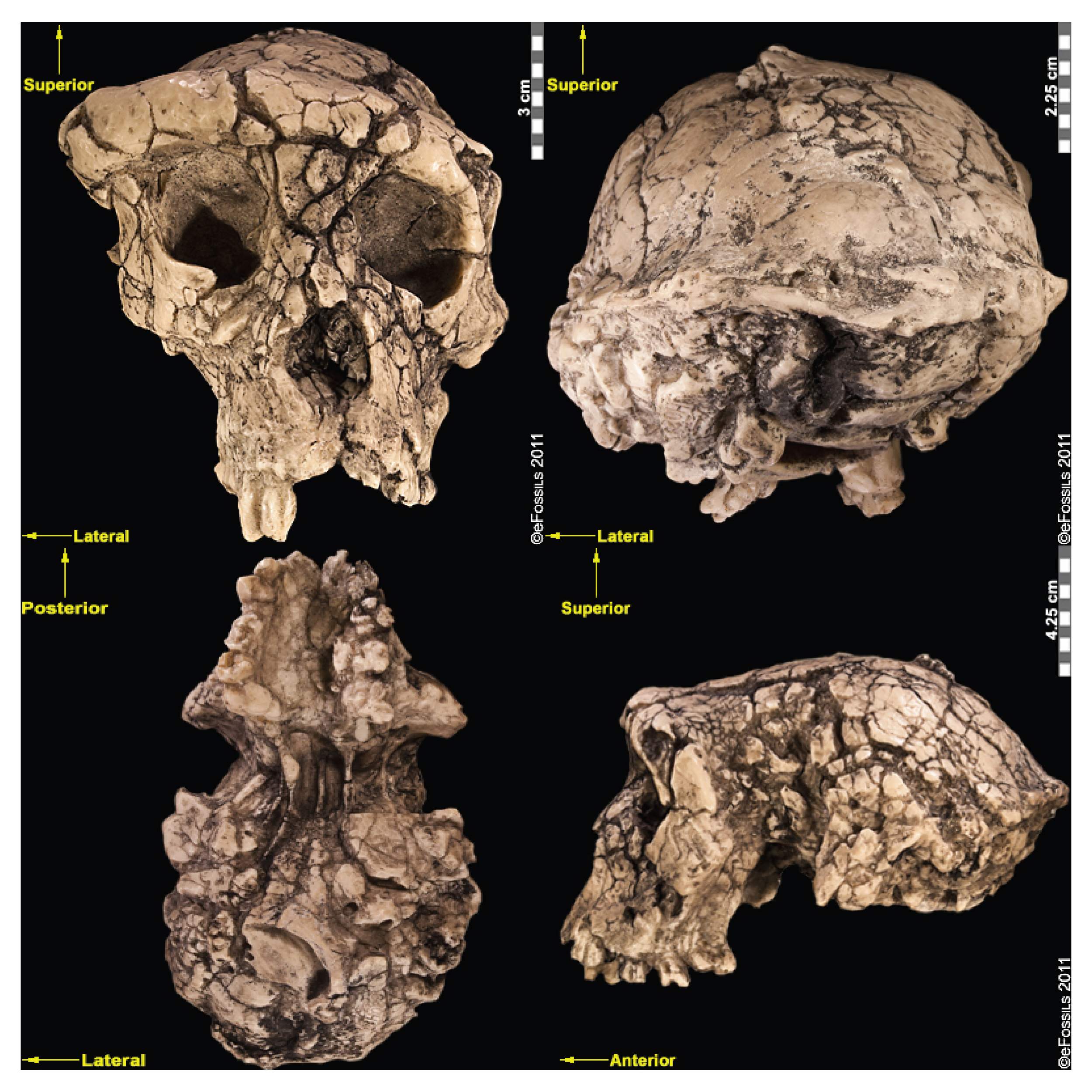
Initially, the inclusion of Sahelanthropus in the hominin family was debated by researchers, since the evidence for bipedalism is based on cranial evidence alone, which is not as convincing as postcranial evidence. Yet, a femur (thigh bone) and ulnae (upper arm bones) thought to belong to Sahelanthropus was discovered in 2001 (although not published until 2022). These bones may support the idea that the hominin was in fact a terrestrial biped with arboreal capabilities and behaviors (Daver et al. 2022).
Orrorin tugenensis (Orrorin meaning “original man”), dated to between 6 mya and 5.7 mya, was discovered near Tugen Hills in Kenya in 2000. Smaller cheek teeth (molars and premolars) than those in even more recent hominins, thick enamel, and reduced, but apelike, canines characterize this species. This is the first species that clearly indicates adaptations for bipedal locomotion, with fragmentary leg, arm, and finger bones having been found but few cranial remains. One of the most important elements discovered was a proximal femur, BAR 1002'00. The femur is the thigh bone, and the proximal part is that which articulates with the pelvis; this is very important for studying posture and locomotion. This femur indicates that Ororrin was bipedal, and recent studies suggest that it walked in a similar way to later Pliocene hominins. Some have argued that features of the finger bones suggest potential tool-making capabilities, although many researchers argue that these features are also consistent with climbing.
Early Hominins: The Genus Ardipithecus
Another genus, Ardipithecus, is argued to be represented by at least two species: Ardipithecus (Ar.) ramidus and Ar. kadabba.
Ardipithecus ramidus (“ramid” means root in the Afar language) is currently the best-known of the earliest hominins (Figure 9.9). Unlike Sahelanthropus and Orrorin, this species has a large sample size of over 110 specimens from Aramis alone. Dated to 4.4 mya, Ar. ramidus was found in Ethiopia (in the Middle Awash region and in Gona). This species was announced in 1994 by American palaeoanthropologist Tim White, based on a partial female skeleton nicknamed “Ardi” (ARA-VP-6/500; White et al. 1994). Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, she had an opposable big toe (hallux), similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. A small brain (300 cc to 350 cc), midfacial projection, and slight prognathism show retained ancestral cranial features, but the cheek bones are less flared and robust than in later hominins.
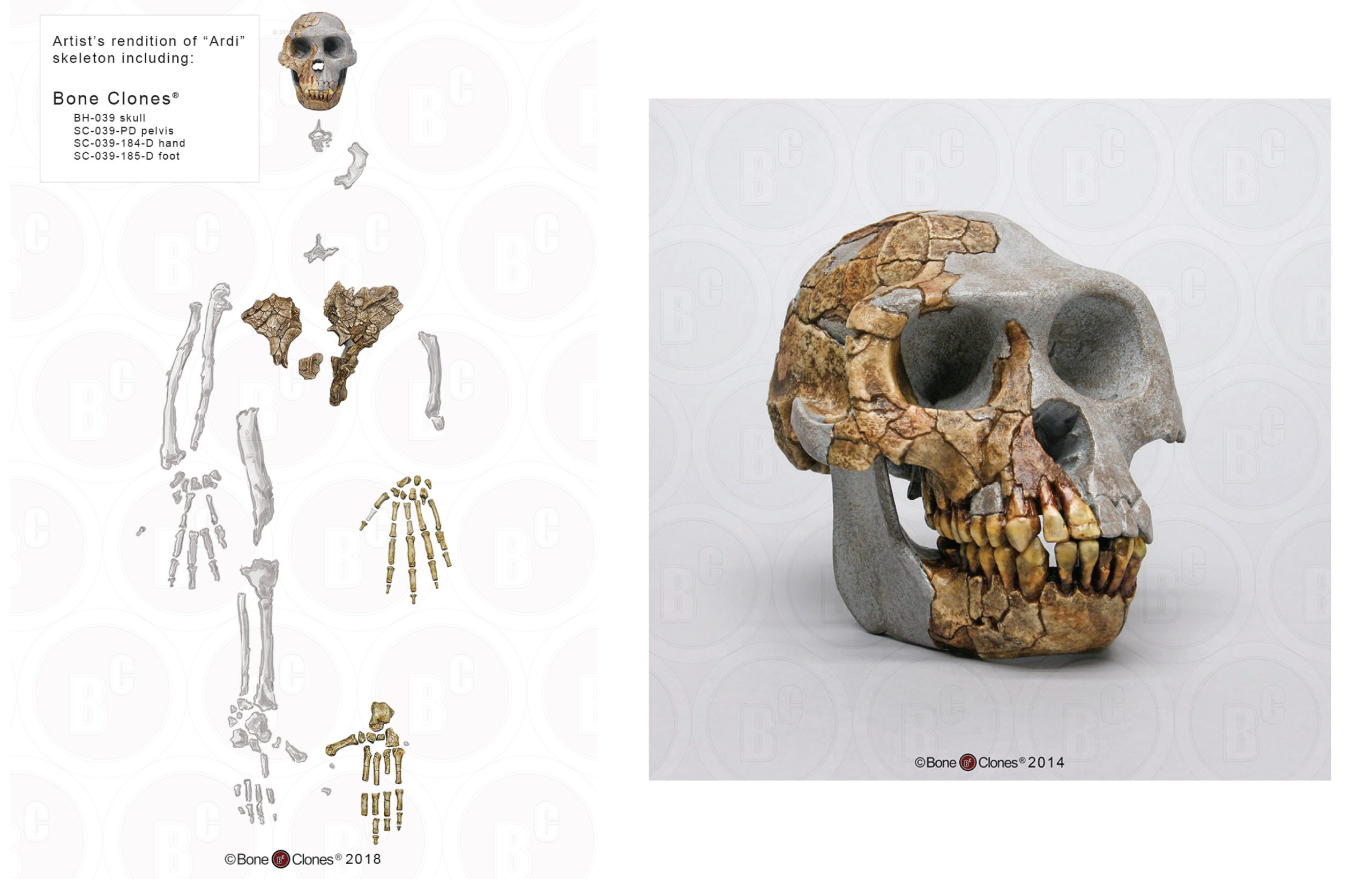
Ardipithecus kadabba (the species name means “oldest ancestor” in the Afar language) is known from localities on the western margin of the Middle Awash region, the same locality where Ar. ramidus has been found. Specimens include mandibular fragments and isolated teeth as well as a few postcranial elements from the Asa Koma (5.5 mya to 5.77 mya) and Kuseralee Members (5.2 mya), well-dated and understood (but temporally separate) volcanic layers in East Africa. This species was discovered in 1997 by paleoanthropologist Dr. Yohannes Haile-Selassie. Originally these specimens were referred to as a subspecies of Ar. ramidus. In 2002, six teeth were discovered at Asa Koma and the dental-wear patterns confirmed that this was a distinct species, named Ar. kadabba, in 2004. One of the postcranial remains recovered included a 5.2 million-year-old toe bone that demonstrated features that are associated with toeing off (pushing off the ground with the big toe leaving last) during walking, a characteristic unique to bipedal walkers. However, the toe bone was found in the Kuseralee Member, and therefore some doubt has been cast by researchers about its association with the teeth from the Asa Koma Member.
Bipedal Trends in Early Hominins: Summary
Trends toward bipedalism are seen in our earliest hominin finds. However, many specimens also indicate retained capabilities for climbing. Trends include a larger, more robust hallux; a more compact foot, with an arch; a robust, long femur, angled at the knee; a robust tibia; a bowl-shaped pelvis; and a more anterior foramen magnum. While the level of bipedality in Salehanthropus tchadenisis is debated since there are few fossils and no postcranial evidence, Orrorin tugenensis and Ardipithecus kadabba show clear indications of some of these bipedal trends. However, some retained ancestral traits, such as an opposable hallux in Ardipithecus, indicate some retention in climbing ability.
Derived Adaptations: Early Hominin Dention
The Importance of Teeth
Teeth are abundant in the fossil record, primarily because they are already highly mineralized as they are forming, far more so than even bone. Because of this, teeth preserve readily. And, because they preserve readily, they are well-studied and better understood than many skeletal elements. In the sparse hominin (and primate) fossil record, teeth are, in some cases, all we have.
Teeth also reveal a lot about the individual from whom they came. We can tell what they evolved to eat, to which other species they may be closely related, and even, to some extent, the level of sexual dimorphism, or general variability, within a given species. This is powerful information that can be contained in a single tooth. With a little more observation, the wearing patterns on a tooth can tell us about the diet of the individual in the weeks leading up to its death. Furthermore, the way in which a tooth is formed, and the timing of formation, can reveal information about changes in diet (or even mobility) over infancy and childhood, using isotopic analyses. When it comes to our earliest hominin relatives, this information is vital for understanding how they lived.
The purpose of comparing different hominin species is to better understand the functional morphology as it applies to dentition. In this, we mean that the morphology of the teeth or masticatory system (which includes jaws) can reveal something about the way in which they were used and, therefore, the kinds of foods these hominins ate. When comparing the features of hominin groups, it is worth considering modern analogues (i.e., animals with which to compare) to make more appropriate assumptions about diet. In this way, hominin dentition is often compared with that of chimpanzees and gorillas (our close ape relatives), as well as with that of modern humans.
The most divergent group, however, is humans. Humans around the world have incredibly varied diets. Among hunter-gatherers, it can vary from a honey- and plant-rich diet, as seen in the Hadza in Tanzania, to a diet almost entirely reliant on animal fat and protein, as seen in Inuits in polar regions of the world. We are therefore considered generalists, more general than the largely frugivorous (fruit-eating) chimpanzee or the folivorous (foliage-eating) gorilla, as discussed in Chapter 5.
One way in which all humans are similar is our reliance on the processing of our food. We cut up and tear meat with tools using our hands, instead of using our front teeth (incisors and canines). We smash and grind up hard seeds, instead of crushing them with our hind teeth (molars). This means that, unlike our ape relatives, we can rely more on developing tools to navigate our complex and varied diets. (We could say) Our brain, therefore, is our primary masticatory organ. Evolutionarily, our teeth have reduced in size and our faces are flatter, or more orthognathic, partially in response to our increased reliance on our hands and brain to process food. Similarly, a reduction in teeth and a more generalist dental morphology could also indicate an increase in softer and more variable foods, such as the inclusion of more meat. These trends begin early on in our evolution. The link has been made between some of the earliest evidence for stone tool manufacture, the earliest members of our genus, and the features that we associate with these specimens.
General Dental Trends in Early Hominins
Several trends are visible in the dentition of early hominins. However, all tend to have the same dental formula. The dental formula tells us how many of each tooth type are present in each quadrant of the mouth. Going from the front of the mouth, this includes the square, flat incisors; the pointy canines; the small, flatter premolars; and the larger hind molars. In many primates, from Old World monkeys to great apes, the typical dental formula is 2:1:2:3. This means that if we divide the mouth into quadrants, each has two incisors, one canine, two premolars, and three molars. The eight teeth per quadrant total 32 teeth in all (although some humans have fewer teeth due to the absence of their wisdom teeth, or third molars).

The morphology of the individual teeth is where we see the most change. Among primates, large incisors are associated with food procurement or preparation (such as biting small fruits), while small incisors indicate a diet that may contain small seeds or leaves (where the preparation is primarily in the back of the mouth). Most hominins have relatively large, flat, vertically aligned incisors that occlude (touch) relatively well, forming a “bite.” This differs from, for instance, the orangutan, whose teeth stick out (i.e., are procumbent).
While the teeth are often aligned with diet, the canines may be misleading in that regard. We tend to associate pointy, large canines with the ripping required for meat, and the reduction (or, in some animals, the absence) of canines as indicative of herbivorous diets. In humans, our canines are often a similar size to our incisors and therefore considered incisiform (Figure 9.10). However, our closest relatives all have very long, pointy canines, particularly on their upper dentition. This is true even for the gorilla, which lives almost exclusively on plants. The canines in these instances reveal more about social structure and sexual dimorphism than diet, as large canines often signal dominance.
Early on in human evolution, we see a reduction in canine size. Sahelanthropus tchadensis and Orrorin tugenensis both have smaller canines than those in extant great apes, yet the canines are still larger and pointier than those in humans or more recent hominins. This implies strongly that, over evolutionary time, the need for display and dominance among males has reduced, as has our sexual dimorphism. In Ardipithecus ramidus, there is no obvious difference between male and female canine size, yet they are still slightly larger and pointier than in modern humans. This implies a less sexually dimorphic social structure in the earlier hominins relative to modern-day chimpanzees and gorillas.
Along with a reduction in canine size is the reduction or elimination of a canine diastema: a gap between the teeth on the mandible that allows room for elongated teeth on the maxilla to “fit” in the mouth. Absence of a diastema is an excellent indication of a reduction in canine size. In animals with large canines (such as baboons), there is also often a honing P3, where the first premolar (also known as P3 for evolutionary reasons) is triangular in shape, “sharpened” by the extended canine from the upper dentition. This is also seen in some early hominins: Ardipithecus, for example, has small canines that are almost the same height as its incisors, although still larger than those in recent hominins.
The hind dentition, such as the bicuspid (two cusped) premolars or the much larger molars, are also highly indicative of a generalist diet in hominins. Among the earliest hominins, the molars are larger than we see in our genus, increasing in size to the back of the mouth and angled in such a way from the much smaller anterior dentition as to give these hominins a parabolic (V-shaped) dental arch. This differs from our living relatives and some early hominins, such as Sahelanthropus, whose molars and premolars are relatively parallel between the left and right sides of the mouth, creating a U-shape.
Among more recent early hominins, the molars are larger than those in the earliest hominins and far larger than those in our own genus, Homo. Large, short molars with thick enamel allowed our early cousins to grind fibrous, coarse foods, such as sedges, which require plenty of chewing. This is further evidenced in the low cusps, or ridges, on the teeth, which are ideal for chewing. In our genus, the hind dentition is far smaller than in these early hominins. Our teeth also have medium-size cusps, which allow for both efficient grinding and tearing/shearing meats.
Understanding the dental morphology has allowed researchers to extrapolate very specific behaviors of early hominins. It is worth noting that while teeth preserve well and are abundant, a slew of other morphological traits additionally provide evidence for many of these hypotheses. Yet there are some traits that are ambiguous. For instance, while there are definitely high levels of sexual dimorphism in Au. afarensis, discussed in the next section, the canine teeth are reduced in size, implying that while canines may be useful indicators for sexual dimorphism, it is also worth considering other evidence.
In summary, trends among early hominins include a reduction in procumbency, reduced hind dentition (molars and premolars), a reduction in canine size (more incisiform with a lack of canine diastema and honing P3), flatter molar cusps, and thicker dental enamel. All early hominins have the ancestral dental formula of 2:1:2:3. These trends are all consistent with a generalist diet, incorporating more fibrous foods.
Special Topic: Contested Species
Many named species are highly debated and argued to have specimens associated with a more variable Au. afarensis or Au. anamensis species. Sometimes these specimens are dated to times when, or found in places in which, there are “gaps” in the palaeoanthropological record. These are argued to represent chronospecies or variants of Au. afarensis. However, it is possible that, with more discoveries, the distinct species types will hold.
Australopithecus bahrelghazali is dated to within the time period of Au. afarensis (3.6 mya; Brunet et al. 1995) and was the first Australopithecine to be discovered in Chad in central Africa. Researchers argue that the holotype, whom discoverers have named “Abel,” falls under the range of variation of Au. afarensis and therefore that A. bahrelghazali does not fall into a new species (Lebatard et al. 2008). If “Abel” is a member of Au. afarensis, the geographic range of the species would be greatly extended.
On a different note, Australopithecus deyiremada (meaning “close relative” in the Ethiopian language of Afar) is dated to 3.5 mya to 3.3 mya and is based on fossil mandible bones discovered in 2011 in Woranso-Mille (in the Afar region of Ethiopia) by Yohannes Haile-Selassie, an Ethiopian paleoanthropologist (Haile-Selassie et al. 2019). The discovery indicated, in contrast to Au. afarensis, smaller teeth with thicker enamel (potentially suggesting a harder diet) as well as a larger mandible and more projecting cheekbones. This find may be evidence that more than one closely related hominin species occupied the same region at the same temporal period (Haile-Selassie et al. 2015; Spoor 2015) or that other Au. afarensis specimens have been incorrectly designated. However, others have argued that this species has been prematurely identified and that more evidence is needed before splitting the taxa, since the variation appears subtle and may be due to slightly different niche occupations between populations over time.
Australopithecus garhi is another species found in the Middle Awash region of Ethiopia. It is currently dated to 2.5 mya (younger than Au. afarensis). Researchers have suggested it fills in a much-needed temporal “gap” between hominin finds in the region, with some anatomical differences, such as a relatively large cranial capacity (450 cc) and larger hind dentition than seen in other gracile Australopithecines. Similarly, the species has been argued to have longer hind limbs than Au. afarensis, although it was still able to move arboreally (Asfaw et al. 1999). However, this species is not well documented or understood and is based on only several fossil specimens. More astonishingly, crude stone tools resembling Oldowan (which will be described later) have been found in association with Au. garhi. While lacking some of the features of the Oldowan, this is one of the earliest technologies found in direct association with a hominin.
Kenyanthopus platyops (the name “platyops” refers to its flatter-faced appearance) is a highly contested genus/species designation of a specimen (KNM-WT 40000) from Lake Turkana in Kenya, discovered by Maeve Leakey in 1999 (Figure 9.11). Dated to between 3.5 mya and 3.2 mya, some have suggested this specimen is an Australopithecus, perhaps even Au. afarensis (with a brain size which is difficult to determine, yet appears small), while still others have placed this specimen in Homo (small dentition and flat-orthognathic face). While taxonomic placing of this species is quite divided, the discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis (Leakey et al. 2001). Some researchers have additionally associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this specimen.

The Genus Australopithecus
The Australopithecines are a diverse group of hominins, comprising various species. Australopithecus is the given group or genus name. It stems from the Latin word Australo, meaning “southern,” and the Greek word pithecus, meaning “ape.” Within this section, we will outline these differing species’ geological and temporal distributions across Africa, unique derived and/or shared traits, and importance in the fossil record.
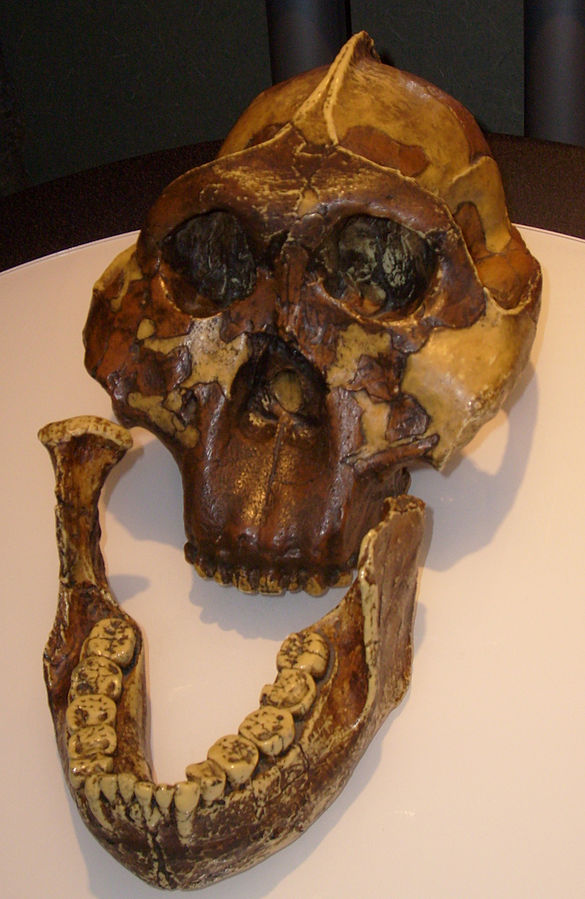
Between 3 mya and 1 mya, there seems to be differences in dietary strategy between different species of hominins designated as Australopithecines. A pattern of larger posterior dentition (even relative to the incisors and canines in the front of the mouth), thick enamel, and cranial evidence for extremely large chewing muscles is far more pronounced in a group known as the robust australopithecines. This pattern is extremely relative to their earlier contemporaries or predecessors, the gracile australopithecines, and is certainly larger than those seen in early Homo, which emerged during this time. This pattern of incredibly large hind dentition (and very small anterior dentition) has led people to refer to robust australopithecines as megadont hominins (Figure 9.12).
Because of these differences, this section has been divided into “gracile” and “robust” Australopithecines, highlighting the morphological differences between the two groups (which many researchers have designated as separate genera: Australopithecus and Paranthropus, respectively) and then focusing on the individual species. It is worth noting, however, that not all researchers accept these clades as biologically or genetically distinct, with some researchers insisting that the relative gracile and robust features found in these species are due to parallel evolutionary events toward similar dietary niches.
Despite this genus’ ancestral traits and small cranial capacity, all members show evidence of bipedal locomotion. It is generally accepted that Australopithecus species display varying degrees of arborealism along with bipedality.
Gracile Australopithecines
This section describes individual species from across Africa. These species are called “gracile australopithecines” because of their smaller and less robust features compared to the divergent “robust” group. Numerous Australopithecine species have been named, but some are only based on a handful of fossil finds, whose designations are controversial.
East African Australopithecines
East African Australopithecines are found throughout the EARS, and they include the earliest species associated with this genus. Numerous fossil-yielding sites, such as Olduvai, Turkana, and Laetoli, have excellent, datable stratigraphy, owing to the layers of volcanic tufts that have accumulated over millions of years. These tufts may be dated using absolute dating techniques, such as Potassium-Argon dating (described in Chapter 7). This means that it is possible to know a relatively refined date for any fossil if the context (i.e., exact location) of that find is known. Similarly, comparisons between the faunal assemblages of these stratigraphic layers have allowed researchers to chronologically identify environmental changes.
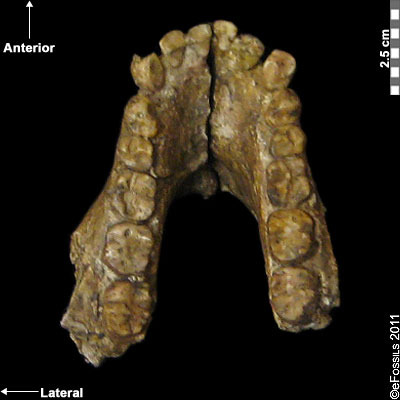
The earliest known Australopithecine is dated to 4.2 mya to 3.8 mya. Australopithecus anamensis (after “Anam,” meaning “lake” from the Turkana region in Kenya; Leakey et al. 1995; Patterson and Howells 1967) is currently found from sites in the Turkana region (Kenya) and Middle Awash (Ethiopia; Figure 9.13). Recently, a 2019 find from Ethiopia, named MRD, after Miro Dora where it was found, was discovered by an Ethiopian herder named Ali Bereino. It is one of the most complete cranial finds of this species (Ward et al. 1999). A small brain size (370 cc), relatively large canines, projecting cheekbones, and earholes show more ancestral features as compared to those of more recent Australopithecines. The most important element discovered with this species is a fragment of a tibia (shinbone), which demonstrates features associated with weight transfer during bipedal walking. Similarly, the earliest found hominin femur belongs to this species. Ancestral traits in the upper limb (such as the humerus) indicate some retained arboreal locomotion.
Some researchers suggest that Au. anamensis is an intermediate form of the chronospecies that becomes Au. afarensis, evolving from Ar. ramidus. However, this is debated, with other researchers suggesting morphological similarities and affinities with more recent species instead. Almost 100 specimens, representing over 20 individuals, have been found to date (Leakey et al. 1995; McHenry 2009; Ward et al. 1999).
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. Au. afarensis (which means “from the Afar region”) is dated to between 2.9 mya and 3.9 mya and is found in sites all along the EARS system, in Tanzania, Kenya, and Ethiopia (Figure 9.14). The most famous individual from this species is a partial female skeleton discovered in Hadar (Ethiopia), later nicknamed “Lucy,” after the Beatles’ song “Lucy in the Sky with Diamonds,” which was played in celebration of the find (Johanson et al. 1978; Kimbel and Delezene 2009). This skeleton was found in 1974 by Donald Johanson and dates to approximately 3.2 mya. In addition, in 2002 a juvenile of the species was found by Zeresenay Alemseged and given the name “Selam” (meaning “peace,” DIK 1-1), though it is popularly known as “Lucy’s Child” or as the “Dikika Child” (Alemseged et al. 2006). Similarly, the “Laetoli Footprints” (discussed in Chapter 7; Hay and Leakey 1982; Leakey and Hay 1979) have drawn much attention.

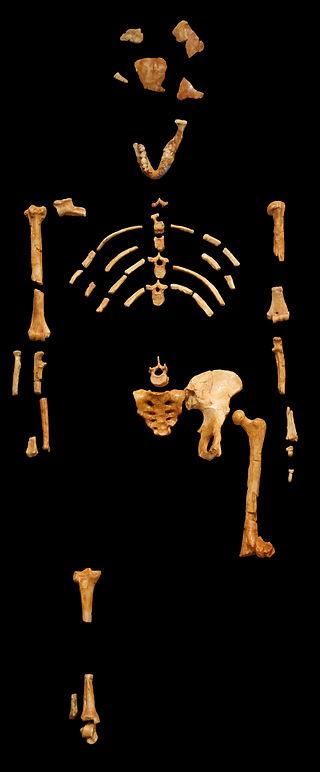
The canines and molars of Au. afarensis are reduced relative to great apes but are larger than those found in modern humans (indicative of a generalist diet); in addition, Au. afarensis has a prognathic face (the face below the eyes juts anteriorly) and robust facial features that indicate relatively strong chewing musculature (compared with Homo) but which are less extreme than in Paranthropus. Despite a reduction in canine size in this species, large overall size variation indicates high levels of sexual dimorphism.
Skeletal evidence indicates that this species was bipedal, as its pelvis and lower limb demonstrate a humanlike femoral neck, valgus knee, and bowl-shaped hip (Figure 9.15). More evidence of bipedalism is found in the footprints of this species. Au. afarensis is associated with the Laetoli Footprints, a 24-meter trackway of hominin fossil footprints preserved in volcanic ash discovered by Mary Leakey in Tanzania and dated to 3.5 mya to 3 mya. This set of prints is thought to have been produced by three bipedal individuals as there are no knuckle imprints, no opposable big toes, and a clear arch is present. The infants of this species are thought to have been more arboreal than the adults, as discovered through analyses of the foot bones of the Dikika Child dated to 3.32 mya (Alemseged et al. 2006).
Although not found in direct association with stone tools, potential evidence for cut marks on bones, found at Dikika, and dated to 3.39 mya indicates a possible temporal/ geographic overlap between meat eating, tool use, and this species. However, this evidence is fiercely debated. Others have associated the cut marks with the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species.
South African Australopithecines
Since the discovery of the Taung Child, there have been numerous Australopithecine discoveries from the region known as “The Cradle of Humankind,” which was recently given UNESCO World Heritage Site status as “The Fossil Hominid Sites of South Africa.” The limestone caves found in the Cradle allow for the excellent preservation of fossils. Past animals navigating the landscape and falling into cave openings, or caves used as dens by carnivores, led to the accumulation of deposits over millions of years. Many of the hominin fossils, encased in breccia (hard, calcareous sedimentary rock), are recently exposed from limestone quarries mined in the previous century. This means that extracting fossils requires excellent and detailed exposed work, often by a team of skilled technicians.
While these sites have historically been difficult to date, with mixed assemblages accumulated over large time periods, advances in techniques such as uranium-series dating have allowed for greater accuracy. Historically, the excellent faunal record from East Africa has been used to compare sites based on relative dating, whereby environmental and faunal changes and extinction events allow us to know which hominin finds are relatively younger or older than others.
The discovery of the Taung Child in 1924 (discussed in the Special Topic box “The Taung Child” below) shifted the focus of palaeoanthropological research from Europe to Africa, although acceptance of this shift was slow (Broom 1947; Dart 1925). The species to which it is assigned, Australopithecus africanus (name meaning “Southern Ape of Africa”), is currently dated to between 3.3 mya and 2.1 mya (Pickering and Kramers 2010), with discoveries from Sterkfontein, Taung, Makapansgat, and Gladysvale in South Africa (Figure 9.16). A relatively large brain (400 cc to 500 cc), small canines without an associated diastema, and more rounded cranium and smaller teeth than Au. afarensis indicate some derived traits. Similarly, the postcranial remains (in particular, the pelvis) indicate bipedalism. However, the sloping face and curved phalanges (indicative of retained arboreal locomotor abilities) show some ancestral features. Although not in direct association with stone tools, a 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities.

Another famous Au. africanus skull (the skull of “Mrs. Ples”) was previously attributed to Plesianthropus transvaalensis, meaning “near human from the Transvaal,” the old name for Gauteng Province, South Africa (Broom 1947, 1950). The name was shortened by contemporary journalists to “Ples” (Figure 9.17). Due to the prevailing mores of the time, the assumed female found herself married, at least in name, and has become widely known as “Mrs. Ples.” It was later reassigned to Au. africanus and is now argued by some to be a young male rather than an adult female cranium (Thackeray 2000, Thackeray et al. 2002).
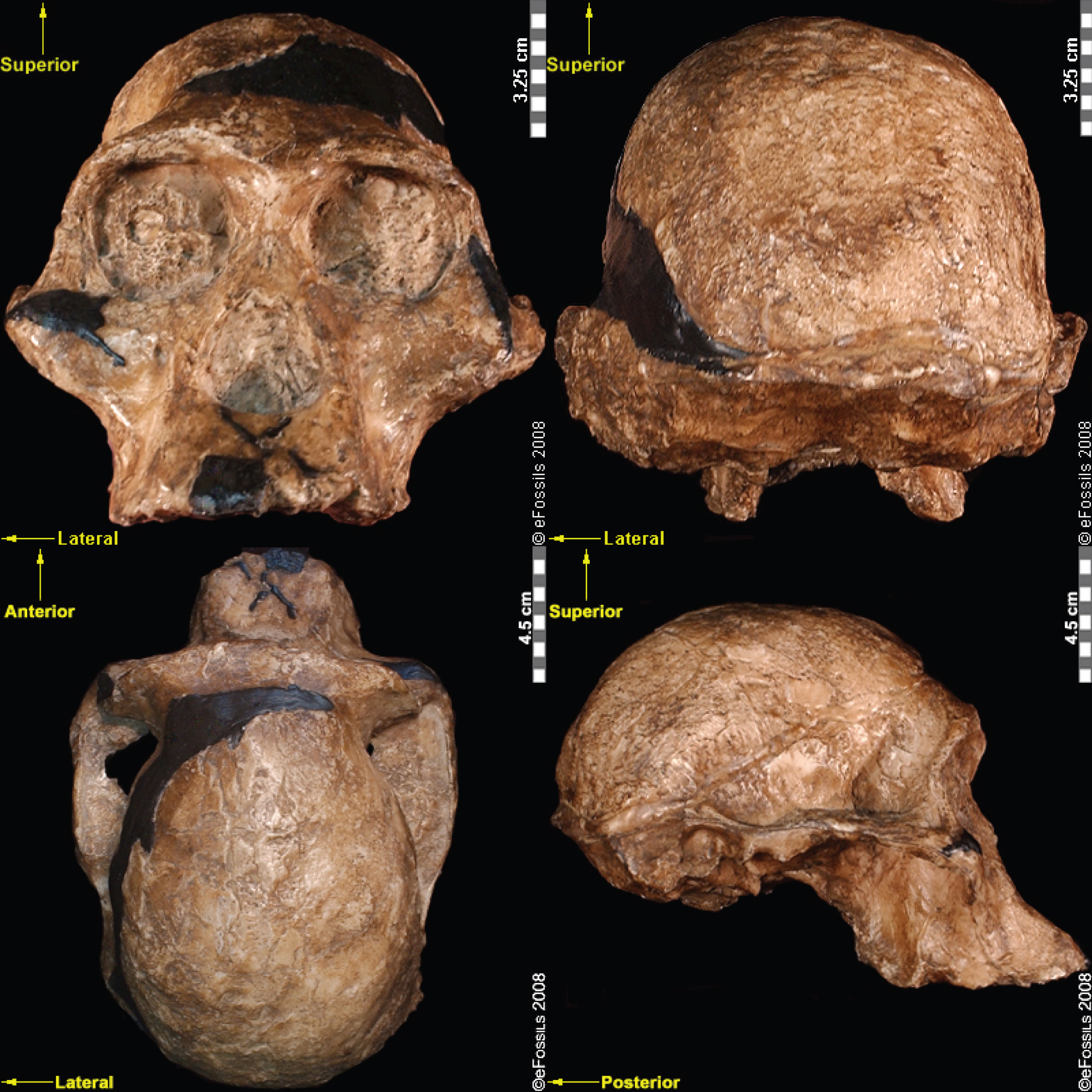
In 2008, nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger, noted a clavicle bone in some leftover mining breccia in the Malapa Fossil Site (South Africa). After rigorous studies, the species, Australopithecus sediba (meaning “fountain” or “wellspring” in the South African language of Sesotho), was named in 2010 (Figure 9.18; Berger et al. 2010). The first type specimen belongs to a juvenile male, Karabo (MH1), but the species is known from at least six partial skeletons, from infants through adults. These specimens are currently dated to 1.97 mya (Dirks et al. 2010). The discoverers have argued that Au. sediba shows mosaic features between Au. africanus and the genus, Homo, which potentially indicates a transitional species, although this is heavily debated. These features include a small brain size (Australopithecus-like; 420 cc to 450 cc) but gracile mandible and small teeth (Homo-like). Similarly, the postcranial skeletons are also said to have mosaic features: scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. Some researchers have argued that Au. sediba shows a modern hand morphology (shorter fingers and a longer thumb), indicating that adaptations to tool manufacture and use may be present in this species.
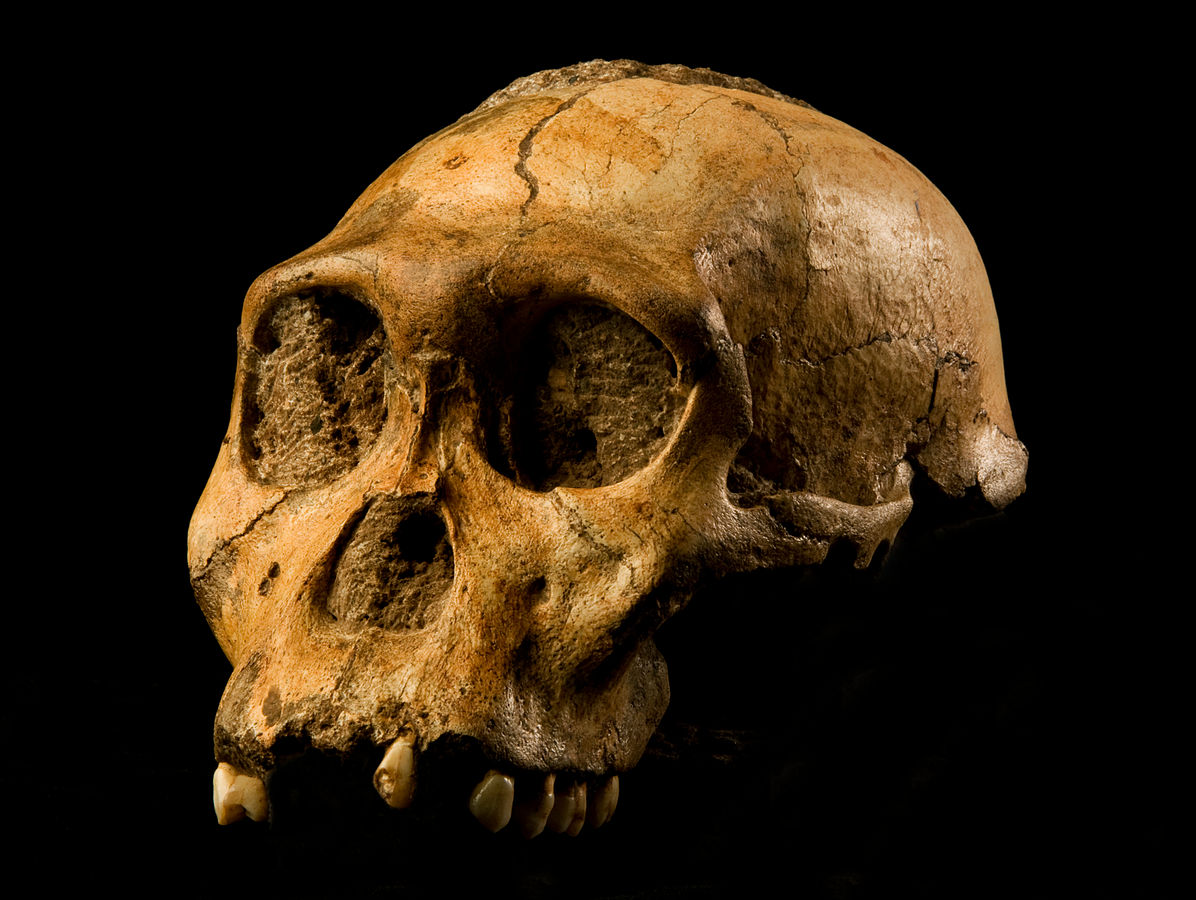
Another famous Australopithecine find from South Africa is that of the nearly complete skeleton now known as “Little Foot” (Clarke 1998, 2013). Little Foot (StW 573) is potentially the earliest dated South African hominin fossil, dating to 3.7 mya, based on radiostopic techniques, although some argue that it is younger than 3 mya (Pickering and Kramers 2010). The name is jokingly in contrast to the cryptid species “bigfoot” and is named because the initial discovery of four ankle bones indicated bipedality. Little Foot was discovered by Ron Clarke in 1994, when he came across the ankle bones while sorting through monkey fossils in the University of Witwatersrand collections (Clarke and Tobias 1995). He asked Stephen Motsumi and Nkwane Molefe to identify the known records of the fossils, which allowed them to find the rest of the specimen within just days of searching the Sterkfontein Caves’ Silberberg Grotto.
The discoverers of Little Foot insist that other fossil finds, previously identified as Au. Africanus, be placed in this new species based on shared ancestral traits with older East African Australopithecines (Clarke and Kuman 2019). These include features such as a relatively large brain size (408 cc), robust zygomatic arch, and a flatter midface. Furthermore, the discoverers have argued that the heavy anterior dental wear patterns, relatively large anterior dentition, and smaller hind dentition of this specimen more closely resemble that of Au. anamensis or Au. afarensis. It has thus been placed in the species Australopithecus prometheus. This species name refers to a previously defunct taxon named by Raymond Dart. The species designation was, through analyzing Little Foot, revived by Ron Clarke, who insists that many other fossil hominin specimens have prematurely been placed into Au. africanus. Others say that it is more likely that Au. africanus is a more variable species and not representative of two distinct species.
Paranthropus “Robust” Australopithecines
In the robust australopithecines, the specialized nature of the teeth and masticatory system, such as flaring zygomatic arches (cheekbones), accommodate very large temporalis (chewing) muscles. These features also include a large, broad, dish-shaped face and and a large mandible with extremely large posterior dentition (referred to as megadonts) and hyper-thick enamel (Kimbel 2015; Lee-Thorp 2011; Wood 2010). Research has revolved around the shared adaptations of these “robust” australopithecines, linking their morphologies to a diet of hard and/or tough foods (Brain 1967; Rak 1988). Some argued that the diet of the robust australopithecines was so specific that any change in environment would have accelerated their extinction. The generalist nature of the teeth of the gracile australopithecines, and of early Homo, would have made them more capable of adapting to environmental change. However, some have suggested that the features of the robust australopithecines might have developed as an effective response to what are known as fallback foods in hard times rather than indicating a lack of adaptability.
There are currently three widely accepted robust australopithecus or, Paranthropus, species: P. aethiopicus, which has more ancestral traits, and P. boisei and P. robustus, which are more derived in their features (Strait et al. 1997; Wood and Schroer 2017). These three species have been grouped together by a majority of scholars as a single genus as they share more derived features (are more closely related to each other; or, in other words, are monophyletic) than the other australopithecines (Grine 1988; Hlazo 2015; Strait et al. 1997; Wood 2010 ). While researchers have mostly agreed to use the umbrella term Paranthropus, there are those who disagree (Constantino and Wood 2004, 2007; Wood 2010).
As a collective, this genus spans 2.7 mya to 1.0 mya, although the dates of the individual species differ. The earliest of the Paranthropus species, Paranthropus aethiopicus, is dated to between 2.7 mya and 2.3 mya and currently found in Tanzania, Kenya, and Ethiopia in the EARS system (Figure 9.19; Constantino and Wood 2007; Hlazo 2015; Kimbel 2015; Walker et al. 1986; White 1988). It is well known because of one specimen known as the “Black Skull” (KNM–WT 17000), so called because of the mineral manganese that stained it black during fossilization (Kimbel 2015). As with all robust Australopithecines, P. aethiopicus has the shared derived traits of large, flat premolars and molars; large, flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle); a sagittal crest (ridge on the top of the skull) for increased muscle attachment of the chewing muscles to the skull; and a robust mandible and supraorbital torus (brow ridge). However, only a few teeth have been found. A proximal tibia indicates bipedality and similar body size to Au. afarensis. In recent years, researchers have discovered and assigned a proximal tibia and juvenile cranium (L.338y-6) to the species (Wood and Boyle 2016).
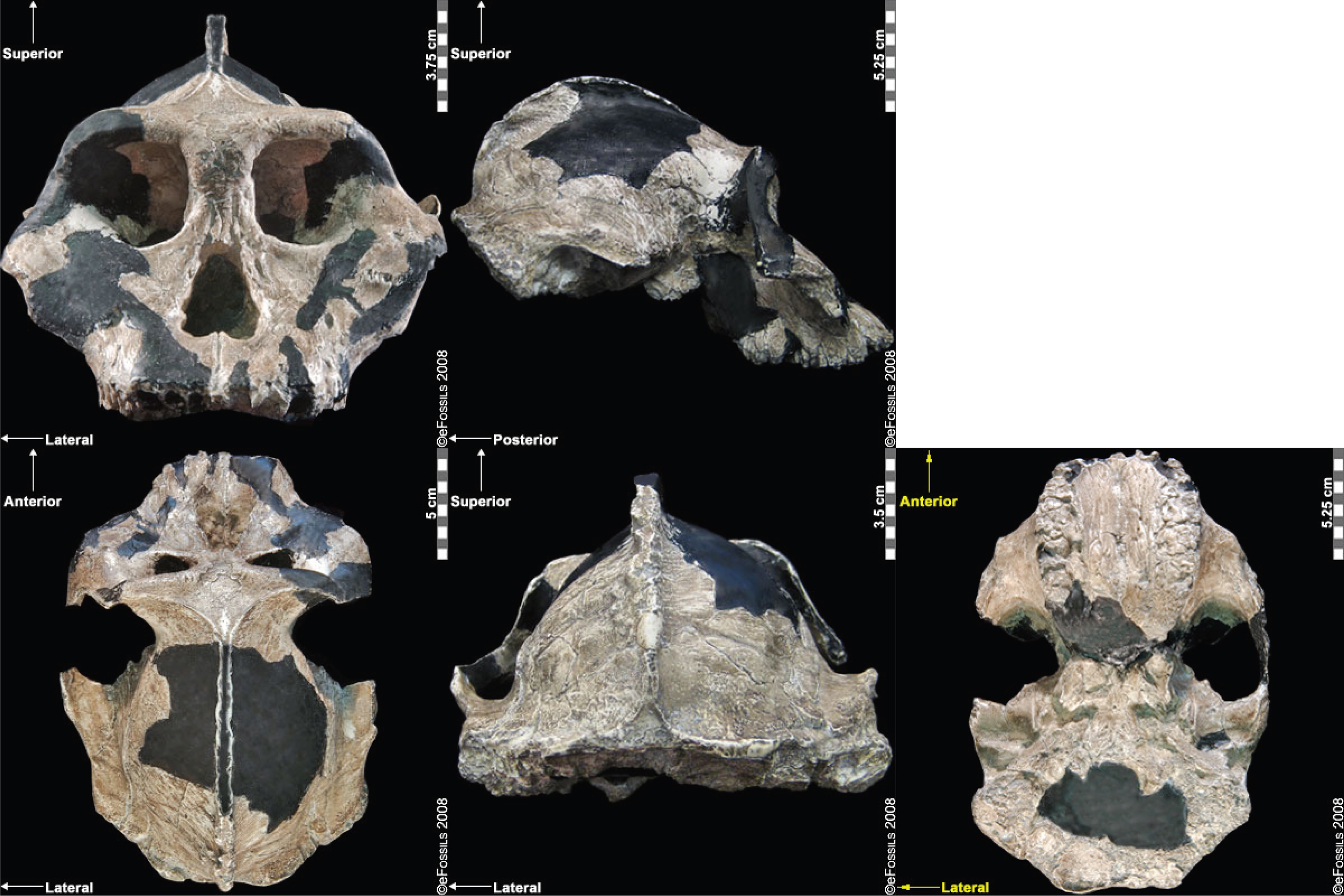
First attributed as Zinjanthropus boisei (with the first discovery going by the nickname “Zinj” or sometimes “Nutcracker Man”), Paranthropus boisei was discovered in 1959 by Mary Leakey (see Figure 9.20 and 9.21; Hay 1990; Leakey 1959). This “robust” australopith species is distributed across countries in East Africa at sites such as Kenya (Koobi Fora, West Turkana, and Chesowanja), Malawi (Malema-Chiwondo), Tanzania (Olduvai Gorge and Peninj), and Ethiopia (Omo River Basin and Konso). The hypodigm, sample of fossils whose features define the group, has been found by researchers to date to roughly 2.4 mya to 1.4 mya. Due to the nature of its exaggerated, larger, and more robust features, P. boisei has been termed hyper-robust—that is, even more heavily built than other robust species, with very large, flat posterior dentition (Kimbel 2015). Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). Another famous specimen from this species is the Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu.

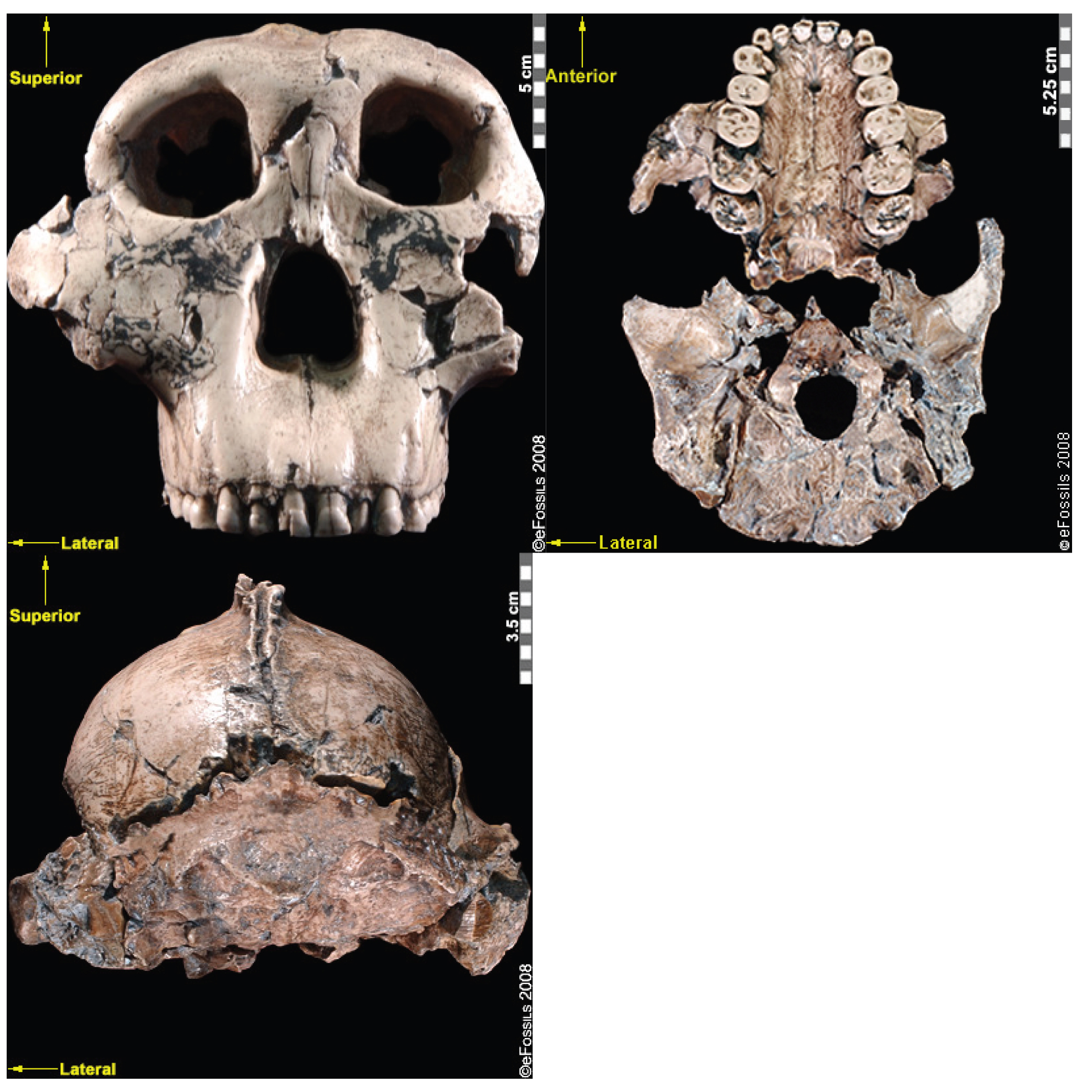
Paranthropus robustus was the first taxon to be discovered within the genus in Kromdraai B by a schoolboy named Gert Terblanche; subsequent fossil discoveries were made by researcher Robert Broom in 1938 (Figure 9.22; Broom 1938a, 1938b, 1950), with the holotype specimen TM 1517 (Broom 1938a, 1938b, 1950; Hlazo 2018). Paranthropus robustus dates approximately from 2.0 mya to 1 mya and is the only taxon from the genus to be discovered in South Africa. Several of these fossils are fragmentary in nature, distorted, and not well preserved because they have been recovered from quarry breccia using explosives. P. robustus features are neither as “hyper-robust” as P. boisei nor as ancestral as P. aethiopicus; instead, they have been described as being less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring; Rak 1983; Walker and Leakey 1988). Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick-enameled dentition.
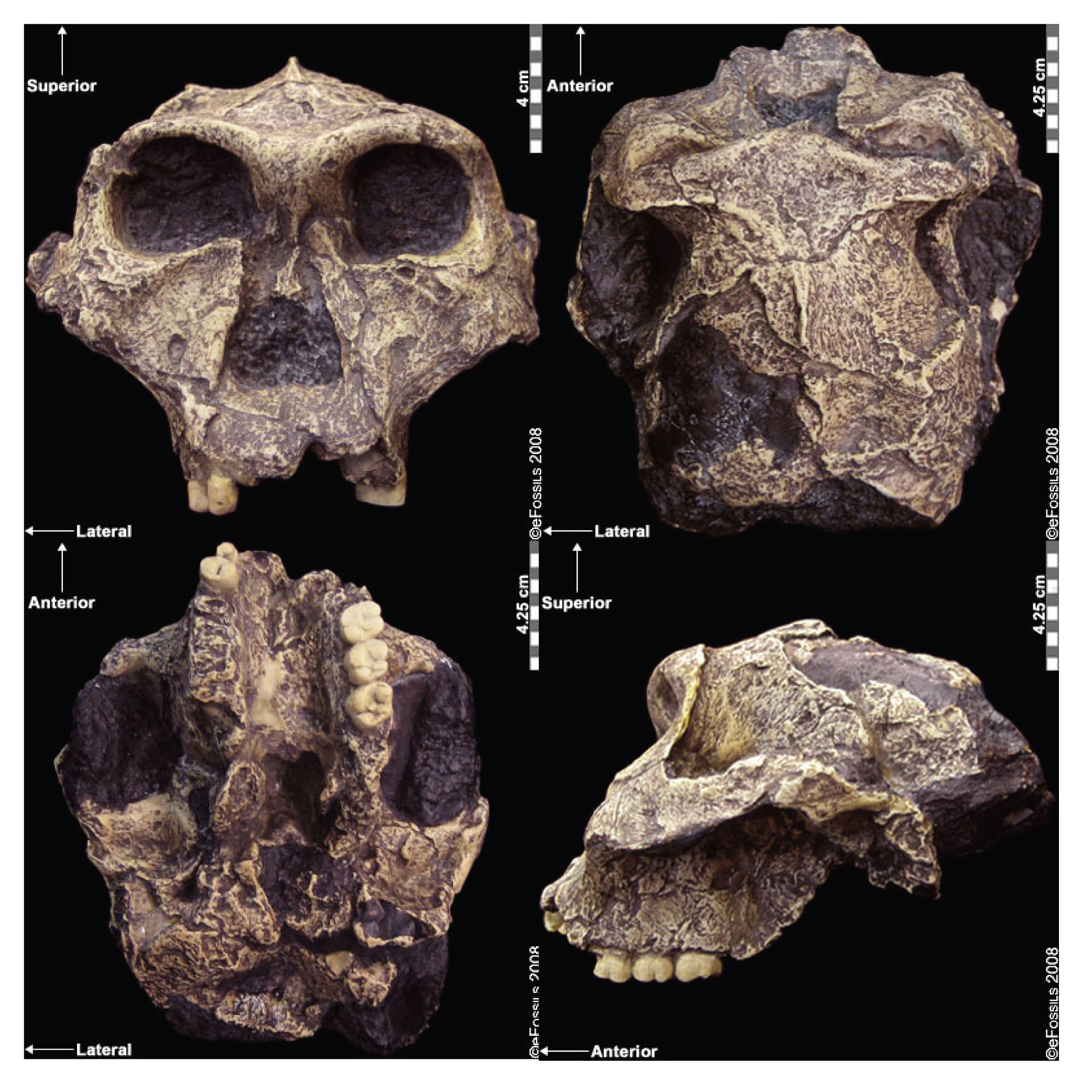
Comparisons between Gracile and Robust Australopiths
Comparisons between gracile and robust australopithecines may indicate different phylogenetic groupings or parallel evolution in several species. In general, the robust australopithecines have large temporalis (chewing) muscles, as indicated by flaring zygomatic arches, sagittal crests, and robust mandibles (jawbones). Their hind dentition is large (megadont), with low cusps and thick enamel. Within the gracile australopithecines, researchers have debated the relatedness of the species, or even whether these species should be lumped together to represent more variable or polytypic species. Often researchers will attempt to draw chronospecific trajectories, with one taxon said to evolve into another over time.
Special Topic: The Taung Child
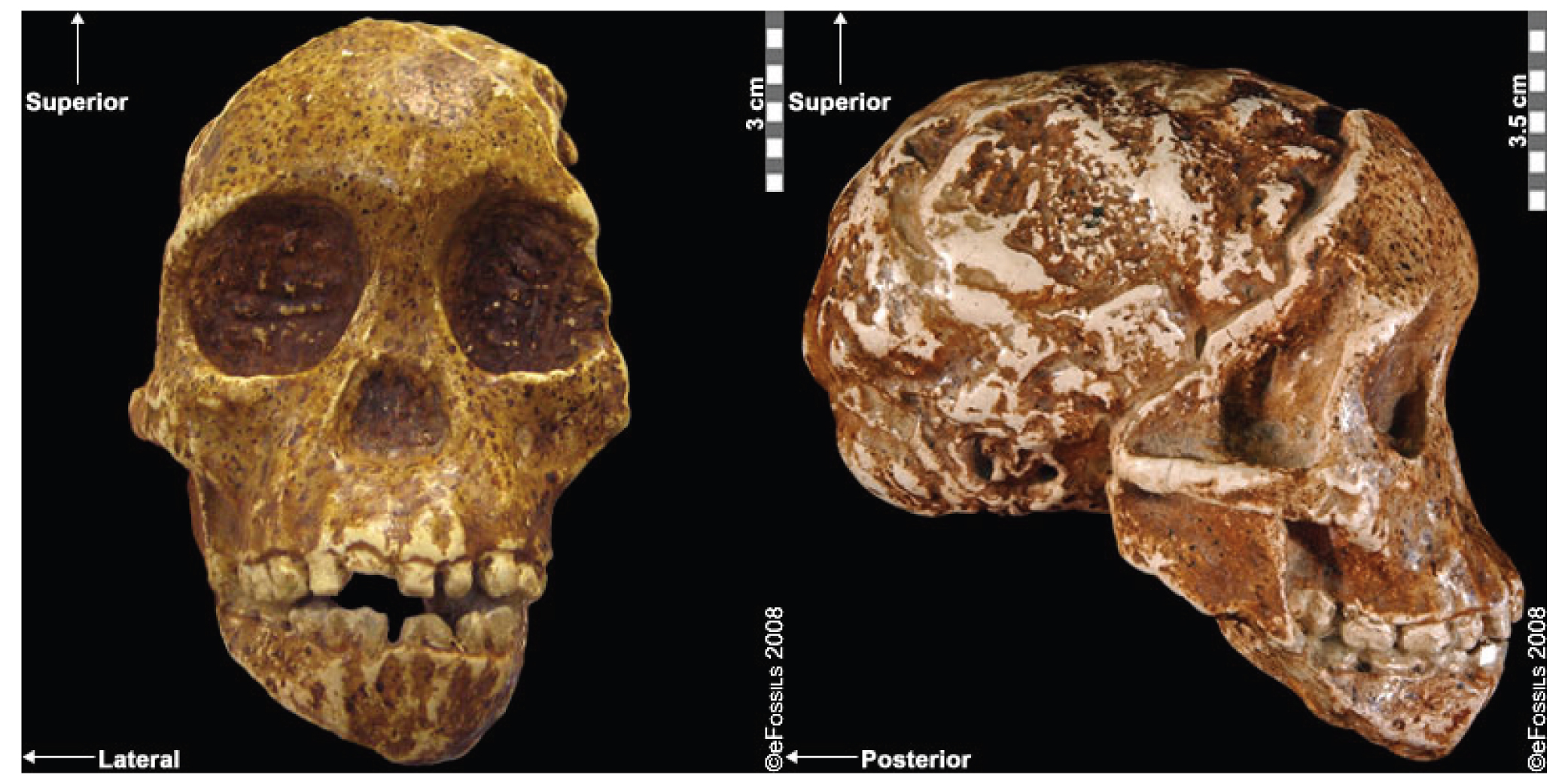
The well-known fossil of a juvenile Australopithecine, the “Taung Child,” was the first early hominin evidence ever discovered and was the first to demonstrate our common human heritage in Africa (Figure 9.23; Dart 1925). The tiny facial skeleton and natural endocast were discovered in 1924 by a local quarryman in the North West Province in South Africa and were painstakingly removed from the surrounding cement-like breccia by Raymond Dart using his wife’s knitting needles. When first shared with the scientific community in 1925, it was discounted as being nothing more than a young monkey of some kind. Prevailing biases of the time made it too difficult to contemplate that this small-brained hominin could have anything to do with our own history. The fact that it was discovered in Africa simply served to strengthen this bias.
Early Tool Use and Technology
Early Stone Age Technology (ESA)
The Early Stone Age (ESA) marks the beginning of recognizable technology made by our human ancestors. Stone-tool (or lithic) technology is defined by the fracturing of rocks and the manufacture of tools through a process called knapping. The Stone Age lasted for more than 3 million years and is broken up into chronological periods called the Early (ESA), Middle (MSA), and Later Stone Ages (LSA). Each period is further broken up into a different techno-complex, a term encompassing multiple assemblages (collections of artifacts) that share similar traits in terms of artifact production and morphology. The ESA spanned the largest technological time period of human innovation from over 3 million years ago to around 300,000 years ago and is associated almost entirely with hominin species prior to modern Homo sapiens. As the ESA advanced, stone tool makers (known as knappers) began to change the ways they detached flakes and eventually were able to shape artifacts into functional tools. These advances in technology go together with the developments in human evolution and cognition, dispersal of populations across the African continent and the world, and climatic changes.
In order to understand the ESA, it is important to consider that not all assemblages are exactly the same within each techno-complex: one can have multiple phases and traditions at different sites (Lombard et al. 2012). However, there is an overarching commonality between them. Within stone tool assemblages, both flakes or cores (the rocks from which flakes are removed) are used as tools. Large Cutting Tools (LCTs) are tools that are shaped to have functional edges. It is important to note that the information presented here is a small fraction of what is known about the ESA, and there are ongoing debates and discoveries within archaeology.
Currently, the oldest-known stone tools, which form the techno-complex the Lomekwian, date to 3.3 mya (Harmand et al. 2015; Toth 1985). They were found at a site called Lomekwi 3 in Kenya. This techno-complex is the most recently defined and pushed back the oldest-known date for lithic technology. There is only one known site thus far and, due to the age of the site, it is associated with species prior to Homo, such as Kenyanthropus platyops. Flakes were produced through indirect percussion, whereby the knappers held a rock and hit it against another rock resting on the ground. The pieces are very chunky and do not display the same fracture patterns seen in later techno-complexes. Lomekwian knappers likely aimed to get a sharp-edged piece on a flake, which would have been functional, although the specific function is currently unknown.
Stone tool use, however, is not only understood through the direct discovery of the tools. Cut marks on fossilized animal bones may illuminate the functionality of stone tools. In one controversial study in 2010, researchers argued that cut marks on a pair of animal bones from Dikika (Ethiopia), dated to 3.4 mya, were from stone tools. The discoverers suggested that they be more securely associated, temporally, with Au. afarensis. However, others have noted that these marks are consistent with teeth marks from crocodiles and other carnivores.
The Oldowan techno-complex is far more established in the scientific literature (Leakey 1971). It is called the Oldowan because it was originally discovered in Olduvai Gorge, Tanzania, but the oldest assemblage is from Gona in Ethiopia, dated to 2.6 mya (Semaw 2000). The techno-complex is defined as a core and flake industry. Like the Lomekwian, there was an aim to get sharp-edged flakes, but this was achieved through a different production method. Knappers were able to actively hold or manipulate the core being knapped, which they could directly hit using a hammerstone. This technique is known as free-hand percussion, and it demonstrates an understanding of fracture mechanics. It has long been argued that the Oldowan hominins were skillful in tool manufacture.
Because Oldowan knapping requires skill, earlier researchers have attributed these tools to members of our genus, Homo. However, some have argued that these tools are in more direct association with hominins in the genera described in this chapter (Figure 9.24).
Invisible Tool Manufacture and Use
The vast majority of our understanding of these early hominins comes from fossils and reconstructed paleoenvironments. It is only from 3 mya when we can start “looking into their minds” and lifestyles by analyzing their manufacture and use of stone tools. However, the vast majority of tool use in primates (and, one can argue, in humans) is not with durable materials like stone. All of our extant great ape relatives have been observed using sticks, leaves, and other materials for some secondary purpose (to wade across rivers, to “fish” for termites, or to absorb water for drinking). It is possible that the majority of early hominin tool use and manufacture may be invisible to us because of this preservation bias.
Chapter Summary
The fossil record of our earliest hominin relatives has allowed paleoanthropologists to unpack some of the mysteries of our evolution. We now know that traits associated with bipedalism evolved before other “human-like” traits, even though the first hominins were still very capable of arboreal locomotion. We also know that, for much of this time, hominin taxa were diverse in the way they looked and what they ate, and they were widely distributed across the African continent. And we know that the environments in which these hominins lived underwent many changes over this time during several warming and cooling phases.
Yet this knowledge has opened up many new mysteries. We still need to better differentiate some taxa. In addition, there are ongoing debates about why certain traits evolved and what they meant for the extinction of some of our relatives (like the robust australopiths). The capabilities of these early hominins with respect to tool use and manufacture is also still uncertain.
Hominin Species Summaries
|
Hominin |
Sahelanthropus tchadensis |
|
Dates |
7 mya to 6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
The initial discovery, made in 2001. |
|
Brain size |
360 cc average |
|
Dentition |
Smaller than in extant great apes; larger and pointier than in humans. Canines worn at the tips. |
|
Cranial features |
A short cranial base and a foramen magnum (hole in which the spinal cord enters the cranium) that is more humanlike in positioning; has been argued to indicate upright walking. |
|
Postcranial features |
Currently little published postcranial material. |
|
Culture |
N/A |
|
Other |
The extent to which this hominin was bipedal is currently heavily debated. If so, it would indicate an arboreal bipedal ancestor of hominins, not a knuckle-walker like chimpanzees. |
|
Hominin |
Orrorin tugenensis |
|
Dates |
6 mya to 5.7 mya |
|
Region(s) |
Tugen Hills (Kenya) |
|
Famous discoveries |
Original discovery in 2000. |
|
Brain size |
N/A |
|
Dentition |
Smaller cheek teeth (molars and premolars) than even more recent hominins (i.e., derived), thick enamel, and reduced, but apelike, canines. |
|
Cranial features |
Not many found |
|
Postcranial features |
Fragmentary leg, arm, and finger bones have been found. Indicates bipedal locomotion. |
|
Culture |
Potential toolmaking capability based on hand morphology, but nothing found directly. |
|
Other |
This is the earliest species that clearly indicates adaptations for bipedal locomotion. |
|
Hominin |
Ardipithecus kadabba |
|
Dates |
5.2 mya to 5.8 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
Discovered by Yohannes Haile-Selassie in 1997. |
|
Brain size |
N/A |
|
Dentition |
Larger hind dentition than in modern chimpanzees. Thick enamel and larger canines than in later hominins. |
|
Cranial features |
N/A |
|
Postcranial features |
A large hallux (big toe) bone indicates a bipedal “push off.” |
|
Culture |
N/A |
|
Other |
Faunal evidence indicates a mixed grassland/woodland environment. |
|
Hominin |
Ardipithecus ramidus |
|
Dates |
4.4 mya |
|
Region(s) |
Middle Awash region and Gona (Ethiopia) |
|
Famous discoveries |
A partial female skeleton nicknamed “Ardi” (ARA-VP-6/500) (found in 1994). |
|
Brain size |
300 cc to 350 cc |
|
Dentition |
Little differences between the canines of males and females (small sexual dimorphism). |
|
Cranial features |
Midfacial projection, slightly prognathic. Cheekbones less flared and robust than in later hominins. |
|
Postcranial features |
Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, an opposable big toe similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. |
|
Culture |
None directly associated |
|
Other |
Over 110 specimens from Aramis |
|
Hominin |
Australopithecus anamensis |
|
Dates |
4.2 mya to 3.8 mya |
|
Region(s) |
Turkana region (Kenya); Middle Awash (Ethiopia) |
|
Famous discoveries |
A 2019 find from Ethiopia, named MRD. |
|
Brain size |
370 cc |
|
Dentition |
Relatively large canines compared with more recent Australopithecines. |
|
Cranial features |
Projecting cheekbones and ancestral earholes. |
|
Postcranial features |
Lower limb bones (tibia and femur) indicate bipedality; arboreal features in upper limb bones (humerus) found. |
|
Culture |
N/A |
|
Other |
Almost 100 specimens, representing over 20 individuals, have been found to date. |
|
Hominin |
Australopithecus afarensis |
|
Dates |
3.9 mya to 2.9 mya |
|
Region(s) |
Afar Region, Omo, Maka, Fejej, and Belohdelie (Ethiopia); Laetoli (Tanzania); Koobi Fora (Kenya) |
|
Famous discoveries |
Lucy (discovery: 1974), Selam (Dikika Child, discovery: 2000), Laetoli Footprints (discovery: 1976). |
|
Brain size |
380 cc to 430 cc |
|
Dentition |
Reduced canines and molars relative to great apes but larger than in modern humans. |
|
Cranial features |
Prognathic face, facial features indicate relatively strong chewing musculature (compared with Homo) but less extreme than in Paranthropus. |
|
Postcranial features |
Clear evidence for bipedalism from lower limb postcranial bones. Laetoli Footprints indicate humanlike walking. Dikika Child bones indicate retained ancestral arboreal traits in the postcrania. |
|
Culture |
None directly, but close in age and proximity to controversial cut marks at Dikika and early tools in Lomekwi. |
|
Other |
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. |
|
Hominin |
Australopithecus bahrelghazali |
|
Dates |
3.6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
“Abel,” the holotype (discovery: 1995). |
|
Brain size |
N/A |
|
Dentition |
N/A |
|
Cranial features |
N/A |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Arguably within range of variation of Au. afarensis. |
|
Hominin |
Australopithecus prometheus |
|
Dates |
3.7 mya (debated) |
|
Region(s) |
Sterkfontein (South Africa) |
|
Famous discoveries |
“Little Foot” (StW 573) (discovery: 1994) |
|
Brain size |
408 cc (Little Foot estimate) |
|
Dentition |
Heavy anterior dental wear patterns, relatively large anterior dentition and smaller hind dentition, similar to Au. afarensis. |
|
Cranial features |
Relatively larger brain size, robust zygomatic arch, and a flatter midface. |
|
Postcranial features |
The initial discovery of four ankle bones indicated bipedality. |
|
Culture |
N/A |
|
Other |
Highly debated new species designation. |
|
Hominin |
Australopithecus deyiremada |
|
Dates |
3.5 mya to 3.3 mya |
|
Region(s) |
Woranso-Mille (Afar region, Ethiopia) |
|
Famous discoveries |
First fossil mandible bones were discovered in 2011 in the Afar region of Ethiopia by Yohannes Haile-Selassie. |
|
Brain size |
N/A |
|
Dentition |
Smaller teeth with thicker enamel than seen in Au. afarensis, with a potentially hardier diet. |
|
Cranial features |
Larger mandible and more projecting cheekbones than in Au. afarensis. |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Contested species designation; arguably a member of Au. afarensis. |
|
Hominin |
Kenyanthopus platyops |
|
Dates |
3.5 mya to 3.2 mya |
|
Region(s) |
Lake Turkana (Kenya) |
|
Famous discoveries |
KNM–WT 40000 (discovered 1999) |
|
Brain size |
Difficult to determine but appears within the range of Australopithecus afarensis. |
|
Dentition |
Small molars/dentition (Homo-like characteristic) |
|
Cranial features |
Flatter (i.e., orthognathic) face |
|
Postcranial features |
N/A |
|
Culture |
Some have associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species/specimen. |
|
Other |
Taxonomic placing of this species is quite divided. The discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis. |
|
Hominin |
Australopithecus africanus |
|
Dates |
3.3 mya to 2.1 mya |
|
Region(s) |
Sterkfontein, Taung, Makapansgat, Gladysvale (South Africa) |
|
Famous discoveries |
Taung Child (discovery in 1994), “Mrs. Ples” (discover in 1947), Little Foot (arguable; discovery in 1994). |
|
Brain size |
400 cc to 500 cc |
|
Dentition |
Smaller teeth (derived) relative to Au. afarensis. Small canines with no diastema. |
|
Cranial features |
A rounder skull compared with Au. afarensis in East Africa. A sloping face (ancestral). |
|
Postcranial features |
Similar postcranial evidence for bipedal locomotion (derived pelvis) with retained arboreal locomotion, e.g., curved phalanges (fingers), as seen in Au. afarensis. |
|
Culture |
None with direct evidence. |
|
Other |
A 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities. |
|
Hominin |
Australopithecus garhi |
|
Dates |
2.5 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
N/A |
|
Brain size |
450 cc |
|
Dentition |
Larger hind dentition than seen in other gracile Australopithecines. |
|
Cranial features |
N/A |
|
Postcranial features |
A femur of a fragmentary partial skeleton, argued to belong to Au. garhi, indicates this species may be longer-limbed than Au. afarensis, although still able to move arboreally. |
|
Culture |
Crude stone tools resembling Oldowan (described later) have been found in association with Au. garhi. |
|
Other |
This species is not well documented or understood and is based on only a few fossil specimens. |
|
Hominin |
Paranthropus aethiopicus |
|
Dates |
2.7 mya to 2.3 mya |
|
Region(s) |
West Turkana (Kenya); Laetoli (Tanzania); Omo River Basin (Ethiopia) |
|
Famous discoveries |
The “Black Skull” (KNM–WT 17000) (discovery 1985). |
|
Brain Size |
410 cc |
|
Dentition |
P. aethiopicus has the shared derived traits of large flat premolars and molars, although few teeth have been found. |
|
Cranial features |
Large flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle), a sagittal crest for increased muscle attachment of the chewing muscles to the skull, and a robust mandible and supraorbital torus (brow ridge). |
|
Postcranial features |
A proximal tibia indicates bipedality and similar size to Au. afarensis. |
|
Culture |
N/A |
|
Other |
The “Black Skull” is so called because of the mineral manganese that stained it black during fossilization. |
|
Hominin |
Paranthropus boisei |
|
Dates |
2.4 mya to 1.4 mya |
|
Region(s) |
Koobi Fora, West Turkana, and Chesowanja (Kenya); Malema-Chiwondo (Malawi), Olduvai Gorge and Peninj (Tanzania); and Omo River basin and Konso (Ethiopia) |
|
Famous discoveries |
“Zinj,” or sometimes “Nutcracker Man” (OH5), in 1959 by Mary Leakey. The Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu. |
|
Brain size |
500 cc to 550 cc |
|
Dentition |
Very large, flat posterior dentition (largest of all hominins currently known). Much smaller anterior dentition. Very thick dental enamel. |
|
Cranial features |
Indications of very large chewing muscles (e.g., flaring zygomatic arches and a large sagittal crest). |
|
Postcranial features |
Evidence for high variability and sexual dimorphism, with estimates of males at 1.37 meters tall and females at 1.24 meters. |
|
Culture |
Richard Leakey and Bernard Wood have both suggested that P. boisei could have made and used stone tools. Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. |
|
Other |
Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). This differs from what is seen in P. robustus. |
|
Hominin |
Australopithecus sediba |
|
Dates |
1.97 mya |
|
Region(s) |
Malapa Fossil Site (South Africa) |
|
Famous discoveries |
Karabo (MH1) (discovery in 2008) |
|
Brain size |
420 cc to 450 cc |
|
Dentition |
Small dentition with Australopithecine cusp-spacing. |
|
Cranial features |
Small brain size (Australopithecus-like) but gracile mandible (Homo-like). |
|
Postcranial features |
Scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (tree climbing, particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. |
|
Culture |
None of direct association, but some have argued that a modern hand morphology (shorter fingers and a longer thumb) means that adaptations to tool manufacture and use may be present in this species. |
|
Other |
It was first discovered through a clavicle bone in 2008 by nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger. |
|
Hominin |
Paranthropus robustus |
|
Dates |
2.3 mya to 1 mya |
|
Region(s) |
Kromdraai B, Swartkrans, Gondolin, Drimolen, and Coopers Cave (South Africa) |
|
Famous discoveries |
SK48 (original skull) |
|
Brain size |
410 cc to 530 cc |
|
Dentition |
Large posterior teeth with thick enamel, consistent with other Robust Australopithecines. Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick enameled dentition. |
|
Cranial features |
P. robustus features are neither as “hyper-robust” as P. boisei or as ancestral in features as P. aethiopicus. They have been described as less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring). |
|
Postcranial features |
Reconstructions indicate sexual dimorphism. |
|
Culture |
N/A |
|
Other |
Several of these fossils are fragmentary in nature, distorted, and not well preserved, because they have been recovered from quarry breccia using explosives. |
Review Questions
- What is the difference between a “derived” versus an “ancestral” trait? Give an example of both, seen in Au. afarensis.
- Which of the paleoenvironment hypotheses have been used to describe early hominin diversity, and which have been used to describe bipedalism?
- Which anatomical features for bipedalism do we see in early hominins?
- Describe the dentition of gracile and robust australopithecines. What might these tell us about their diets?
- List the hominin species argued to be associated with stone tool technologies. Are you convinced of these associations? Why/why not?
Key Terms
Arboreal: Related to trees or woodland.
Aridification: Becoming increasingly arid or dry, as related to the climate or environment.
Aridity Hypothesis: The hypothesis that long-term aridification and expansion of savannah biomes were drivers in diversification in early hominin evolution.
Assemblage: A collection demonstrating a pattern. Often pertaining to a site or region.
Bipedalism: The locomotor ability to walk on two legs.
Breccia: Hard, calcareous sedimentary rock.
Canines: The pointy teeth just next to the incisors, in the front of the mouth.
Cheek teeth: Or hind dentition (molars and premolars).
Chronospecies: Species that are said to evolve into another species, in a linear fashion, over time.
Clade: A group of species or taxa with a shared common ancestor.
Cladistics: The field of grouping organisms into those with shared ancestry.
Context: As pertaining to palaeoanthropology, this term refers to the place where an artifact or fossil is found.
Cores: The remains of a rock that has been flaked or knapped.
Cusps: The ridges or “bumps” on the teeth.
Dental formula: A technique to describe the number of incisors, canines, premolars, and molars in each quadrant of the mouth.
Derived traits: Newly evolved traits that differ from those seen in the ancestor.
Diastema: A tooth gap between the incisors and canines.
Early Stone Age (ESA): The earliest-described archaeological period in which we start seeing stone-tool technology.
East African Rift System (EARS): This term is often used to refer to the Rift Valley, expanding from Malawi to Ethiopia. This active geological structure is responsible for much of the visibility of the paleoanthropological record in East Africa.
Enamel: The highly mineralized outer layer of the tooth.
Encephalization: Expansion of the brain.
Extant: Currently living—i.e., not extinct.
Fallback foods: Foods that may not be preferred by an animal (e.g., foods that are not nutritionally dense) but that are essential for survival in times of stress or scarcity.
Fauna: The animals of a particular region, habitat, or geological period.
Faunal assemblages: Collections of fossils of the animals found at a site.
Faunal turnover: The rate at which species go extinct and are replaced with new species.
Flake: The piece knocked off of a stone core during the manufacture of a tool, which may be used as a stone tool.
Flora: The plants of a particular region, habitat, or geological period.
Folivorous: Foliage-eating.
Foramen magnum: The large hole (foramen) at the base of the cranium, through which the spinal cord enters the skull.
Fossil: The remains or impression of an organism from the past.
Frugivorous: Fruit-eating.
Generalist: A species that can thrive in a wide variety of habitats and can have a varied diet.
Glacial: Colder, drier periods during an ice age when there is more ice trapped at the poles.
Gracile: Slender, less rugged, or pronounced features.
Hallux: The big toe.
Holotype: A single specimen from which a species or taxon is described or named.
Hominin: A primate category that includes humans and our fossil relatives since our divergence from extant great apes.
Honing P3: The mandibular premolar alongside the canine (in primates, the P3), which is angled to give space for (and sharpen) the upper canines.
Hyper-robust: Even more robust than considered normal in the Paranthropus genus.
Hypodigm: A sample (here, fossil) from which researchers extrapolate features of a population.
Incisiform: An adjective referring to a canine that appears more incisor-like in morphology.
Incisors: The teeth in the front of the mouth, used to bite off food.
Interglacial: A period of milder climate in between two glacial periods.
Isotopes: Two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons, giving them the same chemical properties but different atomic masses.
Knappers: The people who fractured rocks in order to manufacture tools.
Knapping: The fracturing of rocks for the manufacture of tools.
Large Cutting Tool (LCT): A tool that is shaped to have functional edges.
Last Common Ancestor (LCA): The hypothetical final ancestor (or ancestral population) of two or more taxa before their divergence.
Lithic: Relating to stone (here to stone tools).
Lumbar lordosis: The inward curving of the lower (lumbar) parts of the spine. The lower curve in the human S-shaped spine.
Lumpers: Researchers who prefer to lump variable specimens into a single species or taxon and who feel high levels of variation is biologically real.
Megadont: An organism with extremely large dentition compared with body size.
Metacarpals: The long bones of the hand that connect to the phalanges (finger bones).
Molars: The largest, most posterior of the hind dentition.
Monophyletic: A taxon or group of taxa descended from a common ancestor that is not shared with another taxon or group.
Morphology: The study of the form or size and shape of things; in this case, skeletal parts.
Mosaic evolution: The concept that evolutionary change does not occur homogeneously throughout the body in organisms.
Obligate bipedalism: Where the primary form of locomotion for an organism is bipedal.
Occlude: When the teeth from the maxilla come into contact with the teeth in the mandible.
Oldowan: Lower Paleolithic, the earliest stone tool culture.
Orthognathic: The face below the eyes is relatively flat and does not jut out anteriorly.
Paleoanthropologists: Researchers that study human evolution.
Paleoenvironment: An environment from a period in the Earth’s geological past.
Parabolic: Like a parabola (parabola-shaped).
Phalanges: Long bones in the hand and fingers.
Phylogenetics: The study of phylogeny.
Phylogeny: The study of the evolutionary relationships between groups of organisms.
Pliocene: A geological epoch between the Miocene and Pleistocene.
Polytypic: In reference to taxonomy, having two or more group variants capable of interacting and breeding biologically but having morphological population differences.
Postcranium: The skeleton below the cranium (head).
Premolars: The smallest of the hind teeth, behind the canines.
Procumbent: In reference to incisors, tilting forward.
Prognathic: In reference to the face, the area below the eyes juts anteriorly.
Quaternary Ice Age: The most recent geological time period, which includes the Pleistocene and Holocene Epochs and which is defined by the cyclicity of increasing and decreasing ice sheets at the poles.
Relative dating: Dating techniques that refer to a temporal sequence (i.e., older or younger than others in the reference) and do not estimate actual or absolute dates.
Robust: Rugged or exaggerated features.
Site: A place in which evidence of past societies/species/activities may be observed through archaeological or paleontological practice.
Specialist: A specialist species can thrive only in a narrow range of environmental conditions or has a limited diet.
Splitters: Researchers who prefer to split a highly variable taxon into multiple groups or species.
Taxa: Plural of taxon, a taxonomic group such as species, genus, or family.
Taxonomy: The science of grouping and classifying organisms.
Techno-complex: A term encompassing multiple assemblages that share similar traits in terms of artifact production and morphology.
Thermoregulation: Maintaining body temperature through physiologically cooling or warming the body.
Ungulates: Hoofed mammals—e.g., cows and kudu.
Volcanic tufts: Rock made from ash from volcanic eruptions in the past.
Valgus knee: The angle of the knee between the femur and tibia, which allows for weight distribution to be angled closer to the point above the center of gravity (i.e., between the feet) in bipeds.
About the Authors

Kerryn Warren, Ph.D.
Grad Coach International, kerryn.warren@gmail.com
Kerryn Warren is a dissertation coach at Grad Coach International and is passionate about stimulating research thinking in students of all levels. She has lectured on multiple topics, including archaeology and human evolution, with her research and science communication interests including hybridization in the hominin fossil record (stemming from research from her Ph.D.) and understanding how evolution is taught in South African schools. She also worked as one of the “Underground Astronauts,” selected to excavate Homo naledi remains from the Rising Star Cave System in the Cradle of Humankind.

K. Lindsay Hunter, M.A., Ph.D. candidate
CARTA, k.lindsay.hunter@gmail.com
Lindsay Hunter is a trained palaeoanthropologist who uses her more than 15 years of experience to make sense of the distant past of our species to build a better future. She received her master’s degree in biological anthropology from the University of Iowa and is completing her Ph.D. in archaeology at the University of the Witwatersrand in Johannesburg, South Africa. She has studied fossil and human bone collections across five continents with major grant support from the National Science Foundation (United States) and the Wenner-Gren Foundation for Anthropological Research. As a National Geographic Explorer, Lindsay developed and managed the National Geographic–sponsored Umsuka Public Palaeoanthropology Project in the Cradle of Humankind World Heritage Site (CoH WHS) in South Africa from within Westbury Township, Johannesburg, between 2016–2019. She currently serves as the Community Engagement & Advancement Director for CARTA: The UC San Diego/Salk Institute Center for Academic Research and Training in Anthropogeny in La Jolla, California.

Navashni Naidoo, M.Sc.
University of Cape Town, nnaidoo2@illinois.edu
Navashni Naidoo is a researcher at Nelson Mandela University, lecturing on physical geology. She completed her Master’s in Science in Archaeology in 2017 at the University of Cape Town. Her research interests include developing paleoenvironmental proxies suited to the African continent, behavioral ecology, and engaging with community-driven archaeological projects. She has excavated at Stone Age sites across Southern Africa and East Africa. Navashni is currently pursuing a PhD in the Department of Anthropology at the University of Illinois.

Silindokuhle Mavuso, M.Sc.
University of Witwatersrand, S.muvaso@ru.ac.za
Silindokuhle has always been curious about the world around him and how it has been shaped. He is a lecturer at Rhodes University of Witwatersrand (Wits), and conducts research on palaeoenvironmental reconstruction and change of the northeastern Turkana Basin’s Pleistocene sequence. Silindokuhle began his education with a B.Sc. (Geology, Archaeology, and Environmental and Geographical Sciences) from the University of Cape Town before moving to Wits for a B.Sc. Honors (geology and paleontology) and M.Sc. in geology. He is currently concluding his PhD Studies. During this time, he has gained more training as a Koobi Fora Fieldschool fellow (Kenya) as well as an Erasmus Mundus scholar (France). Silindokuhle is a Plio-Pleistocene geologist with a specific focus on identifying and explaining past environments that are associated with early human life and development through time. He is interested in a wide range of disciplines such as micromorphology, sedimentology, geochemistry, geochronology, and sequence stratigraphy. He has worked with teams from significant eastern and southern African hominid sites including Elandsfontein, Rising Star, Sterkfontein, Gondolin, Laetoli, Olduvai, and Koobi Fora.
For Further Exploration
The Smithsonian Institution website hosts descriptions of fossil species, an interactive timeline, and much more.
The Maropeng Museum website hosts a wealth of information regarding South African Fossil Bearing sites in the Cradle of Humankind.
This quick comparison between Homo naledi and Australopithecus sediba from the Perot Museum.
This explanation of the braided stream by the Perot Museum.
A collation of 3-D files for visualizing (or even 3-D printing) for homes, schools, and universities.
PBS learning materials, including videos and diagrams of the Laetoli footprints, bipedalism, and fossils.
A wealth of information from the Australian Museum website, including species descriptions, family trees, and explanations of bipedalism and diet.
References
Alemseged, Zeresenay, Fred Spoor, William H. Kimbel, René Bobe, Denis Geraads, Denné Reed, and Jonathan G. Wynn. 2006. “A Juvenile Early Hominin Skeleton from Dikika, Ethiopia.” Nature 443 (7109): 296–301.
Asfaw, Berhane, Tim White, Owen Lovejoy, Bruce Latimer, Scott Simpson, and Gen Suwa. 1999. “Australopithecus garhi: A New Species of Early Hominid from Ethiopia.” Science 284 (5414): 629–635.
Behrensmeyer, Anna K., Nancy E. Todd, Richard Potts, and Geraldine E. McBrinn. 1997. “Late Pliocene Faunal Turnover in the Turkana Basin, Kenya, and Ethiopia.” Science 278 (5343): 637–640.
Berger, Lee R., Darryl J. De Ruiter, Steven E. Churchill, Peter Schmid, Kristian J. Carlson, Paul HGM Dirks, and Job M. Kibii. 2010. “Australopithecus sediba: A New Species of Homo-like Australopith from South Africa.” Science 328 (5975): 195–204.
Bobe, René, and Anna K. Behrensmeyer. 2004. “The Expansion of Grassland Ecosystems in Africa in Relation to Mammalian Evolution and the Origin of the Genus Homo.” Palaeogeography, Palaeoclimatology, Palaeoecology 207 (3–4): 399–420.
Brain, C. K. 1967. “The Transvaal Museum's Fossil Project at Swartkrans.” South African Journal of Science 63 (9): 378–384.
Broom, R. 1938a. “More Discoveries of Australopithecus.” Nature 141 (1): 828–829.
Broom, R. 1938b. “The Pleistocene Anthropoid Apes of South Africa.” Nature 142 (3591): 377–379.
Broom, R. 1947. “Discovery of a New Skull of the South African Ape-Man, Plesianthropus.” Nature 159 (4046): 672.
Broom, R. 1950. “The Genera and Species of the South African Fossil Ape-Man.” American Journal of Physical Anthropology 8 (1): 1–14.
Brunet, Michel, Alain Beauvilain, Yves Coppens, Emile Heintz, Aladji HE Moutaye, and David Pilbeam. 1995. “The First Australopithecine 2,500 Kilometers West of the Rift Valley (Chad).” Nature 378 (6554): 275–273.
Cerling, Thure E., Jonathan G. Wynn, Samuel A. Andanje, Michael I. Bird, David Kimutai Korir, Naomi E. Levin, William Mace, Anthony N. Macharia, Jay Quade, and Christopher H. Remien. 2011. “Woody Cover and Hominin Environments in the Past 6 Million Years.” Nature 476, no. 7358 (2011): 51-56..
Clarke, Ronald J. 1998. “First Ever Discovery of a Well-Preserved Skull and Associated Skeleton of Australopithecus.” South African Journal of Science 94 (10): 460–463.
Clarke, Ronald J. 2013. “Australopithecus from Sterkfontein Caves, South Africa.” In The Paleobiology of Australopithecus, edited by K. E. Reed, J. G. Fleagle, and R. E. Leakey, 105–123. Netherlands: Springer.
Clarke, Ronald J., and Kathleen Kuman. 2019. “The Skull of StW 573, a 3.67 Ma Australopithecus Prometheus Skeleton from Sterkfontein Caves, South Africa.” Journal of Human Evolution 134: 102634.
Clarke, R. J., and P. V. Tobias. 1995. “Sterkfontein Member 2 Foot Bones of the Oldest South African Hominid.” Science 269 (5223): 521–524.
Constantino, P. J., and B. A. Wood. 2004. “Paranthropus Paleobiology”. In Miscelanea en Homenae a Emiliano Aguirre, volumen III: Paleoantropologia, edited by E. G. Pérez and S. R. Jara, 136–151. Alcalá de Henares: Museo Arqueologico Regional.
Constantino, P. J., and B. A. Wood. 2007. “The Evolution of Zinjanthropus boisei.” Evolutionary Anthropology: Issues, News, and Reviews 16 (2): 49–62.
Dart, Raymond A. 1925. “Australopithecus africanus, the Man-Ape of South Africa.” Nature 115: 195–199.
Darwin, Charles. 1871. The Descent of Man: And Selection in Relation to Sex. London: J. Murray.
Daver, Guillaume, F. Guy, Hassane Taïsso Mackaye, Andossa Likius, J-R. Boisserie, Abderamane Moussa, Laurent Pallas, Patrick Vignaud, and Nékoulnang D. Clarisse. 2022. "Postcranial Evidence of Late Miocene Hominin Bipedalism in Chad." Nature 609 (7925): 94–100.
Heinzelin, Jean de, J. Desmond Clark, Tim White, William Hart, Paul Renne, Giday WoldeGabriel, Yonas Beyene, and Elisabeth Vrba. 1999. “Environment and Behavior of 2.5-Million-Year-Old Bouri Hominids.” Science 284 (5414): 625–629.
DeMenocal, Peter B. D. 2004. “African Climate Change and Faunal Evolution during the Pliocene–Pleistocene.” Earth and Planetary Science Letters 220 (1–2): 3–24.
DeMenocal, Peter B. D. and J. Bloemendal, J. 1995. “Plio-Pleistocene Climatic Variability in Subtropical Africa and the Paleoenvironment of Hominid Evolution: A Combined Data-Model Approach.” In Paleoclimate and Evolution, with Emphasis on Human Origins, edited by E. S. Vrba, G. H. Denton, T. C. Partridge, and L. H. Burckle, 262–288. New Haven: Yale University Press.
Dirks, Paul HGM, Job M. Kibii, Brian F. Kuhn, Christine Steininger, Steven E. Churchill, Jan D. Kramers, Robyn Pickering, Daniel L. Farber, Anne-Sophie Mériaux, Andy I. R. Herries, Geoffrey C. P. King, And Lee R. Berger. 2010. “Geological Setting and Age of Australopithecus sediba from Southern Africa.” Science 328 (5975): 205–208.
Faith, J. Tyler, and Anna K. Behrensmeyer. 2013. “Climate Change and Faunal Turnover: Testing the Mechanics of the Turnover-Pulse Hypothesis with South African Fossil Data.” Paleobiology 39 (4): 609–627.
Grine, Frederick E. 1988. “New Craniodental Fossils of Paranthropus from the Swartkrans Formation and Their Significance in ‘Robust’ Australopithecine Evolution.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 223–243. New York: Aldine de Gruyter.
Grine, Frederick E., Carrie S. Mongle, John G. Fleagle, and Ashley S. Hammond. 2022. "The Taxonomic Attribution of African Hominin Postcrania from the Miocene through the Pleistocene: Associations and Assumptions." Journal of Human Evolution 173: 103255.
Haile-Selassie, Yohannes, Luis Gibert, Stephanie M. Melillo, Timothy M. Ryan, Mulugeta Alene, Alan Deino, Naomi E. Levin, Gary Scott, and Beverly Z. Saylor. 2015. “New Species from Ethiopia Further Expands Middle Pliocene Hominin Diversity.” Nature 521 (7553): 432–433.
Haile-Selassie, Yohannes, Stephanie M. Melillo, Antonino Vazzana, Stefano Benazzi, and Timothy M. Ryan. 2019. “A 3.8-Million-Year-Old Hominin Cranium from Woranso-Mille, Ethiopia.” Nature 573 (7773): 214-219.
Harmand, Sonia, Jason E. Lewis, Craig S. Feibel, Christopher J. Lepre, Sandrine Prat, Arnaud Lenoble, Xavier Boës et al. 2015. “3.3-Million-Year-Old Stone Tools from Lomekwi3, West Turkana, Kenya.” Nature 521 (7552): 310–316.
Hay, Richard L. 1990. “Olduvai Gorge: A Case History in the Interpretation of Hominid Paleoenvironments.” In East Africa: Establishment of a Geologic Framework for Paleoanthropology, edited by L. Laporte, 23–37. Boulder: Geological Society of America.
Hay, Richard L., and Mary D. Leakey. 1982. “The Fossil Footprints of Laetoli.” Scientific American 246 (2): 50–57.
Hlazo, Nomawethu. 2015. “Paranthropus: Variation in Cranial Morphology.” Honours thesis, Archaeology Department, University of Cape Town, Cape Town.
Hlazo, Nomawethu. 2018. “Variation and the Evolutionary Drivers of Diversity in the Genus Paranthropus.” Master’s thesis, Archaeology Department, University of Cape Town, Cape Town.
Johanson, D. C., T. D. White, and Y. Coppens. 1978. “A New Species of the Genus Australopithecus (Primates: Hominidae) from the Pliocene of East Africa.” Kirtlandia 28: 1–14.
Kimbel, William H. 2015. “The Species and Diversity of Australopiths.” In Handbook of Paleoanthropology, 2nd ed., edited by T. Hardt, 2071–2105. Berlin: Springer.
Kimbel, William H., and Lucas K. Delezene. 2009. “‘Lucy’ Redux: A Review of Research on Australopithecus afarensis.” American Journal of Physical Anthropology 140 (S49): 2–48.
Kingston, John D. 2007. “Shifting Adaptive Landscapes: Progress and Challenges in Reconstructing Early Hominid Environments.” American Journal of Physical Anthropology 134 (S45): 20–58.
Kingston, John D., and Terry Harrison. 2007. “Isotopic Dietary Reconstructions of Pliocene Herbivores at Laetoli: Implications for Early Hominin Paleoecology.” Palaeogeography, Palaeoclimatology, Palaeoecology 243 (3–4): 272–306.
Leakey, Louis S. B. 1959. “A New Fossil Skull from Olduvai.” Nature 184 (4685): 491–493.
Leakey, Mary 1971. Olduvai Gorge, Vol. 3. Cambridge: Cambridge University Press.
Leakey, Mary D., and Richard L. Hay. 1979. “Pliocene Footprints in the Laetoli Beds at Laetoli, Northern Tanzania.” Nature 278 (5702): 317–323.
Leakey, Meave G., Craig S. Feibel, Ian McDougall, and Alan Walker. 1995. “New Four–Million-Year-Old Hominid Species from Kanapoi and Allia Bay, Kenya.” Nature 376 (6541): 565–571.
Meave G., Fred Spoor, Frank H. Brown, Patrick N. Gathogo, Christopher Kiarie, Louise N. Leakey, and Ian McDougall. 2001. “New Hominin Genus from Eastern Africa Shows Diverse Middle Pliocene Lineages.” Nature 410 (6827): 433–440.
Lebatard, Anne-Elisabeth, Didier L. Bourlès, Philippe Duringer, Marc Jolivet, Régis Braucher, Julien Carcaillet, Mathieu Schuster et al. 2008. “Cosmogenic Nuclide Dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene Hominids from Chad.” Proceedings of the National Academy of Sciences 105 (9): 3226–3231.
Lee-Thorp, Julia. 2011. “The Demise of ‘Nutcracker Man.’” Proceedings of the National Academy of Sciences 108 (23): 9319–9320.
Lombard, Marlize, L. Y. N. Wadley, Janette Deacon, Sarah Wurz, Isabelle Parsons, Moleboheng Mohapi, Joane Swart, and Peter Mitchell. 2012. “South African and Lesotho Stone Age Sequence Updated.” The South African Archaeological Bulletin 67 (195): 123–144.
Maslin, Mark A., Chris M. Brierley, Alice M. Milner, Susanne Shultz, Martin H. Trauth, and Katy E. Wilson. 2014. “East African Climate Pulses and Early Human Evolution.” Quaternary Science Reviews 101: 1–17.
McHenry, Henry M. 2009. “Human Evolution.” In Evolution: The First Four Billion Years, edited by M. Ruse and J. Travis, 256–280. Cambridge: The Belknap Press of Harvard University Press..
Patterson, Bryan, and William W. Howells. 1967. “Hominid Humeral Fragment from Early Pleistocene of Northwestern Kenya.” Science 156 (3771): 64–66.
Pickering, Robyn, and Jan D. Kramers. 2010. “Re-appraisal of the Stratigraphy and Determination of New U-Pb Dates for the Sterkfontein Hominin Site.” Journal of Human Evolution 59 (1): 70–86.
Potts, Richard. 1998. “Environmental Hypotheses of Hominin Evolution.” American Journal of Physical Anthropology 107 (S27): 93–136.
Potts, Richard. 2013. “Hominin Evolution in Settings of Strong Environmental Variability.” Quaternary Science Reviews 73: 1–13.
Rak, Yoel. 1983. The Australopithecine Face. New York: Academic Press.
Rak, Yoel. 1988. “On Variation in the Masticatory System of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by M. Ruse and J. Travis, 193–198. New York: Aldine de Gruyter.
Semaw, Sileshi. 2000. “The World’s Oldest Stone Artefacts from Gona, Ethiopia: Their Implications for Understanding Stone Technology and Patterns of Human Evolution between 2.6 Million Years Ago and 1.5 Million Years Ago.” Journal of Archaeological Science 27(12): 1197–1214.
Shipman, Pat. 2002. The Man Who Found the Missing Link: Eugene Dubois and his Lifelong Quest to Prove Darwin Right. New York: Simon & Schuster.
Spoor, Fred. 2015. “Palaeoanthropology: The Middle Pliocene Gets Crowded.” Nature 521 (7553): 432–433.
Strait, David S., Frederick E. Grine, and Marc A. Moniz. 1997. A Reappraisal of Early Hominid Phylogeny.” Journal of Human Evolution 32 (1): 17–82.
Thackeray, J. Francis. 2000. “‘Mrs. Ples’ from Sterkfontein: Small Male or Large Female?” The South African Archaeological Bulletin 55: 155–158.
Thackeray, J. Francis, José Braga, Jacques Treil, N. Niksch, and J. H. Labuschagne. 2002. “‘Mrs. Ples’ (Sts 5) from Sterkfontein: An Adolescent Male?” South African Journal of Science 98 (1–2): 21–22.
Toth, Nicholas. 1985. “The Oldowan Reassessed.” Journal of Archaeological Science 12 (2): 101–120.
Vrba, E. S. 1988. “Late Pliocene Climatic Events and Hominid Evolution.” In The Evolutionary History of the Robust Australopithecines, edited by F. E. Grine, 405–426. New York: Aldine.
Vrba, Elisabeth S. 1998. “Multiphasic Growth Models and the Evolution of Prolonged Growth Exemplified by Human Brain Evolution.” Journal of Theoretical Biology 190 (3): 227–239.
Vrba, Elisabeth S. 2000. “Major Features of Neogene Mammalian Evolution in Africa.” In Cenozoic Geology of Southern Africa, edited by T. C. Partridge and R. Maud, 277–304. Oxford: Oxford University Press.
Walker, Alan C., and Richard E. Leakey. 1988. “The Evolution of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 247–258. New York: Aldine de Gruyter.
Walker, Alan, Richard E. Leakey, John M. Harris, and Francis H. Brown. 1986. “2.5-my Australopithecus boisei from West of Lake Turkana, Kenya.” Nature 322 (6079): 517–522.
Ward, Carol, Meave Leakey, and Alan Walker. 1999. “The New Hominid Species Australopithecus anamensis.” Evolutionary Anthropology 7 (6): 197–205.
White, Tim D. 1988. “The Comparative Biology of ‘Robust’ Australopithecus: Clues from Content.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 449–483. New York: Aldine de Gruyter.
White, Tim D., Gen Suwa, and Berhane Asfaw. 1994. “Australopithecus ramidus, a New Species of Early Hominid from Aramis, Ethiopia.” Nature 371 (6495): 306–312.
Wood, Bernard. 2010. “Reconstructing Human Evolution: Achievements, Challenges, and Opportunities.” Proceedings of the National Academy of Sciences 10 (2): 8902–8909.
Wood, Bernard, and Eve K. Boyle. 2016. “Hominin Taxic Diversity: Fact or Fantasy?” Yearbook of Physical Anthropology 159 (S61): 37–78.
Wood, Bernard, and Kes Schroer. 2017. “Paranthropus: Where Do Things Stand?” In Human Paleontology and Prehistory, edited by A. Marom and E. Hovers, 95–107. New York: Springer, Cham.
Acknowledgements
All of the authors in this section are students and early career researchers in paleoanthropology and related fields in South Africa (or at least have worked in South Africa). We wish to thank everyone who supports young and diverse talent in this field and would love to further acknowledge Black, African, and female academics who have helped pave the way for us.


